Abstract
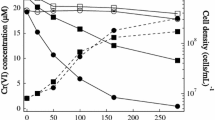
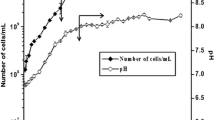
The reduction of hexavalent chromium, Cr(VI), to trivalent chromium, Cr(III), can be an important aspect of remediation processes at contaminated sites. Cellulomonas species are found at several Cr(VI) contaminated and uncontaminated locations at the Department of Energy site in Hanford, Washington. Members of this genus have demonstrated the ability to effectively reduce Cr(VI) to Cr(III) fermentatively and therefore play a potential role in Cr(VI) remediation at this site. Batch studies were conducted with Cellulomonas sp. strain ES6 to assess the influence of various carbon sources, iron minerals, and electron shuttling compounds on Cr(VI) reduction rates as these chemical species are likely to be present in, or added to, the environment during in situ bioremediation. Results indicated that the type of carbon source as well as the type of electron shuttle present influenced Cr(VI) reduction rates. Molasses stimulated Cr(VI) reduction more effectively than pure sucrose, presumably due to presence of more easily utilizable sugars, electron shuttling compounds or compounds with direct Cr(VI) reduction capabilities. Cr(VI) reduction rates increased with increasing concentration of anthraquinone-2,6-disulfonate (AQDS) regardless of the carbon source. The presence of iron minerals and their concentrations did not significantly influence Cr(VI) reduction rates. However, strain ES6 or AQDS could directly reduce surface-associated Fe(III) to Fe(II), which was capable of reducing Cr(VI) at a near instantaneous rate. These results suggest the rate limiting step in these systems was the transfer of electrons from strain ES6 to the intermediate or terminal electron acceptor whether that was Cr(VI), Fe(III), or AQDS.







Similar content being viewed by others
References
Amonette J, Workman D, Kennedy D, Fruchter J, Gorby Y (2000) Dechlorination of carbon tetrachloride by Fe(II) associated with goethite. Environ Sci Technol 34:4606–4613
Anonymous (2005) GAO-06-77R Columbia River contamination from the Hanford site. United States Government Accountability Office, Washington, DC
Barceloux D (1999) Chromium. Clin Toxicol 37:173–194
Borch T, Inskeep W, Harwood J, Gerlach R (2005) Impact of ferrihydrite and anthraquinone-2,6-disulfonate on the reductive transformation of 2,4,6-trinitrotoluene by a gram-positive fermenting bacterium. Environ Sci Technol 39:7126–7133
Brose D, James B (2010) Oxidation-reduction transformations of chromium in aerobic soils and the role of electron-shuttling quinones. Environ Sci Technol 44:9438–9444
Buerge I, Hug S (1997) Kinetics and pH dependence of chromium(VI) reduction by iron(II). Environ Sci Technol 31:1426–1432
Calder L (1988) Chromium contamination of groundwater. In: Niebaer E, Niragu J (eds) Chromium in the natural and human environments. Wiley, New York, pp 215–228
Chen J, Hao O (1998) Microbial chromium(VI) reduction. Crit Rev Environ Sci Technol 28:219–251
Eary L, Rai D (1988) Chromate removal from aqueous wastes by reduction with ferrous ion. Environ Sci Technol 22:972–977
Francisco R, Alpoim M, Morais P (2002) Diversity of chromium-resistant and-reducing bacteria in a chromium-contaminated activated sludge. J Appl Microbiol 92:837–843
Fredrickson J, Zachara J, Kennedy D, Dong H, Onstott T, Hinman N, Li S (1998) Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim Cosmochim Acta 62:3239–3257
Fredrickson J, Kostandarithes H, Li S, Plymale A, Daly M (2000) Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl Environ Microbiol 66:2006–2011
