Abstract
Despite the pernicious impacts that invasive black rats Rattus rattus have on island ecosystems, little is known about their effect upon insular reptiles, which are a highly vulnerable but pivotal element of island biota. To bring to light these effects, we evaluated the threat posed by R. rattus on the critically endangered Canarian spotted lizard Gallotia intermedia by analyzing its frequency of occurrence on rat feces, estimating rat abundance and density, and correlating these parameters with previous lizard censuses. We genetically detected that 14.96% of all rat feces contained G. intermedia, with 27.27% of individual R. rattus consuming this lizard. Rat density varied from 0.740 ± 0.474 to 2.183 ± 1.137 rats/ha and was correlated with larger declines of G. intermedia between past censuses and those of 2019. These results confirm for the first time that R. rattus consumes and impacts this endemic and endangered lizard species. From a broader perspective, this is one of the first studies detecting rat impact on a large-sized reptile, which calls for further attention to the interaction between invasive rats and a highly vulnerable but essential component of island ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The invasive black rat Rattus rattus is a major threat to global biodiversity (Doherty et al. 2016; Bellard et al. 2017; Dueñas et al. 2021) and one of the most pernicious invaders worldwide (Lowe et al. 2000). On islands, they affect native and endemic flora (Pender et al. 2013; Harper and Bunbury 2015), prey on invertebrates (St Clair 2011), cause native vertebrates’ decline and extinction (Towns et al. 2006; Jones et al. 2008; Harris 2009), and eventually trigger cascading ecological effects (Harper and Bunbury 2015; Thoresen et al. 2017). However, experimental evidence on R. rattus impacts is still scarce and often relies on its trophic habit studies (Banks and Hughes 2012). That is why R. rattus impact upon insular reptiles has been generally assumed to be irrelevant, since its diet is mainly based upon plant material, insects, arachnids, and birds (Banks and Hughes 2012; Shiels et al. 2014), but scarcely on reptiles (Shiels et al. 2014). Nevertheless, it is relevant to highlight that R. rattus has been linked to reptile population declines in island ecosystems worldwide (Towns et al. 2006; Smith et al. 2012; Harper and Bunbury 2015), thus understanding the potential impacts of R. rattus is needed to properly plan reptile conservation.
More than one-fifth of all reptile species worldwide are threatened by extinction (Cox et al. 2022), with oceanic islands as hotspots of threatened species (Böhm et al. 2013). Invasive mammals have highly contributed to this situation (Böhm et al. 2013; Doherty et al. 2016; Cox et al. 2022), threatening nearly 20% of all reptiles (Bellard et al. 2017). Island reptile conservation is a priority as they have a key ecological role on island ecosystems—e.g., they sustain native plant pollination and seed dispersal interactions (Olesen and Valido 2003) and are cornerstone in trophic networks (e.g., Schoener and Spiller 2010; Piovia-Scott et al. 2019)—and impacts upon them can have other profound effects. For conservation planning purposes, unraveling menaces to island reptiles is essential to define management actions for both reptiles and invasive species. However, this is not a trivial task; threatened reptiles on islands can be relegated to remote areas with difficult access, often with food shortages, where other invasive species can mask the particular impact of R. rattus.
The Canarian spotted lizard Gallotia intermedia is a large-sized, critically endangered and endemic reptile of Tenerife (Canary Islands) (Mateo-Miras et al. 2009; BOC 2017), that reaches 15 cm of snout-vent length and over 40 cm of total length (Lopez-Darias et al. 2015; Padilla et al. 2019). Listed among the most endangered reptiles of Europe (Cox and Temple 2009), G. intermedia was believed to be extinct until its rediscovery in 1996 (Hernández et al. 2000)— although its distribution was seemingly larger in the past (Rando 2002; BOC 2017; Padilla et al. 2021). The species currently persists only in small and isolated populations (< 500 individuals each) dispersed in two almost inaccessible sea cliffs of the island (Padilla et al. 2019, 2021). Ecological information on the species is mostly lacking, but considering fruits compose over 90% of G. intermedia female diet (Lopez-Darias et al. 2015), it is very likely that it participates in native seed dispersal dynamics similar to other Gallotia species (Pérez-Méndez et al. 2016, 2017). Additionally, as it also consumes invertebrates, it presumably will have an important role in trophic networks, as it has been observed for other giant Gallotia species (Piquet 2022). In 2017, the Canarian Government approved the G. intermedia Recovery Plan, designating six critical areas of conservation and listing a series of priority conservation tasks. Among these tasks, the understanding of the potential effects of rats on G. intermedia population is a priority, as they have been suggested to prey upon eggs and juveniles of endemic lizard species of the archipelago (Rando and López 2001; Traveset et al. 2009; Padilla et al. 2019, 2021). However, no empirical study has yet confirmed this predatory-prey interaction for G. intermedia. To fill this gap, we quantified the occurrence of G. intermedia in R. rattus feces using genetic techniques as a proxy for R. rattus consumption (one of the main objectives of G. intermedia Recovery Plan; BOC 2017) and estimated rat abundance and density in the cliff area where the species is distributed. We then crosschecked this information with the most recent estimates of G. intermedia abundances (Padilla et al. 2019) to further explore the threat posed by R. rattus upon this endangered lizard. Our results will directly contribute to design conservation actions for G. intermedia, while providing further insights on the effect of invasive rats on island reptiles, an impact that remains largely unexplored worldwide.
