Abstract
Wastewaters generated during mining and processing of metal sulfide ores are often acidic (pH < 3) and can contain significant concentrations of nitrate, nitrite, and ammonium from nitrogen based explosives. In addition, wastewaters from sulfide ore treatment plants and tailings ponds typically contain large amounts of inorganic sulfur compounds, such as thiosulfate and tetrathionate. Release of these wastewaters can lead to environmental acidification as well as an increase in nutrients (eutrophication) and compounds that are potentially toxic to humans and animals. Waters from cyanidation plants for gold extraction will often conjointly include toxic, sulfur containing thiocyanate. More stringent regulatory limits on the release of mining wastes containing compounds such as inorganic sulfur compounds, nitrate, and thiocyanate, along the need to increase production from sulfide mineral mining calls for low cost techniques to remove these pollutants under ambient temperatures (approximately 8 °C). In this study, we used both aerobic and anaerobic continuous cultures to successfully couple inorganic sulfur compound (i.e. thiosulfate and thiocyanate) oxidation for the removal of nitrogenous compounds under neutral to acidic pH at the low temperatures typical for boreal climates. Furthermore, the development of the respective microbial communities was identified over time by DNA sequencing, and found to contain a consortium including populations aligning within Flavobacterium, Thiobacillus, and Comamonadaceae lineages. This is the first study to remediate mining waste waters by coupling autotrophic thiocyanate oxidation to nitrate reduction at low temperatures and acidic pH by means of an identified microbial community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wastewaters generated during mining and processing of metal sulfide ores can contain significant concentrations of nitrogenous compounds. These include nitrate (NO3 −), nitrite (NO2 −), and ammonium (NH4 +) that are derived from nitrogen based explosives, such as ‘ammonium nitrate fuel oil’ (ANFO) and watergel-based explosives (Revey 1996). It has been reported that 0.2 to 28% of explosives are undetonated during blasting (best practice is 2–5% for ANFO) (Morin and Hutt 2009) and if the nitrogenous compounds are released, they cause eutrophication in water bodies. Some consequences of eutrophication are an increase in oxygen demand that results in recipient waters turning anoxic (Conley et al. 2011), possible hazards to human health (Knobeloch et al. 2000), and potential toxicity to aquatic animals (Alonso and Camargo 2003). In addition to nitrogenous pollutants, some mining wastewaters are rich in inorganic sulfur compounds (ISCs). These compounds can include toxic thiocyanate (SCN−) generated from cyanide used in gold extraction (Zagury et al. 2004). A further major ISC typically found in mining wastewaters is thiosulfate (S2O3 2−) that can be generated from for example, hydrogen sulfide added during molybdenum flotation (Liljeqvist et al. 2011), ammoniacal thiosulfate leaching (Grosse et al. 2003), and dissolution of metal sulfides such as pyrite (Eq. 1) (Dopson and Johnson 2012; Schippers and Sand 1999). Complete oxidation of ISCs generates sulfuric acid (Eq. 2) that results in serious environmental damage if released due to acidification of recipient water bodies in a process termed ‘acid mine drainage’, abbreviated as AMD (Chen et al. 2016).
A complicating factor in treating mining wastewaters in cold climates, such as in northern Sweden, is that the water temperature rarely increases above approximately 15 °C (Liljeqvist et al. 2011). Due to economic constraints, this requires the development of (bio)remediation processes for contaminant removal that operate at low temperatures.
High concentrations of nitrogen compounds such as NH4 + and NO3 − in mining waters can be treated by physical (e.g. membrane separation), chemical (e.g. electrochemical removal), and biological (e.g. nitrification and denitrification) methods (reviewed in Jermakka et al. 2015). Biological nitrogen removal typically consists of nitrification (NH4 + and NO2 − conversion to NO3 −) followed by denitrification that couples oxidation of an electron donor [e.g. organic carbon, ferrous iron (Fe2+), or sulfide (S2−)] to NO3 − reduction that if completely reduced, eventually forms nitrogen gas (Lu et al. 2014). Recent work has confirmed that organic carbon compounds such as methanol and acetate favor specific denitrifying bacteria and successfully removes NO3 − (Li et al. 2016a). However, the use of methanol as an electron donor produces CaCO3, which increases the pH, and affects the denitrifying microbial community in the system. For example, it has been observed that up to 22.3% of the NO3 − concentration in a methanol fed bioreactor accumulated as NO2 − while increasing the pH up to 9.2. In contrast, at pH 7.6 the accumulation of NO2 − was negligible (Li et al. 2016b). Biological nitrogen removal in wetlands or reactive barriers functions by promoting denitrification processes in sediments or buried organic matter, respectively (Batty and Younger 2004). Nitrogen removal has also been demonstrated in bioreactors with organic (e.g. Papirio et al. 2014; Zou et al. 2014) and inorganic electron donors including ISCs (e.g. Chung et al. 2014; Di Capua et al. 2015; Zhang et al. 2009; Zou et al. 2016). Other studies investigating S2O3 2− oxidation coupled with NO3 − reduction utilized pure cultures of Thiobacillus denitrificans (Justin and Kelly 1978a, b) and Sulfurimonas denitrificans [originally named Thiomicrospira denitrificans (Hoor 1975)]. SCN− oxidation can also be linked to NO3 − reduction by the soda-lake sediment dwelling species Thialkalivibrio thiocyanodenitrificans (Sorokin et al. 2004). In some microorganisms, SCN− can act as an electron donor as well as providing sulfur and nitrogen to the cell (Ogawa et al. 2013). Finally, a novel SCN− degrading pathway has been identified by metagenomics in a Thiobacillus-like strain (Kantor et al. 2015). Advantages of ISC fed autotrophic versus heterotrophic denitrification are that less sludge is produced and costs are reduced as an organic electron donor does not need to be added. A disadvantage is the reduced pH due to generation of sulfuric acid (Eq. 2). However, efficient autotrophic, ISC-fed, low temperature denitrification systems for thiocyanate removal remain to be developed.
