Abstract
Monitoring of environmental impacts of mining activities typically focuses on the main operation footprint, neglecting exploration infrastructure like tracks, roads, and drill pads. These areas are cleared of native vegetation and impacts on the surrounding environment can be both cumulative and enigmatic. Here, we study the impacts of mining exploration infrastructure on habitat characteristics and ground-dwelling arthropod communities in the Midwest region of Western Australia. The study was conducted at three mine sites, each with three infrastructure types: maintained tracks, unmaintained tracks, and drill pads along transects extending 100 m away from the disturbance into remnant vegetation. Habitat characteristics were measured, and arthropods collected from pitfall traps along these transects and identified using COI metabarcoding. The overall arthropod community and two indicator groups, ants (Formicidae) and springtails (Collembola) - were used to measure arthropod responses to changes in response to habitat disturbance. Whilst changes in habitat were only visible to 10 m from the disturbance, impacts on arthropod communities could be detected up to 100 m into the remnant vegetation, and these responses were more complex. In general, we found similar patterns expressed in the compositional changes for arthropods overall and between our chosen indicator groups, but they were not the same across all sites and infrastructure types. Our results demonstrate the utility of bulk arthropod metabarcoding and different arthropod indicator groups for documenting the effects of fine-scale habitat destruction, degradation, or disturbance. They also highlight the need to monitor the negative impacts of mineral exploration on the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main drivers of species extinction across the globe are human activities that cause the destruction, transformation and fragmentation of landscapes (Ellis et al. 2010). Although mining operations only occupy 0.3% of land globally (Hooke et al. 2012), they can have more severe impacts on natural habitats than other visible and wide-ranging activities such as agriculture and urbanisation (Redondo-Vega et al. 2017). This is because mining activities often target geologically unique landscape formations surrounded by pristine and endemic ecosystems (Guijón et al. 2011; Redondo-Vega et al. 2017; Tibbett 2015).
The ecological impacts of habitat disturbance caused by anthropogenic activities including mining are complex and pervasive, affecting population dynamics and community structure of both flora and fauna (Bolger et al. 2000; Fahrig 2003). For example, fragmentation increases the extent of edges around habitats. These edges naturally occur between two habitats with varying light, humidity, and temperature levels, potentially impacting vegetation community structure and the species present (Ries and Sisk 2004; Santos et al. 2008). These biotic and abiotic changes between the contrasting ecotones are known as ‘edge effects’ (Ewers and Didham 2006; Murcia 1995). With the creation of more edges in anthropogenically-altered landscapes, the resulting changes in microclimate can also allow the invasion of weeds and other alien species, leading to changes in overall community structure, and potentially, the functionality of ecosystems (Goosem 2012; Pohlman et al. 2009). The extent to which the impacts of the edge effect can penetrate adjoining habitats can extend from a few metres to several hundreds of metres into remnant habitat (Goosem 2007; Laurance et al. 2009).
In the context of mining, habitat fragmentation and the resulting edge effects are further complicated by the associated mining exploration and linear infrastructure that is constructed. Mining exploration and linear infrastructure encompass haul roads, unpaved tracks, exploration (or seismic) lines, drill pads, power lines, pipelines, railways, and fence lines (Raiter et al. 2014). While the impacts of individual roads and tracks may only require a small area to be cleared, the overall impacts can be significant (Raiter et al. 2014). These impacts are known as ‘cumulative’ or ‘enigmatic impacts’ (Canter and Ross 2010; Raiter et al. 2017; Therivel and Ross 2007). As these impacts are rarely accounted for in impact assessment protocols and regulatory frameworks (McKenney and Kiesecker 2010), it is important to understand the extent of their impact on species and communities and the biophysical drivers behind them to improve how mining operations are managed (Cross et al. 2021).
