Abstract
A mathematical model system was derived to describe the kinetics of ammonium nitrification in a fixed biofilm reactor using dewatered sludge-fly ash composite ceramic particle as a supporting medium. The model incorporates diffusive mass transport and Monod kinetics. The model was solved using a combination of the orthogonal collocation method and Gear’s method. A batch test was conducted to observe the nitrification of ammonium-nitrogen (\({\text{NH}}_{4}^{ + }\)-N) and the growth of nitrifying biomass. The compositions of nitrifying bacterial community in the batch kinetic test were analyzed using PCR–DGGE method. The experimental results show that the most staining intensity abundance of bands occurred on day 2.75 with the highest biomass concentration of 46.5 mg/L. Chemostat kinetic tests were performed independently to evaluate the biokinetic parameters used in the model prediction. In the column test, the removal efficiency of \({\text{NH}}_{4}^{ + }\)-N was approximately 96 % while the concentration of suspended nitrifying biomass was approximately 16 mg VSS/L and model-predicted biofilm thickness reached up to 0.21 cm in the steady state. The profiles of denaturing gradient gel electrophoresis (DGGE) of different microbial communities demonstrated that indigenous nitrifying bacteria (Nitrospira and Nitrobacter) existed and were the dominant species in the fixed biofilm process.









Similar content being viewed by others
References
Alawi M, Lipski A, Sanders T, Pfeiffer EM, Spieck E (2007) Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J 1:256–264
American Public Health Association (2005) Standard methods for the examination of water and wastewater, 21st edn. APHA, Washington, DC
Bock E, Koops HP, Moller UC, Rudert M (1990) A new facultatively nitrite oxidizing bacterium, Nitrobacter vulgaris sp nov. Arch Microbiol 153:105–110
Burrell PC, Keller J, Blackall LL (1998) Microbiology of a nitrite-oxidizing bioreactor. Appl Environ Microbiol 64:1878–1883
Burrell PC, Phalen CM, Hovanec TA (2001) Identification of bacteria responsible for ammonia oxidation in freshwater aquaria. Appl Environ Microbiol 67:5791–5800
Cao XY, Yue QY, Song LY, Li M, Zhao YC (2007) The performance and application of fly ash modified by PDMAAC. J Hazard Mater 147:133–138
Chang YT (2009) Environmental microbiology experiment, 5th edn. Gau-Lih Book Co., Ltd., Taipei, pp 505–526
Cussler EL (1997) Diffusion: mass Transfer in fluid systems. Cambridge University Press, Cambridge
Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M (2001) In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl Environ Microbiol 67:5273–5284
Dinçer AR, Kargi F (2000) Kinetics of sequential nitrification and denitrification processes. Enzyme Microbiol Technol 27:37–42
Finlayson BA (1972) The method of weighted residuals and variational principles. Academic Press, New York
Freitag TE, Chang L, Clegg CD, Prosser JI (2005) Influence of inorganic nitrogen management regime on the diversity of nitrite-oxidizing bacteria in agricultural grassland soils. Appl Environ Microbiol 71:8323–8334
Gaudy AF Jr, Gaudy ET (1980) Microbiology for Environmental Scientists and Engineers. Metcalf and Eddy Inc., McGraw-Hill, New York
Gieseke A, Arnz P, Amann R, Schramm A (2002) Simultaneous P and N removal in a sequencing batch biofilm reactor: insights from reactor- and microscale investigations. Water Res 36:501–509
Grundmann GL, Neyra M, Normand P (2000) High-resolution phylogenetic analysis of NO2–oxidizing Nitrobacter species using the rrs-rrl IGS sequence and rrl genes. Int J Syst Evol Microbiol 50:1893–1898
Gupta A, Flora JR, Sayles GD, Suidan MT (1994) Methanogenesis and sulfate reduction in chemostats-II. Model development and verification. Water Res 28:795–803