Gerlach R, Field E, Viamajala S, Peyton B, Apel W, Cunningham A (2011) Influence of carbon sources and electron shuttles on ferric iron reduction by Cellulomonas sp. strain ES6. Biodegradation 22:983–995
Hayes R (1997) The carcinogenicity of metals in humans. Cancer Causes Control 8:371–385
James B (1994) Hexavalent chromium solubility and reduction in alkaline soils enriched with chromite ore processing residue. J Environ Qual 23:227–233
Jung Y, Choi J, Lee W (2007) Spectroscopic investigation of magnetite surface for the reduction of hexavalent chromium. Chemosphere 68:1968–1975
Krishna K, Philip L (2005) Bioremediation of Cr(VI) in contaminated soils. J Hazard Mater 121:109–117
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Kwon M, Finneran K (2009) Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) reduction is concurrently mediated by direct electron transfer from hydroquinones and resulting biogenic Fe(II) formed during electron shuttle-amended biodegradation. Environ Eng Sci 26:961–971
Li Y, Low G, Scott J, Amal R (2009) The role of iron in hexavalent chromium reduction by municipal landfill leachate. J Hazard Mater 161:657–662
Lichtenthaler F (2002) Unsaturated O- and N-heterocycles from carbohydrate feedstocks. Acc Chem Res 35:728–737
Liu C, Gorby Y, Zachara J, Fredrickson J, Brown C (2002) Reduction kinetics of Fe(III), Co(III), U(VI), Cr(VI), and Tc(VII) in cultures of dissimilatory metal-reducing bacteria. Biotechnol Bioeng 80:637–649
Liu T, Rao P, Lo I (2009) Influences of humic acid, bicarbonate and calcium on Cr(VI) reductive removal by zero-valent iron. Sci Total Environ 407:3407–3414
Lovley D (1997) Microbial Fe(III) reduction in subsurface environments. FEMS Microbiol Rev 20:305–313
Lovley D, Blunt-Harris E (1999) Role of humic-bound iron as an electron transfer agent in dissimilatory Fe(III) reduction. Appl Environ Microbiol 65:4252–4254
Lovley D, Phillips E (1986a) Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol 52:751–757
Lovley D, Phillips E (1986b) Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol 51:683–689
Lovley D, Phillips E (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540
Lovley D, Fraga J, Blunt-Harris E, Hayes L, Phillips E, Coates J (1998) Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol 26:152–157
Nealson K, Saffarini D (1994) Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol 48:311–343
Nevin K, Lovley D (2000) Potential for nonenzymatic reduction of Fe(III) via electron shuttling in subsurface sediments. Environ Sci Technol 34:2472–2478
Newman D (2001) How bacteria respire minerals. Science 292:1312–1313
Nyman J, Caccavo F, Cunningham A, Gerlach R (2002) Biogeochemical elimination of chromium(VI) from contaminated water. Biorem J 6:39–55
Okutman Tas D, Pavlostathis S (2007) The influence of iron reduction on the reductive biotransformation of pentachloronitrobenzene. Eur J Soil Biol 43:264–275
Pettine M, D’Ottone L, Campanella L, Millero F, Passino R (1998) The reduction of chromium(VI) by iron(II) in aqueous solutions. Geochim Cosmochim Acta 62:1509–1519
Prescott S, Dunn C (1983) Prescott and Dunn’s industrial microbiology. AVI, Westport
Roden E, Zachara J (1996) Microbial reduction of crystalline iron(III) oxides: influence of oxide surface area and potential for cell growth. Environ Sci Technol 30:1618–1628
Royer R, Burgos W, Fisher A, Unz R, Dempsey B (2002) Enhancement of biological reduction of hematite by electron shuttling and Fe(II) complexation. Environ Sci Technol 36:1939–1946
Sani R, Peyton B, Smith W, Apel W, Petersen J (2002) Dissimilatory reduction of Cr(VI), Fe(III), and U(VI) by Cellulomonas isolates. Appl Microbiol Biotechnol 60:192–199