To be able to increase production from sulfide mineral mining coupled to more stringent regulatory limits on the release of ISCs and NO3 −, low cost techniques to remove these pollutants under ambient temperatures are required. In this study, autotrophic ISC oxidation coupled to removal of nitrogenous compounds was investigated at low temperature. In addition, the structures of the microbial communities were identified by DNA sequencing.
Materials and methods
Bioreactor set-up
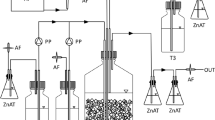
Continuous culture bioreactors (500 mL working volume) were operated with a basal salts medium plus nitrate as electron acceptor. The feed medium was autoclaved before filter sterilized (0.2 µm membrane filter; Sarstedt, Nümbrecht, Germany) ISCs were added, i.e. thiosulfate (0.89 mM) for a thiosulfate reactor and thiocyanate (6.88 mM) for the aerobic and anaerobic reactors (Table 1). For both experiments (described below), the retention time was 9 h and the initial pH in the bioreactors was adjusted to 8.0–8.5 by addition of 1 M Na2CO3. Microbial biomass was retained in the reactors by promoting biofilm growth on 50% (vol/vol) autoclaved high surface area biofilm carriers (AnoxKaldnes). The bioreactors and medium feed were maintained at the desired temperature by placing them in a constant temperature room.
Thiocyanate fed bioreactors
Two SCN− fed bioreactors were operated between June 3rd 2014 and November 9th 2015 (totaling 524 days; Table 2). One of the bioreactors was maintained aerobic (referred to as R1) and the other anaerobic (R2) by sparging with 300 mL sterile filtered air and N2-gas (0.2 µm Acro 50 air filter; PALL Corporation), respectively. The reactors were inoculated with water sampled from a municipal wastewater treatment plant (Tuvanverket), Skellefteå, Sweden. The temperature in the bioreactors was lowered over time: day 0–231 at 21 °C; 232–300 at 15 °C; 301–337 at 10 °C; followed by 8 °C until the end of the experiment. In addition, the pH was lowered with 4.6 M H2SO4 to 5.5 at day 448 and then to 3.8 at day 510.
Thiosulfate fed bioreactor
The S2O3 2− fed bioreactor (R3) was maintained at 8 °C and operated between February 11th 2013 and December 16th 2013 (308 days) in microaerophilic conditions i.e. the reactor was not sparged with air and the medium was stirred gently with a magnetic flea such that the surface of the medium was not broken. The bioreactor was inoculated with a sediment sample removed from an AMD stream 250 m below ground in the Boliden operated Kristineberg mine, Sweden. Details of the AMD stream including the microorganisms present (but not from the underlying sediment used here) are available (Liljeqvist et al. 2015).
Chemical measurements
Measurements for chemical parameters were conducted by opening and sampling the bioreactors. The R1 and R2 SCN− fed bioreactors were measured for pH, redox potential, chemical oxygen demand (COD), NO2 −, NO3 −, NH4 + and SCN− while the R3 bioreactor fed with S2O3 2− was measured for pH, redox potential, NO3 −, protein concentration, S2O3 2−, and tetrathionate (S4O6 2−). pH and redox potential were measured using electrodes (pHenomenal, VWR pH electrode and Ag/AgCl SI Analytics electrode, Mettler Toledo, respectively). Protein concentration was measured using the Bio-Rad protein assay (Bradford protein assay, Bio-Rad). COD (LCI 500), NO2 − (LCK 342), NO3 − (LCK 339), and NH4 + (LCK303) were measured using Hach Lange kits according to the manufacturer’s instructions. S4O6 2− and S2O3 2− were measured by forming an iron-SCN−complex according to Kelly et al. (1969) with the modification of using an ammonium/acetate buffer before the addition of the iron nitrate (Dopson and Lindström 1999). SCN− was measured as described for S4O6 2− and S2O3 2− except that the step of adding cyanide was omitted.
DNA extraction, RFLP, and DNA sequencing of the S2O3 2− bioreactor inoculum
The inoculum of the S2O3 2− fed R3 bioreactor was sampled for DNA extraction by centrifuging 10 mL bioreactor content at 10,000×g for 10 min. The resulting pellet was washed with 100 mM Tris HCl and 10 mM EDTA and re-suspended in 10 mM Tris HCl and 1 mM EDTA. Cells were lysed and genomic DNA extracted according to Dopson and Lindström (2004) and Morales et al. (2005) before the PCR fragments were analyzed by restriction fragment length polymorphism (RFLP) as described in Wu et al. (2013). Briefly, a portion of the bacterial 16S rRNA gene was amplified using the primers GM5F and 907R (Muyzer et al. 1995), the generated PCR fragments were cleaned using a QIAquick PCR Purification Kit (Qiagen), cloned using the pGEM-T Easy Vector System (Promega), and transformed (Hanahan 1983) into Escherichia coli strain DH5α (Sambrook et al. 1989). The resulting transformants were plated onto Luria–Bertani (LB) plates and plasmid DNA extracted using the QIAGEN Plasmid Mini Kit. The plasmids were individually cut using the restriction enzymes HhaI and MspI and fragments separated by agarose gel electrophoresis. Restriction fragment patterns were identified and representative unique clones were Sanger sequenced in both directions by Macrogen. Obtained sequences were checked using DECIPHER (Wright et al. 2012), edited in Geneious version 6.0.6 (Biomatters Ltd Auckland, New Zealand), and compared with the NCBI GenBank database. A phylogenetic tree was created with Molecular Evolutionary Genetic Analysis version 5.1 (Tamura et al. 2011). 16S rRNA gene sequences were submitted to GenBank with the BioProject accession number PRJNA347259.
DNA extraction, high throughput 16S rRNA gene tag sequencing, and bioinformatics
At the end of the experiment (day 308), a sample of the microbial community attached to biofilm carriers in the S2O3 2− fed R1 and R2 bioreactors was detatched by vortexing several biofilm carrier pellets in 5 mL culture medium. DNA was extracted and high-throughput sequencing (Illumina MiSeq) performed as described below. In addition, cells (50 mL culture) from the aerobic R1 and anaerobic R2 SCN− fed reactor inocula were filtered onto a 25 mm 0.2 µm pore-size filter (Supor-200, PALL Corporation). Finally, the microbial communities in the two SCN− fed reactors were sampled (one biofilm carrier for each reactor at each ocassion) for DNA extraction and second generation sequencing as described for the S2O3 2− fed bioreactor biofilm carrier pellets.