Methods
Study area and R. rattus trapping sessions
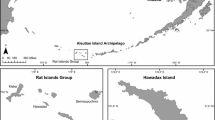
We designated seven study sites on the two sea cliff areas of Tenerife (Canary Islands, Spain) where G. intermedia is present: Teno Rural Park and Natural Monument of Guaza Mountain (Fig. 1) (hereafter Teno and Guaza, respectively). These cliffs are characterized by sparse vegetation from native semi-arid and arid plant communities interspersed with invasive plants (BOC 2017), and present annual mean temperatures of 17.5–20 °C and less than 400 mm rainfall per year (AEMET 2024). To optimize resources and given that most of the sites are only accessible by boat and using climbing techniques, we selected those sites where lizards were most abundant following Padilla et al. (2019), ruling out those excessively difficult to access (Fig. 1).
Location of the Canary Islands, the study areas, and sampling sites, showing Rattus rattus density (black circle) and Gallotia intermedia frequency of occurrence (FO) in rats’ diet. FO was calculated as the number of fecal samples in which the species was detected divided by the total number of fecal samples (FOexc; panel A) and the number of rats that consumed G. intermedia divided by the total number of rats captured (FOind; panel B) in each sampling site. Protected natural areas are shown in each panel as dark grey areas. Map created using QGIS 3.36
We trapped R. rattus to collect their feces and calculate their density in all sites, by performing three trapping sessions in each site in 2020 (13th July to 3rd August, 31st August to 29th September, and 26th October to 9th November), coinciding with lizards’ active period (Rando and Valido 2000; Padilla et al. 2019). We established all trapping sessions on days with favorable weather conditions. Each session consisted of trapping during three consecutive nights, for nine nights per site, using 37 traps distributed among sites (14.89 ± 3.88 traps per site and night). The total number of traps was set to efficiently cover key sites of the species distribution, ensuring manageability given the available human resources and logistics needed to sample these areas. We initially worked with 20 Tomahawk traps (40.64 × 12.7 × 12.7 cm) (Tomahawk Live Trap Co., Tomahawk, Wisconsin, USA), 10 FIXMAN traps (25 × 9 × 9 cm) (FIXMAN Ltd., Bishop’s Stortford, UK), and 7 Sherman folding traps (30.5 × 9.5 × 7.5 cm) (H.B. Sherman Traps Inc., Tallahassee, FL, USA). However, since FIXMAN traps produced tail autotomy in two of the first G. intermedia bycatches, we replaced these with other 10 Tomahawk from the first session onwards. Once installed, we georeferenced all traps, which stayed in the same location for the whole session, and baited them with a mixture of flour and canned sardines, previously shown as attractive for Rattus spp. (authors own data). To minimize non-target and maximize R. rattus captures, we activated traps only during night (activating them at dusk and de-activated them at dawn). We always used latex gloves to handle traps and baits to avoid leaving any human odor that could deter rat captures.
We checked all traps at dawn and noted down those that were closed, empty, or captured R. rattus or non-target species (these latter were immediately released). We transferred captured R. rattus to a handling bag (Koprowski 2002), retrieved fresh fecal pellets from the trap using sterile tweezers to increase subsequent DNA detection and minimize contamination (Ando et al. 2020), and dropped samples in tubes with 100% ethanol, labelled with a specific code for each individual (we never simultaneously captured two individuals in a single trap). We preserved all samples at -20 ºC once in the lab until later analysis. We identified all captured R. rattus using ear-tags (#1005 Size 1 Monel, National Band and Tag Co., Newport, Kentucky, USA) or ear punching (Chen et al. 2016), determined their sex and age (adult vs. juvenile), and released them in the exact same location within a few minutes of handling. We also noted down all individuals that escaped from the trap or during handling and also collected and individually identified any fecal material dropped by them in the trap. We thoroughly cleaned all traps after each capture.
All necessary authorizations to perform this study were available from the competent administration (Cabildo de Tenerife no. AFF 87/20).
Occurrence of G. intermedia in R. rattus feces
After air-drying and homogenizing each fecal sample with a sterile scalpel, we retained 200 mg to extract DNA using the E.Z.N.A. Stool DNA Kit (Omega Bio-tek, Inc. Norcross, Georgia, USA), following manufacturer instructions. We quantified the amount of DNA using NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). To detect the occurrence of G. intermedia in each fecal sample, we amplified a 97 bp-sequence of the mitochondrial 12 S rRNA using the primers L1064 5´-TTGACCACACGAAAGCTTAGA-3´ (Maca-Meyer et al. 2003) and Int12SR2 5´-CTGGCGGAAAGTATTGTATTCA-3´ (M. Hernández, in litt.), this latter previously designed to differentiate G. intermedia from the sympatric species G. galloti. We performed DNA amplification using 20 µL PCR mixes that contained 4 µL of MyTaq 5X tampon (Bioline, London, UK), 0.15 µL of MyTaq DNA polymerase (Bioline), 0.8 µM of each primer, 0.8 µg/µl of BSA (New England Biolabs), and 10–20 ng of DNA. After optimizing the PCR protocol using small pieces of tails and fecal samples from G. intermedia and G. galloti, PCR conditions consisted of an initial step at 95 ºC for 1 min followed by 35 cycles of denaturation for 15 s at 95 ºC, annealing for 15 s at 54 ºC, and elongation for 10 s at 72 ºC, with a final extension step at 72 ºC for 5 min. We carried out all reactions in a BioRad T-100 thermocycler (Bio Rad Laboratories, Hercules, California, USA), and included in each of them a positive and a negative control. To minimize contamination, we performed the entire protocol under a laminar-flow bench and using filtered pipette tips. We visualized PCR products in 1.7% agarose gel stained with RealSafe (Real Biotech Corporation, Taiwan), using a 25–700 bp DNA marker (LADD-DN1-500), and subsequently purified and sequenced the sample at the University of La Laguna Genomic Service (SEGAI). We compared DNA sequences with those available in GenBank for G. intermedia, which we extracted using BLAST + 2.10.1. (Camacho et al. 2009), and aligned using Clustal W in MEGA X (Kumar et al. 2018). We introduced minor corrections by hand and calculated the frequency of occurrence of G. intermedia as a quotient between the number of feces where G. intermedia was detected and the number of rat feces collected (hereafter FOexc), following previous studies on rat diet (e.g., Caut et al. 2008; Zarzoso-Lacoste et al. 2016). Additionally, to provide a better description of rat impacts and identify the proportion of individuals contributing to them, we calculated the proportion of R. rattus individuals consuming G. intermedia from the total of captured rats (including all dead and escaped ones) (hereafter FOind).