Changes in biophysical habitat characteristics, such as vegetation cover and soil composition, can serve as indicators of edge effects and habitat quality (Bolger et al. 2000; Fahrig 2003), and this approach is often combined with surveys of ubiquitous organisms that are responsive to disturbance, such as arthropods (Hodkinson and Jackson 2005; Kwok et al. 2016; McGeoch 1998; Moir et al. 2005; Roy et al. 2003). Arthropods are widely targeted for monitoring in a mining context, for example, tracking restoration trajectories after mining activities (Casimiro et al. 2019; Fernandes et al. 2019; Majer and Nichols 1998; van der Heyde et al. 2022a; Heyde et al. 2022b). Traditionally, arthropod biomonitoring is undertaken by trapping, followed by morphological identification of selected taxa from impacted and reference areas (Yu et al. 2012). However, this methodology is resource-intensive, relying on considerable time and taxonomic expertise to identify large volumes of taxa, especially in biodiverse areas or over large spatial scales (Ji et al. 2013), meaning that only a few selected taxa can be assessed (Pik et al. 1999).
DNA metabarcoding is a useful molecular tool that can address some problems associated with traditional morphology-based monitoring techniques (Ji et al. 2013; Yu et al. 2012). With metabarcoding, it is possible to identify species across multiple taxonomic groups simultaneously and with a fraction of the person-hours of morphology-based techniques (Ji et al. 2013). Thus, DNA metabarcoding of invertebrates could be a beneficial addition to mine site monitoring programs and provide valuable insights into environmental impacts across the whole community of arthropods inhabiting these areas. In recent years, DNA metabarcoding has been used in various systems, including a mine site monitoring context, to help understand and document the impact of environmental changes on invertebrates (for example, using pitfall traps in Fernandes et al. (2019) and van der Heyde et al. (2022a), and using malaise traps in Lynggaard et al. (2020)).
In traditional biomonitoring studies, indicator taxa are often used instead of whole arthropod communities to measure the impacts of disturbance (Majer and Nichols 1998; Heino 2014; Kerr et al. 2000). Indicator taxa are focal groups of organisms with well-known taxonomy and ecology that can be used as representatives of overall changes in the ecosystem. The most common arthropod groups used as indicators in terrestrial ecosystems include beetles, ants, spiders, and butterflies (Oliver and Beattie 1996; Pik et al. 1999; Terlizzi et al. 2003). Ants (Formicidae) are favoured as an indicator taxon due to the consistency of their response to disturbance (Andersen 2019; Hoffmann and Andersen 2003). Another group, springtails (Collembola), has also been recommended as an indicator in Australian systems as they are highly sensitive to disturbance in habitat structure (Greenslade 2007). However, unlike more well-studied focal groups, such as ants, there is limited knowledge on springtail taxonomy, and many species are yet to be described (Jordana and Greenslade 2020). Further, focusing only on certain invertebrate taxa may not be reflective of whole ecosystem dynamics as some groups may not interact with each other spatially or have comparable taxonomic richness (Khosravi and Hemami 2019; Prendergast et al. 1993; van Jaarsveld et al. 1998), and thus they may not fully encapsulate the changes and impacts on the diversity of multiple taxonomic groups and total arthropod assemblages (Siddig et al. 2016; Silva et al. 2017). Therefore, a direct comparison of patterns detected by all arthropods present in samples, along with particular groups such as ants and springtails, is valuable for validating the use of indicator groups.
In this study, we used invertebrate DNA metabarcoding to explore the impacts of mining exploration infrastructure on ground-dwelling arthropod communities associated with banded ironstone formations (BIFs) in the Midwest region of Western Australia. Here we aimed to identify (1) the response of biophysical habitat characteristics and arthropod community diversity and composition to mining exploration disturbances, and (2) to identify whether ants and springtails could function as indicator groups by reflecting patterns in the overall arthropod community.
Materials and methods
Experimental design and study sites
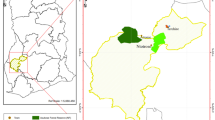
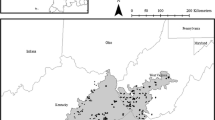
This study was conducted across three iron-ore mines: Koolanooka Hills (KO), Karara (KA), and Mount Gibson (MG) (Fig. 1A) within the Midwest region of Western Australia. The Midwest region is characterised by a dry-warm Mediterranean climate, with mild, wet winters and hot, dry summers, receiving an average rainfall of 300–400 mm, mostly occurring during the winter months (Meissner and Caruso 2008). The average summer and winter daily maximum temperatures are 36.3 °C and 19.1 °C, respectively (Meissner and Caruso 2008). Vegetation communities differed between the three sites, but all were Acacia-dominated shrublands. Vegetation communities in the KO site remnants exhibited considerable disturbance, mainly from feral goats grazing on the natural vegetation but the other two sites were less disturbed.