DNA to be sequenced using high-throughput Illumina methodology was extracted using the PowerWater DNA Isolation Kit (MO BIO Laboratories) and partial 16S rRNA genes amplified with a modified PCR protocol by Hugerth et al. (2014) using primers 341F and 805R (Herlemann et al. 2011). The PCR amplification and Illumina libraries were constructed and sequenced at Science for Life Laboratory, Sweden (www.scilifelab.se) according to Lindh et al. (2015). The UPARSE pipeline (Edgar 2013) was used to process the sequencing data. Clustered OTUs were annotated against the SINA/SILVA database (SILVA 119; Quast et al. 2013), and finally analyzed in Explicet 2.10.5 (Robertson et al. 2013). The amount of pair-end reads received from the sequencing facility, merged and quality trimmed reads, and amount of OTUs clustered can be found in Supplemental File 1. Sequences for phylogenetic analyses were aligned with ClustalW, and built in MEGA 7 as maximum likelihood trees (Kumar et al. 2016). 16S rRNA gene sequences were submitted to GenBank with the BioProject accession number PRJNA347259.
Results
Aerobic SCN− fed bioreactor
Chemical measurements were conducted by sampling the bioreactor. The aerobic SCN− fed R1 bioreactor had SCN− concentrations of 50.8 ± 72.6 mg L−1 (number of data points (n) = 37; ± 1 SD). This corresponded to a 85.2 ± 21.5% decrease in electron donor, i.e. 5.8 ± 1.5 mM. This was the case during most measuring points until the temperature was decreased below 15 °C when the SCN− concentration increased gradually and remained at 152.6 ± 86.9 mg L−1 (n = 51; 62.5 ± 12.9% decrease of electron donor, i.e. 4.3 ± 0.9 mM) until pH was decreased. The NO3 − concentration in the aerobic bioreactor was initially 1230 mg L−1 and after 21 days it had decreased and stabilized at 548.8 ± 176.4 mg L−1 (n = 33; 71.0 ± 10.9% decrease of electron acceptor, i.e. 22.9 ± 3.5 mM) until the temperature was lowered to 15 °C. After the temperature decrease, the NO3 − concentration remained at 436.2 ± 64.0 mg L−1 (n = 51; 77.2 ± 2.0% decrease of electron acceptor, i.e. 24.9 ± 0.6 mM) until pH decrease, with a following slight decrease after pH was lowered. Most of the time in the aerobic reactor the NO2 − concentration was below 10 mg L−1, except peaking between day 133-189 at 18.4 ± 14.2 mg L−1 (n = 13; Fig. 1). The concentration of NH4 + was 75.2 ± 25.4 mg L−1 (n = 38) until it gradually dropped within a range of 14–49 mg L−1 after the temperature was lowered to 15 and 10 °C on day 231 and 300, respectively (Fig. 1). Pearson correlations from the aerobic bioreactor showed that NH4 + positively correlated with NO3 − and NO2 − (p < 0.01; r = 0.592 and 0.519, respectively). For a full list of Pearson correlations see Supplemental File 2. The calculated denitrification (NO3 −–N removal) efficiency in the aerobic reactor was typically in the range of 70–80% throughout the experiment (Fig. 2). Initially, the bioreactor had a pH of 7.8 and remained at 7.2 ± 0.5 until the pH was decreased with H2SO4 to 5.5 and 3.5 at day 448 and 510, respectively (n = 89; Fig. 1). The redox potential remained at 198.5 ± 35.9 mV until the temperature was decreased to 15 °C on day 231 (n = 39). At this point, the redox increased and remained at 233.6 ± 20.7 mV until the end of the experiment (n = 63; Fig. 1). Optical density (460 nm) in the water phase remained low throughout the experiment (<0.01 abs) while COD had a high variation with values of 3–462 mg L−1 before the temperature was decreased to 15 °C and then it eventually stabilized after the pH was decreased to 5.5 at 172–248 mg L−1 (Supplemental File 3).
Chemical measurements from the aerobic R1 (light grey circles) and anaerobic R2 (dark grey circles) SCN− fed bioreactor adapted from 21 °C room temperature to 8 °C over a period of 338 days. The pH was lowered from 8.0–8.5 to 5.5 on day 448 and further lowered to pH 3.8 on day 510. The horizontal black line denotes the input concentration in the medium (electron donor SCN−, electron acceptor NO3 −, and NH4 +). One SCN− outlier value (751.5 mg L−1) has been removed for the anaerobic bioreactor on day 57
Changes in NO3 −–N, SCN−, and NH4 +–N concentrations over time compared to the input (7.1, 6.8, and 0.4 mM, respectively) in the aerobic R1 and anaerobic R2 SCN− fed bioreactor. Denitrification efficiency (% NO3 −–N removed from input) is shown on the right y-axis. One outlier SCN− value (+6.1 mM) was removed for the anaerobic bioreactor on day 57
The molecular phylogeny of the R1 bioreactor inoculum as well as at the end of the 15 and 10 °C temperature changes was investigated (Fig. 3). A full list of annotated OTUs with relative abundance can be found in Supplemental File 4. The aerobic inoculum was dominated by the genera Rhizobacter (relative abundance of 12.7%), Thiobacillus (12.3%), Mycobacterium (11.5%), Isosphaera (5.9%), and the family Hyphomonadaceae (5.8%). After the temperature had been lowered to 15 °C for 69 days the microbial community in the aerobic bioreactor had changed to consist mainly of the genera Flavobacterium (56.0%) and Thiobacillus (30.9%). This high relative abundance of the genus Thiobacillus was further increased after the temperature had been lowered to 10 °C for 38 days. The aerobic microbial community was then dominated by the genera Flavobacterium (43.8%) and Thiobacillus (43.0%). Phylogenetic analysis of closely related species downloaded from NCBI GenBank indicated that the Thiobacillus OTUs from the 16S rRNA gene data were most closely related to T. denitrificans; the Rhizobacter OTU was indicated to be related to Rhizobacter fulvus; and the Flavobacterium OTUs to Flavobacterium hydatis (Fig. 3).