Rattus rattus abundance and density
Following previous research (Fukasawa et al. 2013; Maggs et al. 2015), we calculated R. rattus captures per unit of effort (CPUE) as a proxy of the species abundance in each site using the total number of rats captured divided by the number of active traps per night—i.e., after removing sprung traps and those with by-catches. We calculated CPUE by pooling the data of all traps, as we set a similar number of traps of each type every trapping night in all sites (data not shown). Additionally, we estimated R. rattus density by analyzing capture data and trap location with spatially explicit capture-recapture models performed in secr package (Efford 2023). We divided the capture dataset into seven sessions (one per site) and nine occasions (one per night), and set the detector argument to ‘single’. We determined the most appropriate buffer width using ‘esa.plot’ function and retained its value for subsequent analyses. We modeled density using a half normal detection function, assuming that detection occurred over a constant spatial scale (s) (Piquet and López-Darias 2021), estimating detection probability (g0) according to trap type and maximizing the conditional likelihood. We used Akaike Information Criterion (AIC) and AIC corrected for small sample sizes (Burnham and Anderson 2002) to evaluate the better fit of an alternative model testing for the occurrence of learned responses affecting g0, without finding any support for it (data not shown). We retrieved R. rattus density in each sampling site as Horvitz-Thompson-like estimate following Borchers and Efford (2008) and compared it between Teno and Guaza using Kruskal-Wallis tests. Finally, we evaluated the correlation of rat density with R. rattus abundance, and G. intermedia FOexc and FOind in each site using Spearman rank correlation tests, which are robust to violations of bivariate normality assumptions, non-linear relationships between paired variables and low sample sizes (Bonett and Wright 2000; Quinn and Keough 2002).
Rattus rattus impact on G. Intermedia populations
We performed Spearman rank correlation tests to analyze the relationship of both FOexc and rat density with the following demographic values in each site, extracted from the last available G. intermedia census (Padilla et al. 2019) (see Table 1 for values): (1) the total number of G. intermedia individuals, (2) G. intermedia density, (3) the number of non-reproductive and reproductive G. intermedia—i.e., juvenile and average-sized individuals, and sub-adults and adults in Padilla et al. (2019), respectively— (4) and the lizard population trend in each site, calculated as:
where N and P are the number of G. intermedia detected in each site in the present and the past, respectively.
We performed all analyses using R 4.3.3. (R Core Team 2024) (R code file available under https://doi.org/10.6084/m9.figshare.20455530.v1). Results correspond to mean ± SD, unless stated otherwise.
Results
Captures and sample collection
We captured R. rattus on 123 occasions and collected 127 feces—three belonging to animals that entered the traps but were not captured and another one collected in the vicinity of a trap. We trapped 55 different individuals on all these occasions, three of which deceased during handling and were marked and other three escaped unmarked (Table 2). Therefore, from all trapping occasions, 120 captures corresponded to marked individuals (97.56% of all captures). We captured R. rattus on 46, 45, and 29 occasions in the first, second, and third trapping sessions, respectively. From these, we captured adult females, adult males and juveniles on 49, 43, and 28 occasions, respectively. We recaptured marked individuals an average of 2.31 ± 1.58 times, up to a maximum of 7 recaptures for a single individual (Table 2). Recaptures occurred within the same site where rats were originally captured. Non-target captures included one house mouse Mus musculus domesticus and 11 G. intermedia.
Occurrence of G. intermedia in rodent feces
We detected G. intermedia in 19 fecal samples (14.96% of all samples), belonging to 15 different individual rats (27.27% of captured individuals). All detections had 100% similarity with sequences from GenBank for G. intermedia (AY154913, AY151923) and with one individual of G. simonyi (Z79499). The sequence of 48 bp found between primers allowed us to differentiate between G. intermedia and the sympatric G. galloti lizard. Both juvenile and adult R. rattus consumed G. intermedia (seven and nine detections in female and male feces, respectively, and three in juveniles). The number of detections per site and individuals respectively varied from 1 to 6 and 1 to 3 for the total number of positive feces (FOexc: 5.26 − 42.86% [min-max]; FOind: 11.11 – 75.00% [min-max]) (Table 2). Rattus rattus consumed G. intermedia all throughout the study season, with seven detections in feces during the first session and six during the second and third sessions, respectively (six, five, and six individuals, respectively). We confirmed that R. rattus consumed G. intermedia both in Teno (11 positive feces and 10 individuals) and Guaza (8 positive feces and 5 individuals) (Table 2).
Rattus rattus abundance and density
Rattus rattus CPUE ranged from 0.04 ± 0.08 to 0.16 ± 0.18 captures/trap-night (Table 2), with an average of 0.10 ± 0.14 captures/trap-night for all sites and trapping nights together. Average density was 1.54 ± 0.53 rats/ha, with sites located in Teno and Guaza showing similar values (\(\chi{_{\text{1}}^{\text{2}}}\)= 3.75, P = 0.053) (Table 2)—the highest value was found in Andén de los Ñames (Guaza) (2.18 rats/ha; Table 2). Relative standard errors in all cases exceeded 0.50 (Table 2). We found no significant correlation between R. rattus density and rat abundance, lizard FOexc nor lizard FOind (P > 0.05 in all cases).
Rattus rattus impact on G. Intermedia populations
We found that rat density was negatively correlated to the lizard population trend (rS = -0.96; P = 0.003). No other demographic parameter was correlated to rat density nor FOexc (P > 0.05 in all cases).
