Abstract
This study highlights the conservation problems faced by the tiny freshwater bivalves of the family Sphaeriidae, also known as pea, pill, or fingernail clams (or mussels) in Europe. Despite their global distribution, assumed ecological importance, and potential uses, basic knowledge about their taxonomy, biology, and ecology is very limited and much lower than for the larger freshwater bivalve taxa. Immediate scientific priorities are required to fill knowledge gaps regarding their taxonomy, genetic diversity, distribution, reproductive cycle, ecosystem functions, and population trends. Such fundamental knowledge is necessary to identify specific threats and develop appropriate conservation actions. Deploying environmental DNA analysis at a large scale could be a valuable way to fill gaps in distribution and strengthen monitoring in areas where local taxonomic knowledge is lacking. Until taxon-specific management plans can be developed, we recommend that efforts concentrate on the general protection and restoration of wetland habitats, implementing pollution control measures, and managing invasive species. These actions should be complemented by community engagement through citizen science initiatives. Additionally, prioritising data collection to fill existing knowledge gaps and updating conservation statuses (Red Lists) based on comprehensive assessments will be crucial. Implementing these actions will provide a starting point for the broader protection of freshwater ecosystems, thus benefiting pea clams and other interconnected species within these habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tiny freshwater bivalves of the family Sphaeriidae are also known as pea, fingernail, or pill clams (or mussels), and they are distributed worldwide and inhabit various freshwater ecosystems, from very small ponds to large lakes and rivers (Cox et al. 1969; Kuiper 1983). In Europe and elsewhere, many of the species of pea clams are widespread, and this can be explained by the ability of these tiny animals to travel long distances, which can happen via waterbodies or by clinging on to other animals such as insects, fish, amphibians, birds and mammals, among others (Mackie 1979; Wood et al. 2008; Zelaya and Marinone 2012). Therefore, even from a natural history and biogeographic perspective, this group of animals warrants greater scientific attention given their remarkable variety of dispersal modes.
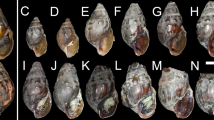
Despite often being overlooked, pea clams are fascinating animals. What sets them apart from other freshwater bivalves are primarily their size ranging from 3 to 25 mm (Fig. 1), possession of a different number of siphons (one for the genus Pisidium), the presence of byssus being limited to larval stages, hermaphroditicity, episodes of self-fertilisation (facilitated by internal fertilisation, although cross-fertilisation is normal), brooding larvae (juveniles develop in brood pouches in the parent's internal demibranchs and are subsequently released into the environment through the siphon), a small number of offspring (usually up to 30), functional gonads upon release (although depending on the species, they still require several months to reach their final size), a lifespan not exceeding five years (some species live much shorter). They also have the ability to live across a wide range of temperatures (from frozen Arctic or high mountain lakes to tropical and arid environments) and habitats, including springs, fens and wet meadows, rivers, lakes, but also anthropogenic ponds, canals and dam reservoirs—from oligotrophic to eutrophic waters (Meier-Brook 1975; Heard 1977; Holopainen and Hanski 1986; Killeen et al. 2004; Korniushin 2006; Piechocki and Wawrzyniak-Wydrowska 2016; Lee 2019). Furthermore, they exhibit an exceptionally high degree of polyploidy for the animal kingdom (most species being polyploid and possessing a high number of mitotic chromosomes ranging from approximately 150 to around 247), although the evolutionary origins and advantages of polyploidy in pea clams are not yet fully understood (Korniushin 2006; Petkevičiūtė et al. 2006, 2007; Kořínková and Morávková 2010; Kořínková and Král 2011).
Like other bivalves, pea clams play a role in maintaining the ecological balance of aquatic environments by filtering water and recycling nutrients (Korniushin and Glaubrecht 2002). Most of their functions and services still remain to be quantitatively assessed (Dittman et al. 2018). However, some examples show that, pea clams can be used for bioremediation due to their ability to accumulate pollutants (Vaughn and Hoellein 2018). These bivalves serve as a food source for organisms at higher trophic levels. They also play a role in the bioturbation of sediments and provide a physical resource for other species that utilise their shells (Cooley and Foighil 2000; Sousa et al. 2011a; Bespalaya et al. 2019). On the other hand, pea clams act as intermediate hosts of a number of trematode species causing disease (Bespalaya et al. 2023).
Despite these important ecological features, and like many other freshwater species, European pea clams face numerous conservation challenges due to habitat loss, pollution, introduction of invasive species, and climate change. Although freshwater bivalves are declining worldwide (Lopes-Lima et al. 2017), there is very little known about the pea clams (Watson and Ormerod 2005; Lopes-Lima et al. 2018). In recent decades, pea clams have been largely overlooked in Europe, especially compared to the bivalves of the family Unionidae (Fig. 2A). Over 40 species of these clams have been found in Europe, represented by five genera (Conventus, Euglesa, Odhneripisidium, Pisidium and Sphaerium) (Bespalaya et al. 2023; Graf and Cummings 2023). However, due to many knowledge gaps, it is difficult to determine their conservation status. A recent large-scale survey in France revealed a worrying conservation status of some species not currently considered endangered (Prié et al. 2023). The current IUCN threat categories (conservation status), which cover only a few European pea clam species, appear outdated (Glöer and Diercking 2010; Cuttelod et al. 2011). The conservation of pea clams in Europe requires a comprehensive understanding of their distribution, biology and ecology, but effective conservation efforts are hindered due to the knowledge gaps. Therefore, in this study, we aim to summarise the threats faced by pea clams, the knowledge gaps that must be filled to conserve this family effectively, and the actions that should be taken to bring pea clam conservation back on the scientific and cultural radar.