Phylogenetic tree of the dominant OTUs in the aerobic SCN− fed R1 bioreactor and stacked bars of single OTUs. The tree with the highest maximum log likelihood (−2032.68) is shown, alongside close relative species (blue text) obtained from NCBI GenBank. DNA was extracted and the 16S rRNA gene was sequenced; from the inoculum; at day 300 after 69 days at 15 °C; and at day 338 after 38 days at 10 °C. The grouping “Other OTUs” comprises OTUs with less than 1% total relative abundance (among all samples summed)
Anaerobic SCN− fed bioreactor
The SCN− concentration in the anaerobic R2 bioreactor was 44.9 ± 63.5 mg L−1 (n = 36; corresponding to a 82.1 ± 34.1% decrease of electron donor, i.e. 5.6 ± 2.3 mM) until the temperature was decreased below 15 °C when the SCN− concentration gradually increased and remained at 170.2 ± 88.9 mg L−1 (n = 51; 59.1 ± 13.7% decrease of electron donor, i.e. 4.0 ± 0.9 mM) until pH was decreased. This pattern starting with the temperature decrease to 15 °C was also observed for COD and NO3 −, and a decrease in NH4 + and NO2 − (Fig. 1). These changes were confirmed with Pearson correlations for the chemical parameters in the anaerobic vessel which showed that SCN- positively correlated with COD and NO3 − (p < 0.01; r = 0.468 and 0.329, respectively). While negatively correlating with NH4 + and NO2 − (p < 0.01; r = − 0.617 and −0.530, respectively).
The NO3 − concentration in the anaerobic R2 bioreactor showed a similar pattern to that of the aerobic R1 reactor, but concentrations remained lower. The initial NO3 − concentration at 21 °C was 1240 mg L−1 that decreased over the initial 21 days and stabilized at 317.7 ± 110.3 mg L−1 (n = 53; 86.9 ± 4.2% decrease of electron acceptor, i.e. 28.0 ± 1.3 mM) until the temperature was lowered below 15 °C. After the temperature decrease, the NO3 − concentration remained at 419.5 ± 64.3 mg L−1 (n = 51; 78.8 ± 2.7% decrease of electron acceptor, i.e. 25.4 ± 0.9 mM) until pH was lowered. This lower NO3 − concentration in the anaerobic R2 reactor at 21 °C indicated a more efficient reduction of NO3 − compared to the aerobic R1 reactor. This difference between the samples could not be seen after the temperature and pH had been lowered (i.e. below 15 °C and pH 8). Up until day 300, the NO2 − concentrations in the anaerobic R2 reactor showed the opposite pattern compared to the aerobic R1 reactor and varied from low (~1 mg L−1) to high (92.4 mg L−1) but with an overall high concentration of 35.6 ± 23.6 mg L−1 (n = 59). This difference between the R1 and R2 reactors disappeared when the temperature was lowered to 10 °C on day 300 (Fig. 1). The NH4 + concentration in the anaerobic R2 reactor showed a similar pattern to the aerobic R1 bioreactor. Concentrations remained at 64.7 ± 23.4 mg L−1 (n = 38) until it gradually dropped to a range of 9–51 mg L−1 after the temperature was lowered to 15 and 10 °C (n = 30; Fig. 1). Pearson correlations from the anaerobic R2 reactor showed that NH4 + correlated positively with NO2 − (p < 0.01; r = 0.774) while negatively correlating with NO3 − (p < 0.01; r = −0.280). The calculated denitrification efficiency in the anaerobic R2 reactor was typically in the range of 70–90% throughout the experiment (Fig. 2). pH measurements were similar to the aerobic R1 reactor throughout the experiment, with an initial pH of 8.1 that remained in the range of 7.1 ± 0.5 until the pH was decreased with H2SO4 (n = 89; Fig. 1). The redox potential showed a similar pattern to the aerobic R1 reactor and remained at 191.9 ± 31.4 mV until the temperature was decreased to 15 °C on day 231 (n = 39). The redox then remained at 235.7 ± 23.3 mV until the pH was decreased to 3.5 at day 510 causing the redox to gradually increase to 309.3 mV (n = 60; Fig. 1). Optical density (460 nm) in the water phase remained low throughout the experiment (<0.01 abs) and COD in the anaerobic R2 bioreactor showed a similar pattern to the aerobic R1 reactor, with a trend to gradually increase during the experiment and stabilize the longer the experiment progressed. COD was measured with a high variation throughout the experiment (8–466 mg L−1) before the 15 °C temperature decrease, to eventually stabilize after pH was decreased to 5.5 at 198–250 mg L−1 (Supplemental File 3).
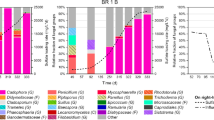
The molecular phylogeny of the anaerobic R2 bioreactor inoculum as well as at the end of the 15 and 10 °C temperature changes was investigated (Fig. 4) and a full list of annotated OTUs plus relative abundance is provided in Supplemental File 4. The anaerobic inoculum mainly consisted of the genera Thiobacillus with a relative abundance of 80.7%, Flavobacterium (6.3%), and Ferruginibacter (4.6%). After 69 days at 15 °C, the community was dominated by the family Comamonadaceae (24.5%) plus the genera Thiobacillus (20.9%), Ferruginibacter (7.1%), Flavobacterium (6.2%), and one OTU annotated as an unclassified proteobacteria (17.6%). After the decrease to 10 °C, the community mainly consisted of the genera Ferruginibacter (34.8%), Thiobacillus (18.4%), and Flavobacterium (15.9%). Phylogenetic analysis of close relative species downloaded from NCBI GenBank indicated that the Thiobacillus OTUs from the 16S rRNA gene data were most closely related to T. denitrificans (Fig. 4).