Discussion
Our results constitute a clear indication of R. rattus consuming the critically endangered G. intermedia. Although invasive rats have been suggested as predators of the Canary Islands endemic lizards (Traveset et al. 2009; Padilla et al. 2019), this is the first empirical evidence confirming this predatory interaction. In light of these results and taking into account the ubiquity of R. rattus in the archipelago (Nogales et al. 2006) and its broad feeding spectrum (Shiels et al. 2014; Harper and Bunbury 2015; Pomeda-Gutiérrez et al. 2021), the consumption of other endemic lizards within the Gallotia genus, including other highly endangered ones (Cox and Temple 2009), seems highly plausible. The critical status of some endemic lizards of the Canary Islands has been frequently attributed to the impact of feral cats (García-Márquez et al. 1997; Nogales et al. 2006; Medina and Nogales 2009); consequently, management efforts within lizards’ distribution areas typically target this invasive species (e.g., Rando and López 2001; Rando 2005). However, our results indicate that R. rattus may have also contributed to the demise of these lizards, and might currently represent an additional obstacle to their recovery. Implementing control actions without considering species interactions can lead to unforeseen and highly detrimental consequences (Rayner et al. 2007; Bergstrom et al. 2009; Ringler et al. 2015). For instance, the initial eradication of cats in Little Barrier Island led to a surge in predation pressure by Pacific rats (R. exulans) and the decline in endemic bird breeding success (Rayner et al. 2007). In the particular case of G. intermedia, and given its critical conservation status (BOC 2017), we suggest promptly undertaking conservation actions to control both feral cats and R. rattus simultaneously. From a broader perspective, this research represents one of the few empirical examples worldwide of R. rattus potentially impacting a large-sized, endemic reptile (Towns et al. 2006; Hayes et al. 2012; Harper and Bunbury 2015), thus calling for further attention to these vulnerable but essential components of island ecosystems.
We detected a high consumption rate of G. intermedia by R. rattus, as the endemic lizard occurred in 14.96% of all samples collected and in all seasons, and in 27.27% of all individual rats captured. Black rats are usually reported to mainly feed on plants and invertebrates, whereas lizard consumption is often low or even completely lacking (Caut et al. 2008; Shiels et al. 2014; Riofrío-Lazo and Páez-Rosas 2015; Pomeda-Gutiérrez et al. 2021). Caut et al. (2008), however, reported the consumption of small-sized Strand litter skink Caledoniscincus haplorhinus, which appeared seasonally in 13% of all gut samples on Surprise Island, a lower FO than that observed in Tenerife for G. intermedia. However, R. rattus diet studies have been mainly performed through gut or intestinal analysis and stable isotopes (Riofrío-Lazo and Páez-Rosas 2015; Clapperton et al. 2019; Nascimento et al. 2019), so a better understanding of reptile consumption might be reached by using genetic techniques, as they increase the likelihood of prey detection compared to other methods (Farrell et al. 2000; Egeter et al. 2015; Zarzoso-Lacoste et al. 2016; Monterroso et al. 2019). Although the use of these techniques has allowed us to confirm G. intermedia consumption, a real quantification of this interaction is difficult as environmental, biological, and methodological factors can affect DNA quantity or quality in samples (Monterroso et al. 2019)—e.g., prey material and DNA can also degrade within hours, preventing accurate prey detection in fecal samples (Egeter et al. 2015). Our estimates do not allow to precisely extrapolate the number of G. intermedia individuals actually consumed, as occurrence could inform about the consumption of one or multiple individuals by the same or different rats (Egeter et al. 2015; Zarzoso-Lacoste et al. 2016). The next steps of this research should try to elucidate whether the detected consumption is reflecting direct predation or could be due to a potential scavenging trophic behavior of R. rattus upon lizard carrion or autotomized tails. However, in the context of these results and the critical conservation status of G. intermedia (BOC 2017), the precautionary principle (IUCN 2018) dictates that R. rattus must be treated as an additional threat to this endemic lizard.
The population density of R. rattus in areas occupied by G. intermedia, reaching 1.54 rats/ha (ranging 0.74–2.18 rats/ha), is lower than that observed in more productive ecosystems like tropical islands (Uchida 1966; Shiels et al. 2014; Harper and Bunbury 2015; Russell et al. 2018) but comparable to, or even exceeds, that found in other areas of low productivity (Clark 1980; Shenbrot et al. 2010; Harper et al. 2011). These low estimates are consistent with what is expected (Clark 1980; Russell et al. 2011; Harper and Bunbury 2015; Carpenter et al. 2022) for an area receiving limited rainfall (AEMET 2024), presenting sparse vegetation, and hosting populations of rat predators (feral cats F. catus) and competitors (M. m. domesticus) (Rando and Valido 2000; BOC 2017; Padilla et al. 2019; Flores Ravelo and Rando Reyes 2021). However, our estimates should be interpreted as the lowest density attained by R. rattus in these areas, as this parameter may substantially increase with greater resource availability after seasonal rains (Clark 1980; Harper et al. 2015; Goedert et al. 2020; Carpenter et al. 2022). Rattus rattus persisting or even thriving in these areas points to the existence of alternative food resources, like invertebrates and seabird chicks and eggs, both of which are commonly consumed elsewhere (Jones et al. 2008; St Clair 2011) (we detected some algae in some R. rattus feces). The high G. intermedia consumption reported here also suggests this species could contribute to sustaining rat populations, although the fact that lizard consumption is unrelated to rat density could be indicative of G. intermedia not being the primary food source. On the other hand, since our density estimates had a degree of uncertainty exceeding the threshold proposed by Efford (2011), these should be interpreted as a proxy of R. rattus abundance—a cost-efficient and useful alternative to precise density calculations (Stephens et al. 2015) that would be extremely labor-intensive, prohibitive, and even dangerous given our study system. Large density uncertainty could explain the lack of relationship between rat density and CPUE, although this finding could also be a consequence of spatially-explicit density estimates considering trap spacing and rat movements (Efford 2004) or variable capture probability affecting CPUE indices (Wiewel et al. 2009). Sites with higher rat density tended however to present greater rat abundance, so that a positive relationship between both parameters may arise under larger sample sizes. Overall, this study provides the first estimation of rat density in Tenerife and the Canary Islands, although rat abundances have already been reported in other habitats of the island (Hernández Martín 1997; Godoy Betancor 2000). More importantly, our results provide baseline estimates of rat abundance and density in areas occupied by G. intermedia, which are essential to managing the impact of invasive species (IUCN 2018).