Comparison of research interest (cumulative number of publications) in every family of freshwater bivalves (Sphaeriidae, Cyrenidae, Dreissenidae, Unionidae and Margaritiferidae) in Europe based on the Scopus database (A) and cumulative number of Scopus publications on Sphaeriidae by different research areas (B). It should be noted that although more papers have been published under species names, the uncertain taxonomy of freshwater bivalves necessitated search terms with a higher taxonomic ranking. Thus, the total papers published in each group may be larger, but the trend and differences between groups are likely the same
Threats to European pea clams
Habitat loss and degradation is a major threat to European pea clam populations. The conversion of wetlands for agriculture, urbanisation, and infrastructure development has resulted in the loss of critical habitats for these bivalves. Global wetland loss has been estimated at more than 50% since 1900. Although the loss rate has slowed in some countries since the 1980s, Europe had already lost more than half of its wetlands by 1990 (EEA 2010). Wetlands are essential for many species of pea clams, providing suitable conditions for their survival and reproduction. The destruction of wetlands disrupts the natural hydrological cycles and reduces the availability of suitable habitats for this group of aquatic organisms (Lopes-Lima et al. 2018). Water pollution, leading to acidification or eutrophication, mainly from agricultural runoff, industrial discharges, and sewage, is a significant threat to all native bivalve species in Europe, including pea clams (Lopes-Lima et al. 2017, 2018). Several studies have shown that the number of pea clam species declines with decreasing pH (Økland and Økland 1986; Meriläinen and Hynynen 1990), as different species have differing tolerance to pH and alkalinity (Økland and Kuiper 1980; Meriläinen and Hynynen 1990). Pollutants such as nutrients, pesticides, and heavy metals can accumulate in the tissues of these bivalves, leading to physiological stress and reduced reproductive success. Additionally, pollution can degrade water quality, affecting the availability of food and oxygen for pea clams (Joyner-Matos et al. 2007, 2011).
Climate change is also a potential threat to the survival of pea clams in Europe. Rising temperatures, changes in precipitation patterns, and increased frequency of extreme climatic events can disrupt the ecological balance of freshwater ecosystems, affecting the survival and reproduction of these bivalves (Sousa et al. 2007, 2008a, 2008b, 2011a). Changes in water temperature and flow regimes can affect the timing of life cycle events of pea clams, potentially leading to population declines, as these phenomena are sometimes exacerbated by the presence of invasive bivalves. For example, during an extreme drought in 2005 in the Minho River (Iberian Peninsula), a freshwater ecosystem heavily invaded by Corbicula fluminea, a complete collapse of a Pisidium amnicum population was observed. Massive mortality of P. amnicum was recorded during this drought, after which the population never recovered due to lack of recruitment and is now considered extirpated (Sousa et al. 2007, 2008a, 2008b, 2011a). Additionally, climate change combined with pollution and other stressors may lead to large-scale disasters, such as in 2022 in the Oder River (the second largest river in Poland), where it is estimated that almost 90% of bivalves in the river died (Szlauer-Łukaszewska et al. 2024).
Invasive species, in addition to the well-documented impact of Corbicula fluminea on Pisidium amnicum in Portugal (Sousa et al. 2007, 2008a, 2011a), can affect pea clams in several ways. Dreissenid bivalves use byssus threads to attach themselves to the valves of pea clams (Lauer and McComish 2001). This fouling by dreissenid bivalves can hinder the ability of pea clams to burrow and move through sediments. Presumably, in some cases, dreissenid bivalves may obstruct the valve movement, thereby impairing their filter feeding, respiration, and reproduction, similar to the impact on unionids (Sousa et al. 2011b; Bódis et al. 2014). This fouling reduces the density of pea clams (Lauer and McComish 2001), potentially leading to their localised extinction in European regions. It has been proven that in addition to native species of fish and invertebrates, invasive ones also feed on pea clams (Meira et al. 2024). For example, invasive species such as Neogobius melanostomus and Orconectes limosus, which are common in Europe, prey on pea clams in North America (Klocker and Strayer 2004; Mack and Andraso 2015). However, there is no evidence of the impact of predation by these and other predator species on European pea clams.
Knowledge gaps about European pea clams
The role of pea clams in ecosystem functioning and the services they provide has yet to be adequately quantified (Rassam et al. 2021). Although they are small in size, they often occur in very high densities, reaching up to 130,000 individuals m−2 for a single species (Dyduch-Falniowska 1982). Therefore, they may play a crucial role in nutrient cycling, water filtration, and sediment stabilisation. For example, the average filtration rate in Sphaerium transversum was estimated to be 15.7 ml hour−1 (Way 1989). They are also likely to play an important role in the interstitial water–sediment interface of aquatic ecosystems (Burton Jr. 1991), which urgently needs to be quantified. Little is also known about the importance of pea clams as a component of the diet of other organisms (Dawidowicz and Gliwicz 1983). Therefore, it seems that pea clams should be of considerable interest to researchers. However, scientific production is ten times less in Sphaeriidae compared to Unionidae (Fig. 2A) (Lopes-Lima et al. 2014). Many issues remain to be resolved, and still little is known about pea clams. To illustrate the scientists' interest in pea clam research, searches were conducted in the Scopus database using research areas proposed for freshwater mussels by Lopes-Lima et al. (Lopes-Lima et al. 2014). Although the investigation did not include articles published in grey literature, articles published in languages other than English, or articles unavailable to this database for different reasons, it revealed a trend in research on pea clams (Fig. 2B). The majority of publications about pea clams focus on their ecology, while more fundamental investigations into taxonomy, genetics, and physiology appear to be neglected (Fig. 2B).