Phylogenetic tree of the dominant OTUs in the anaerobic SCN− fed R2 bioreactor and stacked bars of single OTUs. The tree with the highest maximum log likelihood (−1977.7296) is shown, alongside close relative species (blue text) obtained from NCBI GenBank DNA was extracted and the 16S rRNA gene was sequenced; from the inoculum; at day 300 after 69 days at 15 °C; and at day 338 after 38 days at 10 °C. The grouping “Other OTUs” comprises OTUs with less than 1% total relative abundance (among all samples summed)
Microaerophilic S2O3 2− fed bioreactor
In the S2O3 2− fed R3 bioreactor, the S2O3 2− concentration in the vessel gradually decreased during the experiment from 1.1 mM on day 7 to 0 mM after 266 days. The S4O6 2− concentration in the vessel gradually decreased throughout the experiment from 19.7 to 0 µM on day 91 suggesting little S4O6 2− accumulated from S2O3 2− oxidation. However, a concomitant decrease in NO3 − was not observed in relation to the decrease of S2O3 2− and the concentration of NO3 − concentration was stable at 59.0 ± 6.2 mg L−1 throughout the experiment (n = 69; Fig. 5; corresponding to a 48.6 ± 8.2% decrease of electron acceptor, i.e. 0.9 ± 0.2 mM). However, this concentration was less than the 113.3 mg L−1 in the influent potentially due to denitrification. The pH remained at 6.7 ± 0.4 throughout the experiment (n = 69; Fig. 5) while the redox potential gradually decreased from 203.3 to 50.6 mV for the first 154 days when it sharply increased to 220.5 mV. This increase was probably due to the stirring in the vessel being too high and hence, introducing more oxygen into the vessel. The protein concentration was stable throughout the whole experiment and remained at 10.6 ± 0.44 µg mL−1 (n = 68) expect during the first day (31.2 µg mL−1) when the experiment started (Supplemental File 5).
The inoculum of the S2O3 2− fed R3 reactor was sequenced after RFLP analysis and sequences derived from unique bands from the electrophoresis gel were aligned with known sequences (Fig. 6a). The results indicated that the inoculum consisted of T. denitrificans-like species and/or iron-reducing bacteria. Other sequences clustered tightly with unclassified Gammaproteobacteria and Lysobacter brunescens. DNA extracted from the end of the experiment after 308 days was sequenced on the Illumina platform and showed a microbial community consisting of highly abundant single OTUs belonging to the genera Thiobacillus with a relative abundance of 20.1 ± 0.7% (n = 2, two technical replicates), Thiomonas (18.3 ± 0.1%), Rhodopseudomonas (16.7 ± 1.3%), Cryseobacterium (13.0 ± 0.4%), Rhizobium (6.4 ± 0.3%), and other lower abundant OTUs (Fig. 6b). A full list of high throughput sequenced OTUs with relative abundance can be found in Supplemental File 6. Phylogenetic analysis of close relative species downloaded from NCBI GenBank indicated that the Thiobacillus OTU was most closely related to Thiobacillus plumbophilus and the Bosea OTU to Bosea lupini (Fig. 6b).
Identified partial 16S rRNA genes in the microaerophilic S2O3 2− fed R3 bioreactor inoculum culture and at the end of the experiment. a Phylogenetic tree of species compared to the inoculum from RFLP analysis, samples derived from unique restriction fragment patterns are labelled as Bol-1 to 6. b Phylogenetic tree and stacked bar chart of the dominant OTUs in the microaerophilic S2O3 2− fed bioreactor at the end of the experiment after 308 days. The tree with the maximum highest log likelihood (−2332.68) is shown, alongside close relative species (blue text) obtained from NCBI GenBank. In the stacked bar chart single OTUs are shown as family/genus (two technical replicates). The grouping “Other OTUs” comprises OTUs with less than 1% total relative abundance (among all samples summed)
Discussion
The aerobic SCN− fed R1 bioreactor simulated a pre-denitrification chamber with the goal of oxidizing the SCN− via ammonia/ammonium formation to NO2 − and NO3 − (Eqs. 1–7 in Supplemental File 7) before an anaerobic denitrification chamber. Results from the aerobic R1 bioreactor showed that SCN− and NO3 − were removed throughout the experiment, but the concentration of NO2 − and NO3 − was higher at room temperature, potentially due to ongoing nitrification. In conjunction with this decrease of SCN− and NO3 −, an increase was observed for NH4 + when operated at room temperature (Figs. 1, 2). Three suggested reaction schemes for this process are: (i) formation of NH4 + by direct aerobic oxidation of SCN− (Eq. 1 in Supplemental File 7); (ii) aerobic oxidation of SCN− to cyanate (CNO−) followed by NH4 + formation by CNO− hydrolysis (Eqs. 2–3 in Supplemental File 7) which increases rapidly with a decrease in pH (Warner 1942); or (iii) microbial dissimilatory reduction of NO3 − into NH4 + and NO2 − or N2 inside the biofilm (Eqs. 4 and 5 in Supplemental File 7). The data showed an approximate 1:4 fold molar ratio of SCN−:NO3 − reduction and therefore, suggesting microbial dissimilatory reduction occurred (Eq. 4 in Supplemental File 7). Removal of SCN− in conjunction with NO3 − reduction has been observed previously with the use of biomaterial from a biogas plant (Sahariah and Chakraborty 2012), while dissimilatory nitrate reduction into ammonia has previously been observed in an industrial system operated at 22–28 °C (Villemur et al. 2015). The diverse microbial community attached to the biofilm carriers became dominated by Flavobacterium-like and Thiobacillus-like OTUs (related to T. denitrificans; Fig. 3). Flavobacterium spp. include facultative anaerobic species able to reduce NO3 −, e.g. Flavobacterium denitrificans (Horn et al. 2005). The genus Thiobacillus contains species with a versatile lifestyle that are able grow under aerobic and anaerobic conditions with a wide variety of electron donors such as SCN− (Villemur et al. 2015), S2O3 2− (Trouve and Chazal 1999), and H2S (Subletta 1987) along with NO2 − or NO3 − as an electron acceptor (Justin and Kelly 1978a). Considering that oxygen decreases the growth rate of e.g. T. denitrificans (Subletta 1987), we suggest that part of the microbial community carried out nitrate removal under microaerophilic/anaerobic conditions that are well-known to develop inside biofilms formed on carrier material (Munn 2011). However, compared to laboratory studies conducted at 30 °C, here we have shown that removal of SCN− and NO3 − also occurred in oxic wastewater with the use of biofilm carriers at 20, 15, and 10 °C which are typical for boreal climates. We also observed removal of SCN− and NO3 − after the pH was lowered to pH 5.5 and 3.5. These data indicated that the microbial community carried out these processes during acidic conditions which are typical for some mining wastewaters.