We found a correlation between the density of R. rattus and the decline of G. intermedia populations, suggesting the existence of complex rat-lizard interactions in areas occupied by the endangered lizard. Our results could be explained by direct R. rattus predation (expected under the high FO detected) or resource competition between the invasive species and G. intermedia, as both species are mainly omnivorous (Rando 2002; Harper and Bunbury 2015; Pomeda-Gutiérrez et al. 2021). Alternatively, the impact here detected could derive from R. rattus subsidizing cat populations in the area (Rando and López 2001; Padilla et al. 2019), which would increase predation pressure on the endangered reptile (Courchamp et al. 2000, 2003). This situation could be aggravated by the presence of the congener G. galloti (found in rat feces; data not shown) and the invasive M. musculus, both of which may provide accessible and abundant food resources for F. catus and R. rattus. Indirect interactions involving cats could be particularly relevant considering that G. intermedia occurs in up to 71% of cat feces in certain locations of Teno (Rando and López 2001) and its consumption in Montaña de Guaza has increased in the past years (Flores Ravelo and Rando Reyes 2021 and references therein). In spite of that, G. intermedia FO in rats’ diet exceeds that of cats in most sites (see Rando and López 2001; Flores Ravelo and Rando Reyes 2021), while the only locations holding cat populations in Teno—i.e., La Galera (Rando and López 2001), Punta de Diente de Ajo and Andén de la Amargosa (Padilla et al. 2019)—are those with the highest population recovery of G. intermedia compared to past censuses. Although lizard decline is more pronounced in Montaña de Guaza, where cats are also present (authors’ own data), this area also holds the highest rat density according to our results. Trophic relationships are governed by complex predator-prey cycles (Roemer et al. 2002; Ringler et al. 2015; Cerri et al. 2017), thus further research is needed to fully uncover R. rattus impact and understand how trophic relationships are affecting G. intermedia. However, this research allows considering R. rattus as a potentially serious threat to G. intermedia, whose role in the demise of the endemic lizard may have been masked by multispecies interactions.
Overall, the results presented in this study constitute a call for further attention on R. rattus as a highly detrimental invader potentially impacting endangered lizards on islands worldwide, and represent the first step in unveiling the impact of an interaction long assumed but never proven and whose consequences could be grave for G. intermedia.
Data availability
R code file available under https://doi.org/10.6084/m9.figshare.20455530.v1.
References
AEMET (2024) Standard climate values. In: AEMET. http://www.aemet.es/en/serviciosclimaticos/datosclimatologicos/valoresclimatologicos?k=mur#tab2. Accessed 22 Jan 2024
Ando H, Mukai H, Komura T et al (2020) Methodological trends and perspectives of animal dietary studies by noninvasive fecal DNA metabarcoding. Environ DNA 2:391–406. https://doi.org/10.1002/edn3.117
Banks PB, Hughes NK (2012) A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildl Res 39:78–88. https://doi.org/10.1071/WR11086
Bellard C, Rysman J-F, Leroy B et al (2017) A global picture of biological invasion threat on islands. Nat Ecol Evol 1:1862–1869. https://doi.org/10.1038/s41559-017-0365-6
Bergstrom DM, Lucieer A, Kiefer K et al (2009) Indirect effects of invasive species removal devastate World Heritage Island. J Appl Ecol 46:73–81. https://doi.org/10.1111/j.1365-2664.2008.01601.x
BOC (2017) Decreto 230/2017, de 20 de noviembre, por el que se aprueba de Plan de Recuperación del Lagarto Gigante de Tenerife (Gallotia intermedia)
Böhm M, Collen B, Baillie JEM et al (2013) The conservation status of the world’s reptiles. Biol Conserv 157:372–385. https://doi.org/10.1016/j.biocon.2012.07.015
Bonett DG, Wright TA (2000) Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika 65:23–28. https://doi.org/10.1007/BF02294183
Borchers DL, Efford MG (2008) Spatially explicit maximum likelihood methods for capture-recapture studies. Biometrics 64:377–385. https://doi.org/10.1111/j.1541-0420.2007.00927.x
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-, New York, USA
Camacho C, Coulouris G, Avagyan V et al (2009) BLAST+: architecture and applications. BMC Bioinformatics 10:421. https://doi.org/10.1186/1471-2105-10-421
Carpenter JK, Monks A, Innes J, Griffiths J (2022) Pushing the limits: ship rat (Rattus rattus) population dynamics across an elevational gradient in response to mast seeding and supplementary feeding. Biol Invasions 24:3065–3081. https://doi.org/10.1007/s10530-022-02829-z
Caut S, Angulo E, Courchamp F (2008) Dietary shift of an invasive predator: rats, seabirds and sea turtles. J Appl Ecol 45:428–437. https://doi.org/10.1111/j.1365-2664.2007.01438.x
Cerri J, Ferretti M, Bertolino S (2017) Rabbits killing hares: an invasive mammal modifies native predator–prey dynamics. Anim Conserv 20:511–519. https://doi.org/10.1111/acv.12343
Chen M, Kan L, Ledford BT, He J-Q (2016) Tattooing various combinations of ears, tail, and toes to identify mice reliably and permanently. J Am Assoc Lab Anim Sci 55:189–198