At each site, three exploration infrastructure types were chosen: maintained tracks (MT); unmaintained tracks (UT); and drill pads (Pad). MT was identified as a frequently used and disturbed track with no evidence of vegetation regrowth. By contrast, UT would display vegetation growth, typically along the centre of the track or in old tyre tracks, and it may have vehicular access blocked off. Pads were areas where land had been cleared of all vegetation to drill into the BIF from which iron-ore is extracted. These were small, cleared areas that, once disturbed, had been left relatively untouched, with many having some degree of vegetation regrowth on them. Disturbance age varied across all three sites. The age of the Pads at each site was estimated as follows: KA Pads were constructed after 2012; KO Pads were constructed between 2005 and 2012; and MG Pads were constructed before 2000 (Stephens 2018). The age of the UT and MT is unknown, although MT were still used at the sampling time.
Sampling was carried out along a transect from the interior of each chosen disturbance to around 100 m into the adjacent remnant shrublands along the natural contour of the terrain so as to avoid any potential effects of elevation. For each infrastructure type at each site, four transects were used. Sampling points were located along a transect at six different quadrat locations: (i) disturbance interior; (ii) disturbance adjacent to edge; (iii) edge; (iv) remnant adjacent to edge; (v) 10 m from the natural edge into remnant vegetation; and (vi) remnant interior (Fig. 1B). There were 72 sampling points per site and 216 in total (3 sites x 12 transects per site x 6 sample points per transect). Transects varied slightly in length. This was necessitated because exploration disturbance features vary in shape and size, so the interior of a disturbance and its edges are unique distances apart. Moreover, the edge of exploration land uses is not always clear-cut, so soil disturbance and movement were used as an indication of edge locations. Lastly, due to the fragmented landscape mosaic across these sites, the remnant interior sampling point was also at varying distances from the actual edge. For comparability, the criteria for these sampling points were as follows: at least 100 m away along the same contour from its paired edge sampling point and, if possible, at least 100 m away from any other disturbance edge (Swart et al. 2018).
Sample Collection
Samples were collected between the 30th of October and the 23rd of November 2018. Each sampling point along the transect comprised a 1 m2 quadrat. Within each quadrat, three pitfall traps with a diameter of 4 cm and a capacity of 120 mL, containing ~ 40 mL of ethylene glycol (undiluted commercial engine coolant, “Nulon Premium Long Life Coolant 100% Concentrate”) were dug into the ground, with the rim flush with the soil surface. The pitfall traps were left open for approximately nine days (minimum of eight, maximum of ten days), and once collected, the three pitfall traps were pooled into one sample.
Habitat characteristics
A selection of habitat characteristics was measured and recorded within each 1 m2 quadrat. Slope aspect was measured using a compass and slope angle (degrees) measured using a Suunto PM-5 clinometer. A modified Braun-Blanquet scale (1, < 1% cover; 2, 1–5%; 3, 5–10%; 4, 10–25%; 5, 25–50%; 6, 50–75%; 7, > 75%) was used to approximate total vegetation cover, bare ground cover, rock cover and litter cover within the quadrat. Soil depth (soil compaction) was measured at the centre of each quadrat using a soil probe. Rugosity was measured as the ratio between the length of the contoured surface and a 1 m chain along the 1 m horizontal and 1 m vertical transect, which crossed the centre of the quadrat. All fine woody debris (FWD; 0 < diameter < 0.5 cm) touching the 1 m horizontal transect within the quadrat was counted. All medium woody debris (MWD; 0.5 ≤ diameter < 2.5 cm) was counted along both 1 m horizontal and vertical transects, and all coarse woody debris (CWD; 2.5 ≤ diameter) present in the quadrat had its diameter measured and recorded.