The autecology of various pea clam species in Europe, including their life history, density, biomass, distribution, reproduction, growth, and mortality rates, remains poorly understood. Most of available information comes from studies published over thirty years ago in different languages (e.g. Ladle and Baron 1969; Meier-Brook 1977; Bass 1979; Holopainen 1979; Dyduch-Falniowska 1982, 1983; Holopainen and Hanski 1986; Holopainen and Jónasson 1989; Piechocki 1991; Araujo et al. 1999; Korniushin and Glaubrecht 2002; Mouthon 2005, 2011; Mouthon and Daufresne 2008; Pettinelli and Bicchierai 2009; Korinkova 2011; Bespalaya et al. 2015b, 2019; Myzyk 2017). Significant knowledge gaps exist regarding their anatomy, physiology, reproductive strategies, larval development, and factors influencing their recruitment, growth, survival and dispersal. Moreover, the role of pea clams as hosts of parasites also requires detailed research (Petkevičiūtė et al. 2015). A critical knowledge gaps is the lack of comprehensive data on the diversity, distribution, and abundance of pea clam species throughout Europe. The uneven spread of data is highlighted in Fig. 3 for Euglesa subtruncata, a presumably common species across Europe. Additionally, unsolved taxonomic issues still exist for some groups or recent splits like E. ponderosa, and the taxonomic diversity of Eastern European pea clams (Bespalaya et al. 2015a, 2022, 2023; Vinarski and Kantor 2016; Petkevičiūtė et al. 2018; Groh et al. 2020; Bikashvili et al. 2022). While there is good distributional data for countries and regions such as Britain, Ireland, France, Germany and Scandinavia (in many cases, very old), information is sparse for parts of southern and southeastern Europe. The lack of data from some European countries does not necessarily mean these species are not present but that the they have not been reported. Moreover, data availability is an issue, with some information in museum collections or monographs in national languages needing updating and translation into English, particularly for countries like Ukraine, Belarus, and Poland (Piechocki and Dyduch-Falniowska 1993; Korniushin 1996; Laenko 2012). Pea clams are widely distributed in Europe, with a diversity hotspot mainly in the Balkan Peninsula (Schultheiß et al. 2008). There is limited data on their presence in anthropogenic habitats (Sousa et al. 2021), such as subsidence ponds (Sowa et al. 2019) or even wells (Dumnicka et al. 2017). Despite the global existence of artificial ponds created for various purposes (Fehlinger et al. 2023), there is no evidence that European pea clams utilise these habitats. The substrate and pollution levels in these anthropogenic environments may not support colonisation by pea clams, and frequent maintenance activities like dredging or drying can pose an ecological trap (Sousa et al. 2021).
Uneven pea clam distribution data across Europe. Example of Euglesa subtruncata, a presumably common species throughout Europe. It should be noted that the lack of data from Norway, Italy, Spain or other countries does not necessarily reflect that this species is not present in the country but that the data has not been reported
While some studies have focused mainly on the local or regional scales, such as specific rivers or reservoirs (e.g. Sousa et al. 2008a; Zawal et al. 2016; Urbanič et al. 2020), there is a need for extensive surveys and monitoring programs covering broad geographic areas and diverse habitats. This is essential to assess the presence and population dynamics of pea clams across their entire European range. Further research is needed on their dispersal mechanisms, including by animals (Zelaya and Marinone 2012), plants (Halabowski, personal observation), or with bottom sediments/water (Kappes and Haase 2012). Such research have potential payoffs for meta-population and meta-communities theory regarding pea clams. This is also crucial for identifying conservation priorities areas for implementing effective management strategies.
Pea clams have high sensitivity to acidification, which has led to their use as bioindicators in Finland, Norway, and Sweden (Økland and Økland 1986), where they are included in acidification indices to classify ecological status under the European Union Water Framework Directive. However, these indices only use the family and/or genus level (Norwegian Environment Agency Water Framework Directive Group 2018; Swedish Agency Marine and Water Management 2018a, 2018b; Aroviita et al. 2019). Thus, the pea clams are not used to their full potential and data is not collected at a species level. The use of pea clams as indicators of acidification highlight their potential for use as bioindicators for other environmental parameters, as they are sensitive to changes in flow, oxygen levels, sediment pollution, and habitat alterations (Meier-Brook 1975; Horsák 2006; Bespalaya 2015; Sowa et al. 2019). To fully utilise this potential, understanding and leveraging species-specific differences in tolerance levels is essential.
Surveying and monitoring pea clam populations is problematic because identification of this group of species is difficult. There is a lack of taxonomic expertise, and working in deep, turbid waters with tiny clams completely buried in sediment presents sampling problems. Much use could be made of invertebrate samples collected by environmental agencies for water quality monitoring if the pea clams were passed on to specialists. There is a particular need to train a group of freshwater biologists in Europe to acquire expertise in pea clam identification, including increased taxonomic training. The identification of pea clams using new molecular-based surveys, such as environmental DNA (eDNA) and metabarcoding, have recently been used with great success and should be encouraged (Prié et al. 2023). Although the taxonomy of pea clams using a molecular approach has advanced in recent years (Fig. 2B), a comprehensive taxonomic and systematics revision of European pea clams is urgently needed.
Knowledge of the phylogeny, phylogeography and patterns of genetic diversity and connectivity of European pea clam populations is essential to guide conservation efforts. There is a lack of comprehensive genetic studies on these bivalves (Graf 2013). A recent study by Bespalaya et al. (2023) slightly improves the phylogenetic and taxonomic structure of Sphaeriidae in Europe. However, it is based on a small number of sequences available, mainly in GenBank. We recommend that future molecular research should initially focus on obtaining reference libraries of informative barcodes, such as COI or 16S rDNA, or the whole mitogenome for more accurate species identification, and future work using metabarcoding approaches. The use of novel next-generation sequencing methods (e.g. RAD or whole genome sequencing) to assess genetic diversity within and between populations will provide insights into their evolutionary potential, adaptability, and vulnerability to environmental change. This information is crucial for prioritising populations, species, or clades that represent unique evolutionary lineages for conservation. At the species level, knowledge of phylogeographic and genetic diversity patterns is also of great interest for identifying populations of conservation importance, such as evolutionarily significant units and conservation units. For example, populations with high genetic diversity and unique haplotypes should rank higher regarding conservation priority. It will also help to identify potential inbreeding events and the main genetic flow pathways between populations. Another benefit of knowing the detailed genetic diversity patterns of populations is the identification of potential stock populations for reintroduction or translocation efforts. However, when attempting translocations, care should be taken to minimise the potential for disease or parasite transfer between populations (Brian et al. 2021).
Conservation strategies and action needs for European pea clams
A roadmap to European pea clams conservation encompassing four interrelated, major conceptual areas that need attention. For each conceptual area, the most pressing priorities are listed in Fig. 4.
The basis for pea clam protection should be developing and updating official Red Lists for freshwater molluscs, which many European countries still need to do (Bourlat et al. 2023). The lack of such actions may lead to erroneous conclusions. Namely, it has recently been shown that bivalves are less endangered than freshwater snails (Böhm et al. 2021). This results from the relatively low estimated level of threat in the family Sphaeriidae, for which very few risks have been reported. This, in turn, results from the scarcity of data regarding this group because they are much less frequently studied than other families of molluscs (Böhm et al. 2021). Therefore, there is an urgent need to develop and update local Red Lists, and reevaluate the IUCN category for pea clams.