Clapperton BK, Maddigan F, Chinn W, Murphy EC (2019) Diet, population structure and breeding of Rattus rattus L. In South Island beech forest. N Z J Ecol 43:3370. https://doi.org/10.20417/nzjecol.43.22
Clark DB (1980) Population ecology of Rattus rattus across a desert-montane forest gradient in the Galápagos Islands. Ecology 61:1422–1433
Courchamp F, Langlais M, Sugihara G (2000) Rabbits killing birds: modelling the hyperpredation process. J Anim Ecol 69:154–164. https://doi.org/10.1046/j.1365-2656.2000.00383.x
Courchamp F, Chapuis J-L, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383. https://doi.org/10.1017/S1464793102006061
Cox NA, Temple HJ (2009) European red list of reptiles. Office for Official Publications of the European Communities, Luxembourg
Cox N, Young BE, Bowles P et al (2022) A global reptile assessment highlights shared conservation needs of tetrapods. Nature 605:285–290. https://doi.org/10.1038/s41586-022-04664-7
Doherty TS, Glen AS, Nimmo DG et al (2016) Invasive predators and global biodiversity loss. Proc Natl Acad Sci U S A 113:11261–11265. https://doi.org/10.1073/pnas.1602480113
Dueñas M-A, Hemming DJ, Roberts A, Diaz-Soltero H (2021) The threat of invasive species to IUCN-listed critically endangered species: a systematic review. Glob Ecol Conserv 26:e01476. https://doi.org/10.1016/j.gecco.2021.e01476
Efford M (2004) Density estimation in live-trapping studies. Oikos 106:598–610. https://doi.org/10.1111/j.0030-1299.2004.13043.x
Efford MG (2011) Estimation of population density by spatially explicit capture-recapture analysis of data from area searches. Ecology 92:2202–2207. https://doi.org/10.1890/11-0332.1
Efford MG (2023) secr: spatially explicit capture-recapture models. R package version 4.6.4
Efford MG, Fewster RM (2013) Estimating population size by spatially explicit capture-recapture. Oikos 122:918–928. https://doi.org/10.1111/j.1600-0706.2012.20440.x
Egeter B, Bishop PJ, Robertson BC (2015) Detecting frogs as prey in the diets of introduced mammals: a comparison between morphological and DNA-based diet analyses. Mol Ecol Resour 15:306–316. https://doi.org/10.1111/1755-0998.12309
Farrell LE, Roman J, Sunquist ME (2000) Dietary separation of sympatric carnivores identified by molecular analysis of scats. Mol Ecol 9:1583–1590. https://doi.org/10.1046/j.1365-294X.2000.01037.x
Flores Ravelo AJ, Rando Reyes JC (2021) Trophic ecology of cats (Felis catus) in Montaña de Guaza: implications for the conservation of the critically endangered giant lizard of Tenerife (Gallotia intermedia). Sci Insul Rev Ciencias Nat en islas 4:63–80. https://doi.org/10.25145/j.si.2021.04.04
Fukasawa K, Miyashita T, Hashimoto T et al (2013) Differential population responses of native and alien rodents to an invasive predator, habitat alteration and plant masting. Proc R Soc B Biol Sci 280:20132075. https://doi.org/10.1098/rspb.2013.2075
García-Márquez M, López-Jurado LF, Mateo JA (1997) Predación de Gallotia simonyi por gatos cimarrones. Boletín Asoc Herpetológica Española 8:20–23
Godoy Betancor D (2000) Estudio de la dinámica de la depredación de semillas en el monteverde de Anaga (Tenerife). Thesis from the University of La Laguna
Goedert J, Cochard D, Lenoble A et al (2020) Seasonal demography of different black rat (Rattus rattus) populations under contrasting natural habitats in Guadeloupe (Lesser antilles, Caribbean). Mammal Res 65:793–804. https://doi.org/10.1007/s13364-020-00523-w
Harper GA, Bunbury N (2015) Invasive rats on tropical islands: their population biology and impacts on native species. Glob Ecol Conserv 3:607–627. https://doi.org/10.1016/j.gecco.2015.02.010
Harper GA, Zabala J, Carrion V (2011) Monitoring of a population of Galápagos land iguanas (Conolophus subcristatus) during a rat eradication using brodifacoum. In: Veitch CR, Clout MN, Towns DR (eds) Island invasives: eradication and management. IUCN, Gland, Switzerland, pp 309–312
Harper GA, van Dinther M, Russell JC, Bunbury N (2015) The response of black rats (Rattus rattus) to evergreen and seasonally arid habitats: informing eradication planning on a tropical island. Biol Conserv 185:66–74. https://doi.org/10.1016/j.biocon.2014.11.044
Harris DB (2009) Review of negative effects of introduced rodents on small mammals on islands. Biol Invasions 11:1611–1630. https://doi.org/10.1007/s10530-008-9393-0
Hayes WK, Iverson JB, Knapp CR, Carter RL (2012) Do invasive rodents impact endangered insular iguana populations? Biodivers Conserv 21:1893–1899. https://doi.org/10.1007/s10531-012-0276-4
Hernández E, Nogales M, Martín A (2000) Discovery of a new lizard in the Canary Islands, with a multivariate analysis of Gallotia (Reptilia: Lacertidae). Herpetologica 56:63–76
Hernández Martín MÁ (1997) Éxito reproductor y efecto de la depredación sobre los nidos de las palomas endémicas de la laurisilva canaria, turqué (Columba bollii) y rabiche (Columba junoniae), en la isla de Tenerife. Thesis from the University of La Laguna
IUCN (2018) Guidelines for invasive species planning and management on islands. IUCN, Cambridge, United Kingdom and Gland, Switzerland