Han S, Yue Q, Yue M, Gao B, Zhao Y, Cheng W (2009a) Effect of sludge-fly ash ceramic particles (SFCP) on synthetic wastewater treatment in an A/O combined biological aerated filter. Bioresour Technol 100:1149–1155
Han S, Yue Q, Yue M, Gao B, Li Q, Yu H, Zhao Y, Qi Y (2009b) The characteristics and application of sludge-fly ash ceramic particles (SFCP) as novel filter media. J Hazard Mater 171:809–814
Hem LJ, Rusten B, Ødegaard H (1994) Nitrification in a moving bed biofilm reactor. Water Res 28:1425–1433
Isaka K, Yoshie S, Sumino T, Inamori Y, Tsuneda S (2007) Nitrification of landfill leachate using immobilized nitrifying bacteria at low temperatures. Biochem Eng J 37:49–55
Jianlong W, Ning Y (2004) Partial nitrification under limited dissolved oxygen conditions. Process Biochem 39:1223–1229
Juretschko S, Loy A, Lehner A, Wagner M (2002) The microbial community composition of a nitrifying-denitrifying activated sludge from an industrial sewage treatment plant analyzed by the full-cycle rRNA approach. Syst Appl Microbiol 25:84–99
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Liang CH, Chiang PC (2007) Mathematical model of the non-steady-state adsorption and biodegradation capacities of BAC filters. J Hazard Mater 139:316–322
Liang CH, Chiang PC, Chang EE (2007) Modeling the behaviors of adsorption and biodegradation in biological activated carbon filters. Water Res 41:3241–3250
Mahne I, Prinčič A, Megušar F (1996) Nitrification/denitrification in nitrogen high-strength liquid wastes. Water Res 30:2107–2111
Muyzer G, Brinkhoff T, Nuebel U, Santegoeds C, Schafer H, Wawer C (1997) Denaturing gradient gel electrophoresis (DGGE) in microbial ecology. In: Akkermans ADL, Van Elsas JD, De Bruijn, FJ (eds.), Molecular microbial ecology manual. vol. 3.4.4. Kluwer Academic Publishers, Dordrecht, pp 1–27
Navarro E, Fernandez MP, Grimont F, Claysjosserand A, Bardin R (1992) Genomic heterogeneity of the genus Nitrobacter. Int J Syst Bacteriol 42:105–110
Perry RH, Chilton CH (1973) Chemical Engineers Handbook, 5th edn. McGraw-Hill, New York, pp 3–229
Philips S, Laanbroek HJ, Verstraete W (2002) Origin, causes, and effects of increased nitrite concentrations in aquatic environments. Rev Environ Sci Biotechnol 1:115–141
Purkhold U, Pommerening-Roser A, Juretschko S, Schmid MC, Koops HP, Wagner M (2000) Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl Environ Microbiol 66:5368–5382
Rotthauwe JH, Witzel KP (1997) The ammonia monooxygenase structural gene amoA as a functional maker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Schlegel S, Koester H (2007) Wastewater treatment with submerged fixed bed biofilm reactor system-design rules, operating experiences and ongoing developments. Water Sci Technol 55:83–89
Sheintuch M, Tartakovsky B, Narkis N, Rebhun M (1995) Substrate inhibition and multiple states in a continuous nitrification process. Water Res 29:953–963
Sorokin DY, Muyzer G, Brinkhoff T, Kuenen JG, Jetten MSM (1998) Isolation and characterization of a novel facultatively alkaliphilic Nitrobacter species N. alkalicus sp. Nov. Arch Microbiol 170:345–352
Speitel GE Jr, DiGiano FA (1987) Biofilm shearing under dynamic conditions. J Environ Eng ASCE 113:464–475
Suidan MT, Rittmann BE, Traegner UK (1987) Criteria establishing biofilm-kinetic types. Water Res 21:491–498
Tchobanoglous G, Burton FL (1991) Wastewater engineering: treatment, disposal, reuse, 3rd edn. McGraw Hill, New York
Teske A, Alm E, Regan JM, Toze S, Rittmann BE, Stahl DA (1994) Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J Bacteriol 176:6623–6630
Tsai HH, Ravindran V, Pirbazari M (2005) Model for predicting the performance of membrane bioadsorber reactor process in water treatment applications. Chem Eng Sci 60:5620–5636