The anaerobic SCN− fed R2 bioreactor simulated a denitrification chamber following the aerobic pre-denitrification treatment. Such chambers are common in wastewater treatment plant designs typically consisting of an anaerobic denitrification chamber followed by a cycling of the wastewater in anoxic and oxic chambers (Lu et al. 2014). Similar to the aerobic R1 bioreactor, \({\text{NO}}_{ 3}^{ - }\) and SCN− were removed from the systems but compared to the aerobic R1 reactor no increase of NO3 − was seen at room temperature (Fig. 1). A possible reaction for this is NO3 − reduction to N2-gas in conjunction with SCN− oxidation to CNO− (Eqs. 8 and 9 in Supplemental File 7), followed by hydrolysis of CNO− and NH4 + formation (Eq. 3 in Supplemental File 7). Also similar to the aerobic R1 reactor, SCN− and NO3 − were also removed after the pH had been lowered to 3.5. This was likely as nitrification was inhibited. Compared to the aerobic R1 bioreactor, the NO2 − concentration was higher at room temperature, indicating a more efficient microbial denitrification in the anaerobic reactor. Similar to the aerobic R1 bioreactor, NH4 + concentration also increased in conjunction with a decrease of SCN− and NO3 − (Fig. 2). Through the experiment the microbial community on the biofilm carriers changed from being dominated by Thiobacillus-like OTUs to a more diverse community consisting of Comamonadaceae-like, Thiobacillus-like, Flavobacterium-like, and Ferruginibacter-like OTUs (Fig. 4). In addition to species within the Thiobacillus genus, Comamonadaceae are able to reduce nitrate (Khan et al. 2002) and Flavobacterium spp. grow on NO2 − (Pichinoty et al. 1976). Compared to the aerobic SCN− fed R1 bioreactor the anaerobic R2 reactor had a higher variety of dominant microbial genera, potentially due to higher nitrate removal providing increased concentrations of intermediate nitrogen compounds. All known species belonging to the genus Ferruginibacter are strictly aerobic and incapable of nitrate reduction (Kang et al. 2015; Lee et al. 2014; Lim et al. 2009). Consequently, in this study a potentially novel Ferruginibacter-like population (Fig. 4) was selected that has an unknown role in the anaerobic SCN− fed R2 bioreactor. To our knowledge this is the first study using next-generation sequencing to analyze the microbial community composition in SCN− fed reactors operated at cold temperatures.
In the S2O3 2− fed R3 bioreactor the concentrations of S2O3 2− and NO3 − were always below input levels (Fig. 5) and indicate thiosulfate oxidation in conjunction with nitrate reduction. Similar findings have previously been reported and this process has found to be conducted by species T. denitrificans (Chung et al. 2014; Oh et al. 2000; Trouve and Chazal 1999; Zhang et al. 2009; Zou et al. 2016). Along with Thiobacillus spp., species from the Thiomonas genus are known to oxidize S2O3 2− (Chen et al. 2004) and these populations became abundant in the S2O3 2− fed R3 bioreactor (Fig. 6). It can therefore be concluded that our experimental set-up supported S2O3 2− oxidation coupled to NO3 − reduction and was comparable to previous studies. In addition, the second generation sequencing (Illumina MiSeq) at the end of the experiment showed a higher diversity of taxa compared to that of the RFLP analysis used to sequence the inoculum. This is in accordance to previous studies using second generation sequencing which has a greater power to reveal the complete diversity of microbial taxa (Sinclair et al. 2015).
Removal of NO3 − and ISC in mining wastewaters is feasible (Di Capua et al. 2015) and industrial systems are under development, such as the SCN− removal in the LaRonde gold mine, Canada (Villemur et al. 2015). In this study, we have shown a potential to denitrify mining wastewater with the use of SCN− as an electron donor, and that this can be achieved at sub-optimal temperatures such as 10–15 °C under both oxic and anoxic conditions from pH 8 down to 3.5. Many of these reactions were potentially carried out under microaerophilic/anaerobic conditions inside the biofilm on the biofilm carriers. However, our experiment produced large amounts of NH4 + from this process which would need to undergo further nitrification. It is suggested that following SCN− depletion a re-cycling of the wastewater through oxic and anoxic conditions should be conducted. This would hypothetically nitrify the produced NH4 + followed by anaerobic denitrification of produced NO3 −. The room temperature SCN− and NO3 − removal rates were comparable to a published study carried out at mesophilic temperatures (Sahariah and Chakraborty 2012) but additionally, we report efficient SCN− and NO3 − removal at temperatures typical for the boreal climate (15 and 10 °C) and a pH typical for mining wastewaters (Table 3).