Jones HP, Tershy BR, Zavaleta ES et al (2008) Severity of the effects of invasive rats on seabirds: a global review. Conserv Biol 22:16–26. https://doi.org/10.1111/j.1523-1739.2007.00859.x
Koprowski JL (2002) Handling tree squirrels with a safe and efficient restraint. Wildl Soc Bull 30:101–103
Kumar S, Stecher G, Li M et al (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lopez-Darias M, Vanhooydonck B, Cornette R, Herrel A (2015) Sex-specific differences in ecomorphological relationships in lizards of the genus Gallotia. Funct Ecol 29:506–514. https://doi.org/10.1111/1365-2435.12353
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. The invasive species specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the. World Conservation Union (IUCN), Auckland, New Zealand
Maca-Meyer N, Carranza S, Rando JC et al (2003) Status and relationships of the extinct giant Canary Island lizard Gallotia goliath (Reptilia: Lacertidae), assessed using ancient mtDNA from its mummified remains. Biol J Linn Soc 80:659–670. https://doi.org/10.1111/j.1095-8312.2003.00265.x
Maggs G, Nicoll M, Zuël N et al (2015) Rattus management is essential for population persistence in a critically endangered passerine: combining small-scale field experiments and population modelling. Biol Conserv 191:274–281. https://doi.org/10.1016/j.biocon.2015.06.039
Mateo-Miras JA, Pérez-Mellado V, Martínez-Solano I (2009) Gallotia intermedia. The IUCN Red List of Threatened Species 2009. e.T61505A12494026. https://doi.org/https://dx.doi.org/10.2305/IUCN.UK.2009.RLTS.T61505A12494026.en
Medina FM, Nogales M (2009) A review on the impacts of feral cats (Felis silvestris catus) in the Canary Islands: implications for the conservation of its endangered fauna. Biodivers Conserv 18:829–846. https://doi.org/10.1007/s10531-008-9503-4
Monterroso P, Godinho R, Oliveira T et al (2019) Feeding ecological knowledge: the underutilised power of faecal DNA approaches for carnivore diet analysis. Mamm Rev 49:97–112. https://doi.org/10.1111/mam.12144
Nascimento T, Oliveira N, Fagundes AI et al (2019) Diet selection of introduced black rats Rattus rattus L. in relation to plant availability on Berlenga Island, Portugal. Ecol Mediterr 45:15–29. https://doi.org/10.3406/ecmed.2019.2072
Nogales M, Rodríguez-Luengo JL, Marrero P (2006) Ecological effects and distribution of invasive non-native mammals on the Canary Islands. Mamm Rev 36:49–65. https://doi.org/10.1111/j.1365-2907.2006.00077.x
Olesen JM, Valido A (2003) Lizards as pollinators and seed dispersers: an island phenomenon. Trends Ecol Evol 18:177–181. https://doi.org/10.1016/S0169-5347(03)00004-1
Padilla DP, Carmona JM, Martín Carbajal J (2019) Censo de las poblaciones de lagarto gigante de Tenerife (Gallotia intermedia). Cabildo de Tenerife and GESPLAN S.A., official report
Padilla DP, Martín-Carbajal J, Carmona JMM et al (2021) Sobrevivir en el abismo. El lagarto gigante de Tenerife: del olvido a la lucha por su conservación. InDiferente 23:58–75
Pender RJ, Shiels AB, Bialic-Murphy L, Mosher SM (2013) Large-scale rodent control reduces pre- and post-dispersal seed predation of the endangered hawaiian lobeliad, Cyanea superba subsp. superba (Campanulaceae). Biol Invasions 15:213–223. https://doi.org/10.1007/s10530-012-0280-3
Pérez-Méndez N, Jordano P, García C, Valido A (2016) The signatures of Anthropocene defaunation: cascading effects of the seed dispersal collapse. Sci Rep 6:24820. https://doi.org/10.1038/srep24820
Pérez-Méndez N, Jordano P, Valido A (2017) Persisting in defaunated landscapes: reduced plant population connectivity after seed dispersal collapse. J Ecol 106:936–947. https://doi.org/10.1111/1365-2745.12848
Piovia-Scott J, Yang LH, Wright AN et al (2019) Pulsed seaweed subsidies drive sequential shifts in the effects of lizard predators on island food webs. Ecol Lett 22:1850–1859. https://doi.org/10.1111/ele.13377
Piquet JC (2022) The perils of an invasive snake: the California kingsnake in the Canary Islands. PhD Thesis
Piquet JC, López-Darias M (2021) Invasive snake causes massive reduction of all endemic herpetofauna on Gran Canaria. Proceeding R Soc B Biol Sci 288:20211939. https://doi.org/10.1098/rspb.2021.1939
Pomeda-Gutiérrez F, Medina FM, Nogales M, Vargas P (2021) Diet of the black rat (Rattus rattus) in a Canary laurel forest: species identification based on morphological markers and DNA sequences. J Nat Hist 55:629–648. https://doi.org/10.1080/00222933.2021.1915400
Quinn GP, Keough MJ (2002) Experimental design and data anlysis for biologists. Cambridge University Press, Cambridge, United Kingdom
R Core Team (2024) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rando JC (2002) Gallotia intermedia Hernández 2000. Lagarto canario moteado, Caimán. In: Pleguezuelos JM, Márquez R, Lizana M (eds) Atlas y libro rojo de los anfibios y reptiles de España. Ministerio de Medio Ambiente, Dirección General de Conservación de la Naturaleza, Madrid, Spain, pp 204–206
Rando JC (2005) Actuaciones para el control de las colonias de gatos (Felis catus) del Barranco De Masca. Cabildo Insular de Tenerife and GESPLAN S.A. Unpublished Report
Rando JC, López M (2001) Acciones para la conservación del lagarto canario moteado (Gallotia intermedia). Viceconsejería de Medio Ambiente, Gobierno de Canarias, Official Report