Vayenas DV, Lyberatos G (1994) A novel model for nitrifying trickling filters. Water Res 28:1275–1284
Wang S, Boyjoo Y, Choueib A, Zhu ZH (2005) Removal of dyes from aqueous solution using fly ash and red mud. Water Res 39:129–138
Watson SW, Bock E, Harms H, Koops HP, Hooper AB (1989) Nitrifying bacteria. In: Stanley JT (ed) Bergey’s manual of systematic bacteriology, vol 3. Williams and Wilkins, Baltimore, pp 1808–1834
Wilke CE, Chang P (1955) Correlation of diffusion coefficients in dilute solutions. AIChE J 1:264–270
Williamson K, McCarty PL (1976) Verification studies of the biofilm model for bacterial substrate utilization. J Water Pollut Control Fed 48:281–296
Yurt N, Beyenal H, Sears J, Lewandowski Z (2003) Quantifying selected growth parameters of Leptothrix discophora SP-6 in biofilms from oxygen concentration profiles. Chem Eng Sci 58:4557–4566
Zhang T, Fang HHP (2000) Digitization of DGGE (denaturing gradient gel electrophoresis) profile and cluster analysis of microbial communities. Biotechnol Lett 22:399–405
Zhu S, Chen S (2001) Impacts of Reynolds number on nitrification biofilm kinetics. Aquacult Eng 24:213–229
Zorpas AA, Arapoglou D, Panagiotis K (2003) Waste paper and clinoptilolite as a bulking material with dewatered anaerobically stabilized primary sewage sludge (DASPSS) for compost production. Waste Manage 23:27–35
Acknowledgments
The authors would like to thank the Ministry of Science and Technology of Taiwan for supporting this research under Contract No. NSC 100-2221-E-166-004-MY2. Ted Knoy is appreciated for his editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Conceptual basis of biofilm
A schematic diagram represented the ammonium utilization by attached and suspended nitrifying biomasses in the biological reactor is illustrated in Fig. 10. For the attached biomass, the ammonium concentration profile is established in the biofilm due to the diffusional resistance to the transport of the ammonium. In the biofilm reactor, the attached biomass accumulates to form a biofilm and sheared-off biofilm mass is assumed to be in dispersed form, increasing the suspended biomass concentration (Tsai et al. 2005). The shear loss of attached biomass into the flowing liquid increased the amount of suspended biomass in the bulk liquid.
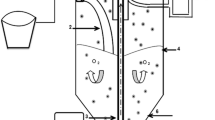
Appendix 2: Bioreactor setup
The detailed design of a fixed biofilm reactor packing with dewater sludge-fly ash ceramic particle is presented in Fig. 11. The reactor was inoculated by charging an 80 mL nitrifying biomass with 4.8 mg VSS/L. The fixed biofilm reactor with a high recycle flow rate (Q r /Q = 25) for maintaining a completely mixed stirred tank reactor was applied to conduct the experiments of ammonium nitrification. The use of a completely mixed flow reactor greatly simplifies the mathematical description of a fixed biofilm reactor because \({\text{NH}}_{4}^{ + }\)-N concentration profiles along the reactor need not to be considered. The reactor was fed with an initial \({\text{NH}}_{4}^{ + }\)-N concentration of 120 mg/L at 15 L/day, which yield a hydraulic residence time of 5.6 h. The working volume of reactor was 3.5 L. The dewatered sludge-fly ash ceramic particles were places in the fixed biofilm reactor between two plates used for fixation. Dissolved oxygen (DO) was controlled at a value above 4.5 mg/L for nitrification by an adjustable air pump. The air supply was sterilized by filtration through polytetrafluoroethylene (PTFE) membranes with a pore size of 0.2 μm. The pH was controlled at 7.2 ± 0.1 by adding \({\text{HPO}}_{4}^{ 2- }\)/\({{{{\text{H}}_{2}}{\text{PO}}}}_{4}^{ 2- }\) in the feed solution (Hem et al. 1994).