References
Alonso A, Camargo AJ (2003) Short-term toxicity of ammonia, nitrite, and nitrate to the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bull Environ Contam Toxicol 70:1006–1012. doi:10.1007/s00128-003-0082-5
Batty LC, Younger PL (2004) The use of waste materials in the passive remediation of mine water pollution Surveys. Geophysics 25:55–67. doi:10.1023/B:GEOP.0000015387.12390.ab
Chen XG, Geng AL, Yan R, Gould WD, Ng YL, Liang DT (2004) Isolation and characterization of sulphur-oxidizing Thiomonas sp. and its potential application in biological deodorization. Lett Appl Microbiol 39:495–503. doi:10.1111/j.1472-765X.2004.01615.x
Chen LX, Huang LN, Mendez-Garcia C, Kuang JL, Hua ZS, Liu J, Shu WS (2016) Microbial communities, processes and functions in acid mine drainage ecosystems. Curr Opin Biotechnol 38:150–158. doi:10.1016/j.copbio.2016.01.013
Chung J, Amin K, Kim S, Yoon S, Kwon K, Bae W (2014) Autotrophic denitrification of nitrate and nitrite using thiosulfate as an electron donor. Water Res 58:169–178. doi:10.1016/j.watres.2014.03.071
Conley DJ et al (2011) Hypoxia is increasing in the coastal zone of the Baltic Sea. Environ Sci Technol 45:6777–6783. doi:10.1021/es201212r
Di Capua F, Papirio S, Lens PNL, Esposito G (2015) Chemolithotrophic denitrification in biofilm reactors Chem Eng J 280:643–657. doi:10.1016/j.cej.2015.05.131
Dopson M, Johnson DB (2012) Biodiversity, metabolism and applications of acidophilic sulfur-metabolizing microorganisms. Environ Microbiol 14:2620–2631. doi:10.1111/j.1462-2920.2012.02749.x
Dopson M, Lindström EB (1999) Potential role of Thiobacillus caldus in arsenopyrite bioleaching. Appl Environ Microbiol 65:36–40
Dopson M, Lindström EB (2004) Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite, and chalcopyrite. Microb Ecol 48:19–28
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. doi:10.1038/nmeth.2604
Grosse AC, Dicinoski GW, Shaw MJ, Haddad PR (2003) Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 69:1–21
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Herlemann DP, Labrenz M, Jurgens K, Bertilsson S, Waniek JJ, Andersson AF (2011) Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J 5:1571–1579. doi:10.1038/ismej.2011.41
Hoor AT-T (1975) A new type of thiosulphate oxidizing, nitrate reducing microorganism: Thiomicrospira denitrificans sp. nov Netherlands. J Sea Res 9:344–350. doi:10.1016/0077-7579(75)90008-3
Horn MA, Ihssen J, Matthies C, Schramm A, Acker G, Drake HL (2005) Dechloromonas denitrificans sp. nov., Flavobacterium denitrificans sp. nov., Paenibacillus anaericanus sp. nov. and Paenibacillus terrae strain MH72, N2O-producing bacteria isolated from the gut of the earthworm Aporrectodea caliginosa. Int J Syst Evol Microbiol 55:1255–1265. doi:10.1099/ijs.0.63484-0
Hugerth LW et al (2014) DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies. Appl Environ Microbiol 80:5116–5123. doi:10.1128/aem.01403-14
Jermakka J, Wendling L, Sohlberg E, Heinonen H, Vikman M (2015) Potential technologies for the removal and recovery of nitrogen compounds from mine and quarry waters in subarctic conditions. Crit Rev Environ Sci Technol 45:703–748
Justin P, Kelly DP (1978a) Growth kinetics of Thiobacillus denitrificans in anaerobic and aerobic chemostat culture. Microbiology 107:123–130. doi:10.1099/00221287-107-1-123
Justin P, Kelly DP (1978b) Metabolic changes in Thiobacillus denitrificans accompanying the transition from aerobic to anaerobic growth in continuous chemostat culture. Microbiology 107:131–137. doi:10.1099/00221287-107-1-131
Kang H, Kim H, Joung Y, Jang TY, Joh K (2015) Ferruginibacter paludis sp. nov., isolated from wetland freshwater, and emended descriptions of Ferruginibacter lapsinanis and Ferruginibacter alkalilentus. Int J Syst Evol Microbiol 65:2635–2639. doi:10.1099/ijs.0.000311
Kantor RS, van Zyl AW, van Hille RP, Thomas BC, Harrison STL, Banfield JF (2015) Bioreactor microbial ecosystems for thiocyanate and cyanide degradation unravelled with genome-resolved metagenomics. Environ Microbiol 17:4929–4941. doi:10.1111/1462-2920.12936
Kelly DP, Chambers LA, Trudinger PA (1969) Cyanolysis and spectrophotometric estimation of trithionate in mixture with thiosulfate and tetrathionate. Anal Chem 41:898–901
Khan ST, Horiba Y, Yamamoto M, Hiraishi A (2002) Members of the family Comamonadaceae as primary poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-degrading denitrifiers in activated sludge as revealed by a polyphasic approach. Appl Environ Microbiol 68:3206–3214. doi:10.1128/aem.68.7.3206-3214.2002
Knobeloch L, Salna B, Hogan A, Postle J, Anderson H (2000) Blue babies and nitrate-contaminated well water. Environ Health Perspect. 108:675–678
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol 3:1870–1874. doi:10.1093/molbev/msw054
Lee B-I, Kang H, Kim H, Joung Y, Joh K (2014) Ferruginibacter yonginensis sp. nov., isolated from a mesotrophic artificial lake. Int J Syst Evol Microbiol 64:846–850. doi:10.1099/ijs.0.057083-0
Li W et al (2016a) Enrichment of denitratating bacteria from a methylotrophic denitrifying culture. Appl Microbiol Biotechnol 100:10203–10213. doi:10.1007/s00253-016-7859-z
Li W et al (2016b) Effect of self-alkalization on nitrite accumulation in a high-rate denitrification system: performance, microflora and enzymatic activities. Water Res 88:758–765. doi:10.1016/j.watres.2015.11.003
Liljeqvist M, Sundkvist J-E, Saleh A, Dopson M (2011) Low temperature removal of inorganic sulfur compounds from mining process waters. Biotechnol Bioeng 108:1251–1259
Liljeqvist M et al (2015) Metagenomic analysis reveals adaptations to a cold-adapted lifestyle in a low-temperature acid mine drainage stream. FEMS Microb Ecol. doi:10.1093/femsec/fiv1011
Lim JH, Baek SH, Lee ST (2009) Ferruginibacter alkalilentus gen. nov., sp. nov. and Ferruginibacter lapsinanis sp. nov., novel members of the family ‘Chitinophagaceae’ in the phylum Bacteroidetes, isolated from freshwater sediment. Int J Syst Evol Microbiol 59:2394–2399. doi:10.1099/ijs.0.009480-0