The protection and restoration of wetland habitats are essential for the conservation of pea clams in Europe. Efforts should focus on identifying and designating important wetlands as protected areas. These protected areas can provide a refuge for populations of pea clams and ensure the preservation of their habitats. Additionally, restoration projects can improve degraded freshwater habitats and provide new suitable habitats for pea clam populations. However, it is important to emphasise that the protection of freshwater ecosystems and their biodiversity requires a management strategy for entire river catchments (Abell et al. 2007; Nogueira et al. 2021a, 2021b, 2023) and so all dimensions of connectivity should be taken into account.
The quality of the substrate plays an important role for pea clams. Habitat preferences vary among species, but generally in rivers and lakes they prefer the detritus-covered bottom in the ripal and littoral zones, respectively. However, other species are found in clean, sandy substrates or alternatively in muddy substrates (Piechocki and Wawrzyniak-Wydrowska 2016). Being filter-feeders, they are exposed to the pollutants and accumulation of heavy metals such as aluminium, cadmium, lead, and mercury released from the sediments under appropriate conditions (e.g. lowered pH) (Szczęsny 1990). A multi-faceted approach is needed to restore damaged and polluted habitats for pea clams. It should include both increasing the diversity of substrates and removing undesirable substances (various types of pollutants, e.g. heavy metals) using for example phytoremediation (Hering et al. 2015; Samal et al. 2019; Debnath et al. 2021). Improving water quality is essential for the conservation of pea clams in Europe. Implementing and enforcing regulations to reduce pollution from agricultural and industrial sources can help minimise the negative impacts of water pollution on these bivalves. Additionally, promoting sustainable agricultural practices, such as reducing the use of fertilisers and pesticides and increasing the riparian vegetation, can help mitigate the pollution risks to pea clams and other freshwater organisms.
Efforts should be made to control and manage invasive species that threaten European pea clams, including competing bivalves and known molluscivorous predators, such as some crayfish and fish species. Therefore, it is crucial to develop and implement early detection and rapid response programmes to prevent the establishment and spread of harmful invasive species.
Mitigation of the potential impacts of climate change is also important for the long-term conservation of pea clams in Europe (Sousa et al. 2008a). This may involve implementing measures to increase the resilience of freshwater ecosystems, such as restoring riparian vegetation, maintaining or restoring natural flow regimes, and promoting sustainable water management practices. Additionally, monitoring and research efforts should focus on understanding the specific vulnerabilities of pea clams to climate change and developing adaptive management strategies to mitigate these impacts.
Understanding and assessing the extent and impact of these threats on pea clam populations are essential to developing effective conservation strategies and prioritising conservation actions (Watson and Ormerod 2005). In addition, conservation strategies and governance needs for pea clams are similar to those described for freshwater mussels (Sousa et al. 2023). Therefore, by implementing a more comprehensive approach, conservation success can be achieved for both freshwater mussels and pea clams. Additionally, understanding the pea clam's ecological interactions and contributions to ecosystem services is crucial for assessing their conservation value and integrating their protection into broader ecosystem management strategies.
The use of citizen science for the conservation of pea clams can help scientists and stakeholders obtain valuable information about these organisms. In particular, the local community can provide knowledge about distribution, habitat, and environmental changes, which can be valuable for conservation planning and management. Engaging with local communities and travellers/naturalists and incorporating their knowledge can improve the effectiveness of conservation and management efforts. Efforts should therefore be made to raise people's awareness of the existence of these fascinating animals and to increase the knowledge about them, the ecosystem services they provide, and the threats they face.
Conclusions
Often overlooked and undervalued, pea clams may play a crucial role in freshwater ecosystems. However, their hidden existence is threatened by various anthropogenic activities and environmental changes. Conservation efforts must prioritise understanding their biology, ecological role, and the threats they face. Implementing effective management strategies that include habitat protection, pollution control, invasive species control, and mitigation of climate change effects is essential. Bridging knowledge gaps through comprehensive research across European countries and engaging local communities can ensure the continued existence and contributions of pea clams to freshwater ecosystems at different scales. Raising awareness and support for the conservation of these remarkable organisms is essential to preserve their biodiversity and maintain healthy freshwater ecosystems.
Data Availability
No datasets were generated or analysed during the current study.