Appendix 3
- a :
-
The specific surface area of bed (L−1)
- α :
-
The conversion factor for the utilization of \({\text{NH}}_{4}^{ + }\) to nitrate (Ms \({\text{M}}_{s}^{-1}\))
- ϕb :
-
Association parameters
- μ:
-
Secific growth rate of nitrifying biomass (T−1)
- A:
-
Total surface area of ceramic particles (L2)
- b:
-
Decay coefficient of nitrifying biomass (T−1)
- bs :
-
Shear-loss coefficient of nitrifying biomass (T−1)
- Df :
-
Diffusion coefficient of ammonium in biofilm (L2T−1)
- dp :
-
Diameter of the ceramic particle (L)
- Dw :
-
Diffusion coefficient of ammonium in bulk liquid (L2T−1)
- Jf :
-
Flux of ammonium from bulk liquid into nitrifying biofilm (MsL−2T−1)
- K:
-
Monod maximum specific utilization rate of ammonium (MsMxT−1)
- Kf :
-
Liquid film transfer coefficient of ammonium (LT−1)
- Ks :
-
Monod half-velocity coefficient of ammonium (MsL−3)
- Lf :
-
Thickness of nitrifying biofilm (L)
- Lf0 :
-
Initial thickness of nitrifying biofilm (L)
- M:
-
Mass in general
- Mb :
-
Molecular weight of solvent (M)
- Ms :
-
Mass of \({\text{NH}}_{4}^{ + }\)
- Mx :
-
Mass of nitrifying biomass
- Q:
-
Influent flow rate (L3T-1)
- Re :
-
Reynolds number
- S:
-
Effluent concentration of \({\text{NH}}_{4}^{ + }\) in the aeration tank (MsL−3)
- S0 :
-
Influent concentration of \({\text{NH}}_{4}^{ + }\) in the aeration tank (MsL−3)
- Sb :
-
Concentration of \({\text{NH}}_{4}^{ + }\) in bulk liquid (MsL−3)
- Sb0 :
-
concentration of \({\text{NH}}_{4}^{ + }\) in the feed (MsL−3)
- Sc :
-
Schmidt number
- Sf :
-
Concentration of \({\text{NH}}_{4}^{ + }\) in biofilm (MsL−3)
- Sn :
-
The nitrate concentration in the effluent (MsL−3)
- Ss :
-
Concentration of \({\text{NH}}_{4}^{ + }\) at liquid/biofilm interface (MsL-3)
- T:
-
Absolute temperature (K)
- T:
-
Time (T)
- U:
-
Specific rate of nitrification (Ms \({\text{M}}_{x}^{ -1 }\)T-1)
- V:
-
Effective working volume of reactor (L3)
- Vb :
-
Molar volume of solute as liquid at its normal boiling point (L3mol−1)
- Vs :
-
Superficial flow velocity through column (LT−1)
- X:
-
biomass concentration in the aeration tank (MxL−3)
- Xb :
-
concentration of nitrifying biomass in bulk liquid (MxL−3)
- Xb0 :
-
initial concentration of nitrifying biomass in bulk liquid (MxL−3)
- Xf :
-
Density of nitrifying biofilm (MxL−3)
- Y:
-
Growth yield of nitrifying biomass (MxMs -1)
- Zf :
-
Radial distance in biofilm (L)
- ε:
-
Reactor porosity (dimensionless)
- θc :
-
Sludge age (T)
- θ:
-
Hydraulic residence time (T)
- μb :
-
Absolute viscosity of solvent (ML−1T−1)
- μmax :
-
Monod maximum specific growth rate (T−1)
- μw :
-
Viscosity of water (L2T−1)
- υ:
-
Kinematic viscosity (L2T−1)
Rights and permissions
About this article
Cite this article
Lee, MC., Lin, YH. & Yu, HW. Kinetics of nitrification in a fixed biofilm reactor using dewatered sludge-fly ash composite ceramic particle as a supporting medium. Biodegradation 25, 849–865 (2014). https://doi.org/10.1007/s10532-014-9705-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-014-9705-2