Rando JC, Valido A (2000) Distribución, tamaño de población y propuesta de plan de recuperación para el lagarto canario moteado (Gallotia intermedia). Viceconsejería de Medio Ambiente, Gobierno de Canarias, Official Report
Rayner MJ, Hauber ME, Imber MJ et al (2007) Spatial heterogeneity of mesopredator release within an oceanic island system. Proc Natl Acad Sci U S A 104:20862–20865. https://doi.org/10.1073/pnas.0707414105
Ringler D, Russell JC, Le Corre M (2015) Trophic roles of black rats and seabird impacts on tropical islands: mesopredator release or hyperpredation? Biol Conserv 185:75–84. https://doi.org/10.1016/j.biocon.2014.12.014
Riofrío-Lazo M, Páez-Rosas D (2015) Feeding habits of introduced black rats, Rattus rattus, in nesting colonies of Galapagos petrel on San Cristóbal Island, Galapagos. PLoS ONE 10:e0127901. https://doi.org/10.1371/journal.pone.0127901
Roemer GW, Donlan CJ, Courchamp F (2002) Golden eagles, feral pigs, and insular carnivores: how exotic species turn native predators into prey. Proc Natl Acad Sci U S A 99:791–796. https://doi.org/10.1073/pnas.012422499
Russell JC, Ringler D, Trombini A, Le Corre M (2011) The island syndrome and population dynamics of introduced rats. Oecologia 167:667–676. https://doi.org/10.1007/s00442-011-2031-z
Russell JC, Abrahão CR, Silva JCR, Dias RA (2018) Management of cats and rodents on inhabited islands: an overview and case study of Fernando De Noronha, Brazil. Perspect Ecol Conserv 16:193–200. https://doi.org/10.1016/J.PECON.2018.10.005
Schoener TW, Spiller DA (2010) Trophic cascades on islands. In: Terborgh J, Estes JA (eds) Trophic cascades: predators, prey and the changing dynamics of nature. Island, Washington, DC, U.S.A., pp 179–202
Shenbrot G, Krasnov B, Burdelov S (2010) Long-term study of population dynamics and habitat selection of rodents in the Negev Desert. J Mammal 91:776–786. https://doi.org/10.1644/09-MAMM-S-162.1
Shiels AB, Pitt WC, Sugihara RT, Witmer GW (2014) Biology and impacts of pacific island invasive species. 11. Rattus rattus, the black rat (Rodentia: Muridae). Pac Sci 68:145–184. https://doi.org/10.2984/68.2.1
Smith MJ, Cogger H, Tiernan B et al (2012) An oceanic island reptile community under threat: the decline of reptiles on Christmas Island, Indian Ocean. Herpetol Conserv Biol 7:206–218
St Clair JJH (2011) The impacts of invasive rodents on island invertebrates. Biol Conserv 144:68–81. https://doi.org/10.1016/j.biocon.2010.10.006
Stephens PA, Pettorelli N, Barlow J et al (2015) Management by proxy? The use of indices in applied ecology. J Appl Ecol 52:1–6. https://doi.org/10.1111/1365-2664.12383
Thoresen JJ, Towns D, Leuzinger S et al (2017) Invasive rodents have multiple indirect effects on seabird island invertebrate food web structure. Ecol Appl 27:1190–1198. https://doi.org/10.1002/eap.1513
Towns DR, Atkinson IAE, Daugherty CH (2006) Have the harmful effects of introduced rats on islands been exaggerated ? Biol Invasions 8:863–891. https://doi.org/10.1007/s10530-005-0421-z
Traveset A, Nogales M, Alcover JA et al (2009) A review on the effects of alien rodents in the Balearic (Western Mediterranean Sea) and Canary Islands (Eastern Atlantic Ocean). Biol Invasions 11:1653–1670. https://doi.org/10.1007/s10530-008-9395-y
Uchida TA (1966) Observations on the monitor lizard, Varanus indicus (Daudin), as a rat-control agent on Ifaluk, Western Caroline Islands. Bull World Health Organ 35:976–980
Wiewel AS, Yackel Adams AA, Rodda GH (2009) Evaluating abundance estimate precision and the assumptions of a count-based index for small mammals. J Wildl Manage 73:761–771. https://doi.org/10.2193/2008-180
Zarzoso-Lacoste D, Bonnaud E, Corse E et al (2016) Improving morphological diet studies with molecular ecology: an application for invasive mammal predation on island birds. Biol Conserv 193:134–142. https://doi.org/10.1016/j.biocon.2015.11.018
Acknowledgements
We are deeply grateful to M. Hernández for generously providing us with the primers required for this research, and for his insightful guidance along this study. We are equally thankful to E. Expósito, A. Salas, and T. Acosta for sharing lab equipment and supplies during the COVID-19 pandemic. We also wish to thank M. Efford for assisting us with SECR analyses. CABILDO DE TENERIFE funded this study. MLD was funded under the Program TF INNOVA 2016-21 (with MEDI & FDCAN Funds).
Funding
CABILDO DE TENERIFE funded this study. MLD was funded under the Program TF INNOVA 2016-21 (with MEDI & FDCAN Funds).
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
MLD conceptualized the study, designed the methodology, was in charge of funding acquisition, resources and supervision. DPP and JMC performed fieldwork. MLG carried out all genetic procedures. MLD, MLG and JCP analyzed the data and wrote the original draft. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Indraneil Das.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López-Darias, M., López-González, M., Padilla, D.P. et al. Invasive black rats menacing endangered lizards. Biodivers Conserv 33, 2775–2789 (2024). https://doi.org/10.1007/s10531-024-02882-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-024-02882-1