References
Abell R, Allan JD, Lehner B (2007) Unlocking the potential of protected areas for freshwaters. Biol Conserv 134:48–63
Araujo R, Ramos MÁ, Molinet R (1999) Growth pattern and dynamics of a southern peripheral population of Pisidium amnicum (Müller, 1774) (Bivalvia: Sphaeriidae) in Spain. Malacologia 41:119–137
Aroviita J, Mitikka S, Vienonen S (2019) Status classification and assessment criteria of surface waters in the third river basin management cycle
Bass J (1979) Growth and fecundity of Pisidium amnicum (Müller) (Bivalvia: Sphaeriidae) in the Tadnoll Brook, Dorset, England. J Conchol 30:129–134
Bespalaya Y (2015) Molluscan fauna of an Arctic lake is dominated by a cosmopolitan Pisidium species. J Molluscan Stud 81:294–298
Bespalaya Y, Bolotov I, Aksenova O, Kondakov A, Gofarov M, Paltser I (2015a) Occurrence of a Sphaerium species (Bivalvia: Sphaeriidae) of Nearctic origin in European Arctic Russia (Vaigach Island) indicates an ancient exchange between freshwater faunas across the Arctic. Polar Biol 38:1545–1551
Bespalaya Y, Bolotov I, Aksenova O, Kondakov A, Paltser I, Gofarov M (2015b) Reproduction of Pisidium casertanum (Poli, 1791) in Arctic lake. R Soc Open Sci 2:140212
Bespalaya Y, Joyner-Matos J, Bolotov I, Aksenova O, Gofarov M, Sokolova S, Shevchenko A, Travina O, Zubriy N, Aksenov A, Kosheleva A, Ovchinnikov D (2019) Reproductive ecology of Pisidium casertanum (Poli, 1791) (Bivalvia: Sphaeriidae) in Arctic lakes. J Molluscan Stud 85:11–23
Bespalaya YV, Travina OV, Tomilova AA, Khrebtova IS, Aksenova OV, Aksenov AS, Vinarskii MV, Kondakov AV, Nekhaev IO, Palatov DM, Spitsyn VM, Shevchenko AR, Bolotov IN (2022) Species diversity, settlement routes, and ecology of freshwater mollusks of Kolguev Island (Barents Sea, Russia). Inland Water Biol 15:836–849
Bespalaya YV, Vinarski MV, Aksenova OV, Babushkin ES, Gofarov MY, Kondakov AV, Konopleva ES, Kropotin AV, Mabrouki Y, Ovchankova NB, Palatov DM, Sokolova SE, Shevchenko AR, Travina OV, Taybi AF, Soboleva AA, Zubrii NA, Bolotov IN (2023) Phylogeny, taxonomy, and biogeography of the Sphaeriinae (Bivalvia: Sphaeriidae). Zool J Linn Soc 201: 305–338
Bikashvili A, Kachlishvili N, Japoshvili B, Mumladze L (2022) Species diversity and DNA barcode library of freshwater molluscs of south caucasus. Biodivers Data J 10:84887
Bódis E, Tóth B, Sousa R (2014) Impact of Dreissena fouling on the physiological condition of native and invasive bivalves: interspecific and temporal variations. Biol Invasions 16:1373–1386
Böhm M, Dewhurst-Richman NI, Seddon M, Ledger SEH, Albrecht C, Allen D, Bogan AE, Cordeiro J, Cummings KS, Cuttelod A, Darrigran G, Darwall W, Fehér Z, Gibson C, Graf DL, Köhler F, Lopes-Lima M, Pastorino G, Perez KE, Smith K, van Damme D, Vinarski MV, von Proschwitz T, von Rintelen T, Aldridge DC, Aravind NA, Budha PB, Clavijo C, Van Tu D, Gargominy O, Ghamizi M, Haase M, Hilton-Taylor C, Johnson PD, Kebapçı Ü, Lajtner J, Lange CN, Lepitzki DAW, Martínez-Ortí A, Moorkens EA, Neubert E, Pollock CM, Prié V, Radea C, Ramirez R, Ramos MA, Santos SB, Slapnik R, Son MO, Stensgaard A-S, Collen B (2021) The conservation status of the world’s freshwater molluscs. Hydrobiologia 848:3231–3254
Bourlat SJ, Tschan GF, Martin S, Iqram M, Leidenberger S (2023) A red listing gap analysis of molluscs and crustaceans in Northern Europe: what has happened in the last 10 years? Biol Conserv 286:110247
Brian JI, Ollard IS, Aldridge DC (2021) Don’t move a mussel? Parasite and disease risk in conservation action. Conserv Lett 14:12799
Burton GA Jr (1991) Assessing the toxicity of freshwater sediments. Environ Toxicol Chem 10:1585–1627
Cooley L, Foighil D (2000) Phylogenetic analysis of the Sphaeriidae (Mollusca: Bivalvia) based on partial mitochondrial 16S rDNA gene sequences. Invertebr Biol 119:299–308
Cox L, Newell N, Boyd D (1969) Bivalvia. Treatise on Invertebrate Paleonthology. Mollusca. University of Kansas and Geological Society of America, Lawrence
Cuttelod A, Seddon MB, Neubert E (2011) European red list of non-marine molluscs. Publications Office of the European Union, Luxembourg
Dawidowicz P, Gliwicz M (1983) Food of brook charr in extreme oligotrophic conditions of an alpine lake. Environ Biol Fishes 8:55–60
Debnath A, Singh PK, Chandra Sharma Y (2021) Metallic contamination of global river sediments and latest developments for their remediation. J Environ Manage 298:113378
Dittman DE, Johnson JH, Nack CC (2018) Microhabitat and biology of Sphaerium striatinum in a central New York stream. Hydrobiologia 810:367–374
Dumnicka E, Galas J, Krodkiewska M (2017) Patterns of Benthic Fauna Distribution in Wells: The Role of Anthropogenic Impact and Geology. Vadose Zone J 16: 1–9
Dyduch-Falniowska A (1982) Oscillations in density and diversity of Pisidium communities in two biotopes in Southern Poland. Hydrobiol Bull 16:123–132
Dyduch-Falniowska A (1983) Age structure of the populations of Pisidium species from two localities in southern Poland. Hydrobiol Bull 17:111–117
EEA (2010) The European environment: state and outlook 2010.