Seaman J, Bertsch P, Schwallie L (1999) In situ Cr(VI) reduction within coarse-textured, oxide-coated soil and aquifer systems using Fe(II) solutions. Environ Sci Technol 33:938–944
Smith W, Apel W, Peterson J, Peyton B (2002) Effect of carbon and energy source on bacterial chromate reduction. Biorem J 6:205–215
Tseng J, Bielefeldt A (2002) Low-temperature chromium(VI) biotransformation in soil with varying electron acceptors. J Environ Qual 31:1831–1841
Turick C, Apel W, Carmiol N (1996) Isolation of hexavalent chromium-reducing anaerobes from hexavalent-chromium-contaminated and noncontaminated environments. Appl Microbiol Biotechnol 44:683–688
Turick C, Tisa L, Caccavo F Jr (2002) Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl Environ Microbiol 68:2436–2444
Tzou Y, Wang M, Loppert R (2003) Sorption of phosphate and Cr(VI) by Fe(III) and Cr(III) hydroxides. Arch Environ Contam Toxicol 44:445–453
Vázquez-Morillas A, Vaca-Mier M, Alvarez P (2006) Biological activation of hydrous ferric oxide for reduction of hexavalent chromium in the presence of different anions. Eur J Soil Biol 42:99–106
Viamajala S, Peyton B, Apel W, Peterson J (2002) Chromate reduction in Shewanella oneidensis MR-1 is an inducible process associated with anaerobic growth. Biotechnol Prog 18:290–295
Viamajala S, Smith W, Sani R, Apel W, Petersen J, Neal A, Roberto F, Newby D, Peyton B (2007) Isolation and characterization of Cr(VI) reducing Cellulomonas spp. from subsurface soils: implications for long-term chromate reduction. Bioresour Technol 98:612–622
Viamajala S, Peyton B, Gerlach R, Sivaswamy V, Apel W, Petersen J (2008) Permeable reactive biobarriers for in situ Cr(VI) reduction: bench scale tests using Cellulomonas sp. strain ES6. Biotechnol Bioeng 101:1150–1162
Wielinga B, Mizuba M, Hansel C, Fendorf S (2001) Iron promoted reduction of chromate by dissimilatory iron-reducing bacteria. Environ Sci Technol 35:522–527
Workman D, Woods S, Gorby Y, Fredrickson J, Truex M (1997) Microbial reduction of vitamin B12 by Shewanella alga strain BrY with subsequent transformation of carbon tetrachloride. Environ Sci Technol 31:2292–2297
Xu W, Liu Y, Zeng G, Li X, Tang C, Yuan X (2005) Enhancing effect of iron on chromate reduction by Cellulomonas flavigena. J Hazard Mater 126:17–22
Yassi A, Nieboer E (1988) Carcinogenicity of chromium compounds. In: Niragu J, Niebaer E (eds) Chromium in the natural and human environments. Wiley, New York, pp 443–478
Zachara J, Fredrickson J, Li S, Kennedy D, Smith S, Gassman P (1998) Bacterial reduction of crystalline Fe3+ oxides in single phase suspensions and subsurface materials. Am Mineral 83:1426–1443
Acknowledgments
The authors would like to thank Lindsey Hopper, Kristy Weaver, Nicholas Ballor and Crystal Russell for their various contributions in the laboratory. This research was supported by the U.S. Department of Energy, Office of Science, Subsurface Biogeochemical Research Program, under Grant Nos. DE-FG02-03ER63582 and DOE-NE Idaho Operations Office Contract DE-AC07-05ID14517. Partial financial support was provided by a grant from the Inland Northwest Research Alliance (INRA) under contract MSU 002. We thank Thomas Borch, Matthew Marcus (ALS) and Matthew Ginder-Vogel for their help with synchrotron-based analyses. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Field, E.K., Gerlach, R., Viamajala, S. et al. Hexavalent chromium reduction by Cellulomonas sp. strain ES6: the influence of carbon source, iron minerals, and electron shuttling compounds. Biodegradation 24, 437–450 (2013). https://doi.org/10.1007/s10532-012-9600-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-012-9600-7