Lu HJ, Chandran K, Stensel D (2014) Microbial ecology of denitrification in biological wastewater treatment. Water Res 64:237–254. doi:10.1016/j.waters.2014.06.42
Morales TA, Dopson M, Athar R, Herbert RB Jr (2005) Analysis of bacterial diversity in acidic pond water and compost after treatment of artificial acid mine drainage for metal removal. Biotechnol Bioeng 90:543–551. doi:10.1002/bit.20421
Morin KA, Hutt NM (2009) Mine-water leaching of nitrogen species from explosive residues. In: Proceedings of GeoHalifax, pp 20–24
Munn C (2011) Marine microbiology: Ecology and applications. Garland Science,
Muyzer G, Teske A, Wirsen CO, Jannasch HW (1995) Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol 164:165–172. doi:10.1007/bf02529967
Ogawa T et al (2013) Carbonyl sulfide hydrolase from Thiobacillus thioparus strain THI115 is one of the beta-carbonic anhydrase family enzymes. J Am Chem Soc 135:3818–3825. doi:10.1021/ja307735e
Oh SE, Kim KS, Choi HC, Cho J, Kim IS (2000) Kinetics and physiological characteristics of autotrophic dentrification by denitrifying sulfur bacteria. Water Sci Technol 42:59–68
Papirio S, Ylinen A, Zou G, Peltola M, Esposito G, Puhakka JA (2014) Fluidized-bed denitrification for mine waters. Part I: low pH and temperature operation. Biodegradation 25:425–435. doi:10.1007/s10532-013-9671-0
Pichinoty F, Bigliardi-Rouvier J, Mandel M, Greenway B, Metenier G, Garcia JL (1976) The isolation and properties of a denitrifying bacterium of the genus Flavobacterium. Antonie Van Leeuwenhoek 42:349–354
Quast C et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucl Acids Res 41:D590–D596. doi:10.1093/nar/gks1219
Revey GF (1996) Practical methods to control explosives losses and reduce ammonia and nitrate levels in mine water. Min Engin 48:61–64
Robertson CE et al (2013) Explicet: graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics 29:3100–3101. doi:10.1093/bioinformatics/btt526
Sahariah BP, Chakraborty S (2012) Effect of feed concentration and hydraulic retention time on removal of phenol, thiocyanate, and nitrate–nitrogen in anoxic-fed batch moving bed reactor. Toxicol Environ Chem 94:1629–1645. doi:10.1080/02772248.2012.727817
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. vol v. 1. Cold Spring Harbor Laboratory, New York
Schippers A, Sand W (1999) Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microbiol 65:319–321
Sinclair L, Osman OA, Bertilsson S, Eiler A (2015) Microbial community composition and diversity via 16S rRNA gene amplicons: evaluating the Illumina platform. PLoS ONE 10:e0116955. doi:10.1371/journal.pone.0116955
Sorokin DY, Tourova TP, Antipov AN, Muyzer G, Kuenen JG (2004) Anaerobic growth of the haloalkaliphilic denitrifying sulfur-oxidizing bacterium Thialkalivibrio thiocyanodenitrificans sp. nov. with thiocyanate. Microbiology 150:2435–2442. doi:10.1099/mic.0.27015-0
Subletta KL (1987) Aerobic oxidation of hydrogen sulfide by Thiobacillus denitrificans. Biotechnol Bioeng 29:690–695. doi:10.1002/bit.260290605
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Trouve C, Chazal PM (1999) Autotrophic denitrification by Thiobacillus denitrificans witht thiosulphate as sole energy source. pilot scale experiments at low temperature. Environ Technol 20:387–395. doi:10.1080/09593332008616831
Villemur R, Juteau P, Bougie V, Menard J, Deziel E (2015) Development of four-stage moving bed biofilm reactor train with a pre-denitrification configuration for the removal of thiocyanate and cyanate. Bioresour Technol 181:254–262. doi:10.1016/j.biortech.2015.01.051
Warner RC (1942) The kinetics of the hydrolysis of urea and of arginine. J Biol Chem 142:705–723
Wright ES, Yilmaz LS, Noguera DR (2012) DECIPHER, a search-based approach to chimera identification for 16S rRNA sequences. Appl Environ Microbiol 78:717–725. doi:10.1128/aem.06516-11
Wu X, Wong ZL, Sten P, Engblom S, Österholm P, Dopson M (2013) Microbial community potentially responsible for acid and metal release from an Ostrobothnian acid sulfate soil. FEMS Microbiol Ecol 84:555–563
Zagury GJ, Oudjehani K, Deschênes L (2004) Characterization and availability of cyanide in solid mine tailings from gold extraction plants. Sci Total Environ 320:211–224. doi:10.1016/j.scitotenv.2003.08.012
Zhang Z, Lei Z, He X, Zhang Z, Yang Y, Sugiura N (2009) Nitrate removal by Thiobacillus denitrificans immobilized on poly(vinyl alcohol) carriers. J Hazard Mater 163:1090–1095. doi:10.1016/j.jhazmat.2008.07.062
Zou G, Papirio S, Ylinen A, Di Capua F, Lakaniemi AM, Puhakka JA (2014) Fluidized-bed denitrification for mine waters. Part II: effects of Ni and Co. Biodegradation 25:417–423. doi:10.1007/s10532-013-9670-1
Zou G, Papirio S, Lakaniemi AM, Ahoranta SH, Puhakka JA (2016) High rate autotrophic denitrification in fluidized-bed biofilm reactors. Chem Eng J 284:1287–1294. doi:10.1016/j.cej.2015.09.074
Acknowledgements
We acknowledge the National Genomics Infrastructure (NGI) and UPPMAX for providing assistance in massive parallel sequencing and computational infrastructure. The research was funded by Boliden AB, Sweden, 2012–2016. The authors acknowledge support from Science for Life Laboratory and the National Genomics Infrastructure for providing assistance in massive parallel sequencing and computational infrastructure. The computations were performed on resources provided by SNIC through the Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project b2013127.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was supported by grants from Boliden AB, Sweden.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Broman, E., Jawad, A., Wu, X. et al. Low temperature, autotrophic microbial denitrification using thiosulfate or thiocyanate as electron donor. Biodegradation 28, 287–301 (2017). https://doi.org/10.1007/s10532-017-9796-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-017-9796-7