Fehlinger L, Misteli B, Morant D, Juvigny-Khenafou N, Cunillera-Montcusí D, Chaguaceda F, Stamenković O, Fahy J, Kolář V, Halabowski D, Nash LN, Jakobsson E, Nava V, Tirozzi P, Cordero PU, Mocq J, Santamans AC, Zamora-Marín JM, Marle P, Chonova T, Bonacina L, Mathieu-Resuge M, Suarez E, Osakpolor SE, Timoner P, Evtimova V, Nita D, Carreira BM, Tapolczai K, Martelo J, Gerber R, Dinu V, Henriques J, Selmeczy GB, Rimcheska B (2023) The ecological role of permanent ponds in Europe: a review of dietary linkages to terrestrial ecosystems via emerging insects. Inland Waters 13:30–46
Glöer P, Diercking R (2010) Atlas der Süßwassermollusken: Rote Liste, Verbreitung, Ökologie, Bestand und Schutz. Freie und Hansestadt Hamburg, Behörde für Stadtentwicklung und Umwelt, Amt für Natur- und Ressourcenschutz, Abt. Naturschutz,
Graf DL (2013) Patterns of freshwater bivalve global diversity and the state of phylogenetic studies on the Unionoida, Sphaeriidae, and Cyrenidae. Am Malacol Bull 31:135–153
Graf DL, Cummings K (2023) The Freshwater Mussels (Unionoida) of the World (and Other Less Consequential Bivalves), updated 14 Febrary 2023, 2023. MUSSEL Project Web Site. http://www.mussel-project.net/
Groh K, Bössneck U, Clewing C, Albrecht C, Richling I (2020) A new pill clam from an unusual habitat: the interstitial Pisidium interstitialis n. sp. (Bivalvia: Sphaeriidae) from southwestern and central Germany. J Molluscan Stud 86:104–119
Heard W (1977) Reproduction of fingernail clams (Sphaeriidae: Sphaerium & Musculium). Malacologia 16:421–455
Hering D, Aroviita J, Baattrup-Pedersen A, Brabec K, Buijse T, Ecke F, Friberg N, Gielczewski M, Januschke K, Köhler J, Kupilas B, Lorenz AW, Muhar S, Paillex A, Poppe M, Schmidt T, Schmutz S, Vermaat J, Verdonschot PFM, Verdonschot RCM, Wolter C, Kail J (2015) Contrasting the roles of section length and instream habitat enhancement for river restoration success: a field study of 20 European restoration projects. J Appl Ecol 52:1518–1527
Holopainen IJ, Hanski I (1986) Life history variation in Pisidium (Bivalvia: Pisidiidae). Holarct Ecol 9:85–98
Holopainen IJ, Jónasson PM (1989) Reproduction of Pisidium (Bivalvia, Sphaeriidae) at different depths in Lake Esrom, Denmark. Arch Für Hydrobiol 116:85–95
Holopainen IJ (1979) Population dynamics and production of Pisidium species (Bivalvia, Sphaeriidae) in the oligotrophic and mesohumic Lake Paajarvi, Southern Finland. Archiv für Hydrobiologie Supplement 54:466–508
Horsák M (2006) Habitat requirements of the Czech Pisidium species (Mollusca: Bivalvia) and possible application to bioindication. Verhandlungen Int Ver Limnol 29:1767–1769
Joyner-Matos J, Chapman LJ, Downs CA, Hofer T, Leeuwenburgh C, Julian D (2007) Stress response of a freshwater clam along an abiotic gradient: too much oxygen may limit distribution. Funct Ecol 21:344–355
Joyner-Matos J, Richardson H, Sammeli T, Chapman LJ (2011) A fingernail clam (Sphaerium sp.) shows higher reproductive success in hypoxic waters. Can J Zool 89:161–168
Kappes H, Haase P (2012) Slow, but steady: dispersal of freshwater molluscs. Aquat Sci 74:1–14
Killeen I, Aldridge DC, Oliver G (2004) Freshwater Bivalves of Britain and Ireland. Telford
Klocker CA, Strayer DL (2004) Interactions among an Invasive Crayfish (Orconectes rusticus), a Native Crayfish (Orconectes limosus), and Native Bivalves (Sphaeriidae and Unionidae). Northeast Nat 11:167–178
Korinkova T (2011) A complex view of breeding strategy and life-history in one population of Sphaerium corneum linnaeus 1758 (Bivalvia: Sphaeriidae). J Conchol 40:527–536
Kořínková T, Král J (2011) Structure and meiotic behaviour of B chromosomes in Sphaerium corneum/S. nucleus complex (Bivalvia: Sphaeriidae). Genetica 139:155–165
Kořínková T, Morávková A (2010) Does polyploidy occur in central European species of the family Sphaeriidae (Mollusca: Bivalvia)? Cent Eur J Biol 5:777–784
Korniushin AV (2006) Non-unionid freshwater bivalves (Sphaeriidae, Corbiculidae, Dreissenidae) of North American Fauna. Vestn Zool 41:13–22
Korniushin AV, Glaubrecht M (2002) Phylogenetic analysis based on the morphology of viviparous freshwater clams of the family Sphaeriidae (Mollusca, Bivalvia, Veneroida). Zool Scr 31:415–459
Korniushin AV (1996) Bivalve mollusks of the superfamily Pisidioidea in the Palaearctic region: Fauna, systematics, phylogeny. Schmalhausen Institute of Zoology, Kiev
Kuiper JGJ (1983) The sphaeriidae of Australia. Basteria 47:3–52
Ladle M, Baron F (1969) Studies on three species of Pisidium (Mollusca: Bivalvia) from a Chalk Stream. J Anim Ecol 38:407–413
Laenko TM (2012) Fauna of the aquatic mollusks of Belarus. Minsk
Lauer TE, McComish TS (2001) Impact of zebra mussels (Dreissena polymorpha) on fingernail clams (Sphaeriidae) in extreme Southern Lake Michigan. J Gt Lakes Res 27:230–238
Lee T (2019) Sphaeriidae. Freshwater Mollusks of the World: A Distribution Atlas. Johns Hopkins University Press, Baltimore,
Lopes-Lima M, Teixeira A, Froufe E, Lopes A, Varandas S, Sousa R (2014) Biology and conservation of freshwater bivalves: past, present and future perspectives. Hydrobiologia 735:1–13
Lopes-Lima M, Sousa R, Geist J, Aldridge DC, Araujo R, Bergengren J, Bespalaya Y, Bódis E, Burlakova L, Van Damme D, Douda K, Froufe E, Georgiev D, Gumpinger C, Karatayev A, Kebapçi Ü, Killeen I, Lajtner J, Larsen BM, Lauceri R, Legakis A, Lois S, Lundberg S, Moorkens E, Motte G, Nagel K-O, Ondina P, Outeiro A, Paunovic M, Prié V, von Proschwitz T, Riccardi N, Rudzīte M, Rudzītis M, Scheder C, Seddon M, Şereflişan H, Simić V, Sokolova S, Stoeckl K, Taskinen J, Teixeira A, Thielen F, Trichkova T, Varandas S, Vicentini H, Zajac K, Zajac T, Zogaris S (2017) Conservation status of freshwater mussels in Europe: state of the art and future challenges: conservation of European freshwater mussels. Biol Rev 92:572–607
Lopes-Lima M, Burlakova LE, Karatayev AY, Mehler K, Seddon M, Sousa R (2018) Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia 810:1–14
Mack TN, Andraso G (2015) Ostracods and other prey survive passage through the gut of round goby (Neogobius melanostomus). J Gt Lakes Res 41:303–306
Mackie G (1979) Dispersal mechanisms in Sphaeriidae (Mollusca: Bivalvia). Bull Am Malacol Union 45:17–21
Meier-Brook C (1975) Der ökologische indikatorwert mitteleuropäischer pisidium-arten (Mollusca, Eulamellibranchiata). Eiszeitalt Ggw 26:190–195
Meier-Brook C (1977) Intramarsupial suppression of fetal development in sphaeriid clams. Malacol Rev 10:53–58
Meira A, Byers JE, Sousa R (2024) A global synthesis of predation on bivalves. Biol Rev 99:1015–1057
Meriläinen J, Hynynen J (1990) Benthic invertebrates in relation to acidity in Finnish forest lakes. Acidification in Finland. Finnish Acidification Research Programme HAPRO 1985–1990. Springer-Verlag, Berlin, pp 1029–1049
Mouthon J (2005) Life cycle and population dynamics of Pisidium subtruncatum Malm (Bivalvia: Sphaeriidae) in the Saone, a large lowland river, at Lyon (France): environmental influences. Arch Für Hydrobiol 163:539–554
Mouthon J (2011) Response of bivalve populations to drying disturbance and life history traits of two Pisidium species (Bivalvia: Sphaeriidae) in a reservoir of the French Upper Rhone river. Ann Limnol - Int J Limnol 47:175–184
Mouthon J, Daufresne M (2008) Population dynamics and life cycle of Pisidium amnicum (Müller) (Bivalvia : Sphaeriidae) and Valvata piscinalis (Müller) (Gastropoda : Prosobranchia) in the Saône river, a nine-year study. Ann Limnol - Int J Limnol 44:241–251
Myzyk S (2017) On the reproduction of Pisidium C. Pfeiffer, 1821 (Bivalvia: Sphaeriidae) from Sąpolno (NW. Poland). Folia Malacol 25:175–194
Nogueira JG, Sousa R, Benaissa H, De Knijf G, Ferreira S, Ghamizi M, Gonçalves DV, Lansdown R, Numa C, Prié V, Riccardi N, Seddon M, Urbańska M, Valentini A, Vikhrev I, Varandas S, Teixeira A, Lopes-Lima M (2021a) Alarming decline of freshwater trigger species in western Mediterranean key biodiversity areas. Conserv Biol 35:1367–1379
Nogueira JG, Teixeira A, Varandas S, Lopes-Lima M, Sousa R (2021b) Assessment of a terrestrial protected area for the conservation of freshwater biodiversity. Aquat Conserv Mar Freshw Ecosyst 31:520–530
Nogueira JG, Lopes-Lima M, Beja P, Filipe AF, Froufe E, Gonçalves DV, da Silva JP, Sousa R, Teixeira A, Varandas S, Hermoso V (2023) Identifying freshwater priority areas for cross-taxa interactions. Sci Total Environ 864:161073
Norwegian Environment Agency Water Framework Directive Group (2018) Classification of environmental status in waters. Ecological and chemical classification system for coastal waters, groundwater, lakes and rivers.
Økland KA, Kuiper JGJ (1980) Small mussels (Sphaeriidae) in fresh water in Norway. Distribution, ecology, and relation to acidification of lakes. SNSF-project. Oslo-Ås Nor IR 61–80
Økland J, Økland KA (1986) The effects of acid deposition on benthic animals in lakes and streams. Experientia 42:471–486
Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G (2006) Polymorphism of the Sphaerium corneum (Bivalvia, Veneroida, Sphaeriidae) revealed by cytogenetic and sequence comparison. Biol J Linn Soc 89:53–64
Petkevičiūtė R, Stanevičiūtė G, Stunžėnas V, Lee T, Foighil Ó, D, (2007) Pronounced karyological divergence of the North American congeners Sphaerium rhomboideum and S. occidentale (Bivalvia: Veneroida: Sphaeriidae). J Molluscan Stud 73:315–321
Petkevičiūtė R, Kudlai O, Stunžėnas V, Stanevičiūtė G (2015) Molecular and karyological identification and morphological description of cystocercous cercariae of Phyllodistomum umblae and Phyllodistomum folium (Digenea, Gorgoderidae) developing in European sphaeriid bivalves. Parasitol Int 64:441–447
Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G (2018) Comments on species divergence in the genus Sphaerium (Bivalvia) and phylogenetic affinities of Sphaerium nucleus and S. corneum var. mamillanum based on karyotypes and sequences of 16S and ITS1 rDNA. PLOS ONE. 13:0191427
Pettinelli R, Bicchierai M (2009) Life cycle of Pisidium henslowanum (Sheppard, 1823) (Bivalvia, Veneroida, Sphaeriidae) from Piediluco Lake (Umbria, Italy). Fundam Appl Limnol Arch Für Hydrobiol 175:79–92
Piechocki A, Dyduch-Falniowska A (1993) Mięczaki (Mollusca), małże (Bivalvia). Państwowe Wydawnictwo Naukowe, Warszawa
Piechocki A, Wawrzyniak-Wydrowska B (2016) Guide to Freshwater and Marine Mollusca of Poland. Bogucki Wydawnictwo Naukowe, Poznań
Piechocki A (1991) Systematics, biology and ecology of the Polish pill-clams (Pisidium Pfeiff.) (Bivalvia, Eulamellibranchia). Acta Univ Lodz Folia Limnol 3–31
Prié V, Danet A, Valentini A, Lopes-Lima M, Taberlet P, Besnard A, Roset N, Gargominy O, Dejean T (2023) Conservation assessment based on large-scale monitoring of eDNA: application to freshwater mussels. Biol Conserv 283:110089
Rassam H, Ghamizi M, Benaissa H, Clewing C, Albrecht C (2021) The fingernail clams (Bivalvia: Veneroida: Sphaeriidae) of Morocco: diversity, distribution and conservation status. Biodivers Data J 9:73346
Samal K, Kar S, Trivedi S (2019) Ecological floating bed (EFB) for decontamination of polluted water bodies: design, mechanism and performance. J Environ Manage 251:109550
Schultheiß R, Albrecht C, Bößneck U, Wilke T (2008) The neglected side of speciation in ancient lakes: phylogeography of an inconspicuous mollusc taxon in lakes Ohrid and Prespa. Hydrobiologia 615:141–156
Sousa R, Antunes C, Guilhermino L (2007) Species composition and monthly variation of the Molluscan fauna in the freshwater subtidal area of the River Minho estuary. Estuar Coast Shelf Sci 75:90–100
Sousa R, Morais P, Antunes C, Guilhermino L (2008a) Factors affecting Pisidium amnicum (Müller, 1774; Bivalvia: Sphaeriidae) distribution in the River Minho Estuary: consequences for its conservation. Estuaries Coasts 31:1198–1207
Sousa R, Nogueira A, Antunes C, Guilhermino L (2008b) Growth and production of Pisidium amnicum in the freshwater tidal area of the River Minho estuary. Estuar Coast Shelf Sci 79:467–474
Sousa R, Ilarri M, Souza AT, Antunes C, Guilhermino L (2011a) Rapid decline of the greater European peaclam at the periphery of its distribution. Ann Limnol—Int J Limnol 47:211–219
Sousa R, Pilotto F, Aldridge DC (2011b) Fouling of European freshwater bivalves (Unionidae) by the invasive zebra mussel (Dreissena polymorpha). Freshw Biol 56:867–876
Sousa R, Halabowski D, Labecka AM, Douda K, Aksenova O, Bespalaya Y, Bolotov I, Geist J, Jones HA, Konopleva E, Klunzinger MW, Lasso CA, Lewin I, Liu X, Lopes-Lima M, Mageroy J, Mlambo M, Nakamura K, Nakano M, Österling M, Pfeiffer J, Prié V, Paschoal LRP, Riccardi N, Santos R, Shumka S, Smith AK, Son MO, Teixeira A, Thielen F, Torres S, Varandas S, Vikhrev IV, Wu X, Zieritz A, Nogueira JG (2021) The role of anthropogenic habitats in freshwater mussel conservation. Glob Change Biol 27:2298–2314
Sousa R, Zając T, Halabowski D, Aksenova OV, Bespalaya YV, Carvalho F, Castro P, Douda K, da Silva JP, Ferreira-Rodríguez N, Geist J, Gumpinger C, Labecka AM, Lajtner J, Lewin I, Lopes-Lima M, Meira A, Nakamura K, Nogueira JG, Ondina P, Ożgo M, Reis J, Riccardi N, Shumka S, Son MO, Teixeira A, Thielen F, Urbańska M, Varandas S, Wengström N, Zając K, Zieritz A, Aldridge DC (2023) A roadmap for the conservation of freshwater mussels in Europe. Conserv Biol 37:e13994
Sowa A, Krodkiewska M, Halabowski D, Lewin I (2019) Response of the mollusc communities to environmental factors along an anthropogenic salinity gradient. Sci Nat 106:60
Swedish Agency Marine and Water Management (2018a) Benthic fauna and lakes. Guidance document for status classification.
Swedish Agency Marine and Water Management (2018b) Benthic fauna in water courses. Guidance document for status classification.
Szczęsny B (1990) Benthic macroinvertebrates in acid streams of the Świętokrzyski National Park (central Poland). Acta Hydrobiol 32:155–169
Szlauer-Łukaszewska A, Ławicki Ł, Engel J, Drewniak E, Ciężak K, Marchowski D (2024) Quantifying a mass mortality event in freshwater wildlife within the Lower Odra River: Insights from a large European river. Sci Total Environ 907:167898
Urbanič G, Mihaljević Z, Petkovska V, Pavlin Urbanič M (2020) Disentangling the effects of multiple stressors on large rivers using benthic invertebrates—a study of Southeastern European Large Rivers with implications for management. Water 12:621
Vaughn CC, Hoellein TJ (2018) Bivalve impacts in freshwater and marine ecosystems. Annu Rev Ecol Evol Syst 49:183–208
Vinarski M, Kantor Y (2016) Analytical catalogue of fresh and brackish water molluscs of Russia and adjacent countries. Litres, Moscow
Watson AM, Ormerod SJ (2005) The distribution and conservation of threatened Sphaeriidae on British grazing marshland. Biodivers Conserv 14:2207–2220
Way CM (1989) Dynamics of filter-feeding in Musculium transversum (Bivalvia:Sphaeriidae). J North Am Benthol Soc 8:243–249
Wood L, Griffiths R, Groh K, Engel E, Schley L (2008) Interactions between freshwater mussels and newts: a novel form of parasitism? Amphib-Reptil 29:457–462
Zawal A, Lewin I, Stępień E, Szlauer-Łukaszewska A, Buczyńska E, Buczyński P, Stryjecki R (2016) The influence of the landscape structure within buffer zones, catchment land use and instream environmental variables on mollusc communities in a medium-sized lowland river. Ecol Res 31:853–867
Zelaya DG, Marinone MC (2012) A Case of Phoresis of Sphaeriids by Corixids: First Report for the Americas. Malacologia 55:363–367
Acknowledgements
This publication is based upon work from COST Action CA18239, supported by COST (European Cooperation in Science and Technology). D.H. was supported by the Polish National Science Grant 2022/06/X/NZ8/00696. J.H.M. was supported by the Norwegian Institute for Nature Research, both through internal funding and funding from the Research Council of Norway. D.A.C is supported by the Woolf Fisher Scholarship and Whitten Studentship.
Funding
European Cooperation in Science and Technology, CA18239; Narodowe Centrum Nauki, 2022/06/X/NZ8/00696; Norsk institutt for naturforskning; Woolf Fisher Scholarship and Whitten Studentship.
Author information
Authors and Affiliations
Contributions
D.H.: conceptualisation, investigation, visualisation, writing—original draft, writing—review and editing; R.S., M.L.-L., I.K., K.Z., D.A., J.H.M., D.A.C., M.U. and M.Ö.: investigation, writing—original draft, writing—review and editing; V.P.: investigation, data collection, visualisation, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Communicated by Michael Joy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halabowski, D., Sousa, R., Lopes-Lima, M. et al. Off the conservation radar: the hidden story of Europe's tiny pea clams (Bivalvia: Sphaeriidae). Biodivers Conserv (2024). https://doi.org/10.1007/s10531-024-02921-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10531-024-02921-x