Metabarcoding methodology
Arthropod samples were visually size sorted; any organisms larger than the size of a honey bee were removed and two legs were removed and added to the overall sample (as per Ji et al. 2013; Meier et al. 2016). Samples were homogenised and then processed using a modified DNEasy Blood and Tissue kit (Qiagen, Netherlands), with a 400 µL starting digest volume and a 100 µL double elution in EB buffer, resulting in 200 µL of the eluate. All extractions were carried out on an automated Qiacube (Qiagen). DNA extraction controls were included in every 23 samples.
A qPCR assay was run using an invertebrate mitochondrial COI primer set: fwhF2 (5’-GGDACWGGWTGAACWGTWTAYCCHCC-3’) and fwhR2n (5’-GTRATWGCHCCDGCTARWACWGG-3’) targeting a 205 bp region (Vamos et al. 2017). Samples were amplified using ‘fusion primers’, gene-specific primers labelled on both the forward and reverse with 6–8 bp molecular identification (MID) tags and Illumina sequencing adaptors (Bohmann et al. 2022). Samples were amplified in duplicate, using the same tag combinations for each sample, including negative controls. Samples were pooled in approximate equimolar concentrations. The library pool was sequenced on an Illumina MiSeq (Illumina, USA) using a single-end 300-cycle V2 kit per the manufacturer’s directions. For detailed methods, see Appendix 1.
Bioinformatic processing
Bioinformatic processing was conducted using the ‘eDNAFlow’ pipeline (Mousavi-Derazmahalleh et al. 2021). Briefly, raw sequences were demultiplexed to their sample using MID-tag combinations and length filtered using ‘OBITools’ (Boyer et al. 2016) with a minimum length of 50 base pairs. Sequences were then quality filtered with errors and chimeras removed using USEARCH (Edgar 2010) with a minimum Phred quality score of 20 and a minimum abundance of 8 sequences for zero-distance operational taxonomic unit (zOTU) creation. A zOTU approach was used because it is a highly sensitive method to characterise biodiversity, without the need for reliance on complete reference databases. zOTUs were curated using ‘LULU’ at default parameters (Frøslev et al. 2017) on R v 3.6.1 (R Core Team 2019). Appropriate sample depth per sample was assessed using rarefaction curves with the ‘rarecurve’ function in vegan (Oksanen et al. 2019) and samples with less than 2,000 sequences were removed. A 0.05% minimum abundance filtering threshold was used to combat false, low abundance zOTUS (Prodan et al. 2020). Low occurrence zOTUs with less than five sequences and occurring in only one sample were also removed. zOTUs were matched to the GenBank Reference database (www.ncbi.nlm.nih.gov/genbank/) and the Barcode of Life Database (Ratnasingham and Hebert 2007) using the Basic Local Alignment Search Tool (BLAST) for taxonomic assignment. BLASTn results returned the top ten hits with a minimum query coverage of 95% and a minimum percentage identity of 85% to account for low representation of Australian arthropod species in reference databases. zOTUs present in the extraction and PCR controls (Fungal zOTUs) were removed from the dataset. The dataset was divided into three categories; firstly, all zOTUs that were able to be identified to the phylum Arthropoda (overall arthropods), secondly all zOTUs belonging to the family Formicidae (ants), and lastly all zOTUs belonging to the class Collembola (springtails).
Statistical analysis
Statistical analysis was performed using PRIMER v.7 (Clarke and Gorley 2015) with the PERMANOVA + add-on (Anderson et al. 2008). Firstly, to illustrate the scale of environmental disturbance the environmental variables from all samples were normalised and Draftsman’s plots were generated to check for correlation between every habitat characteristic. Those that were correlated were not both included, and a representative of the correlating pair was instead chosen for the analysis. A principal component analysis (PCA) was performed with Euclidean distance on the centroids of quadrat number located at each treatment and each site to illustrate the main differences in patterns. A Pearson’s correlation test was conducted comparing the normalised habitat characteristic values to the quadrat number. A permutational ANOVA (PERMANOVA) was then conducted on the Euclidian distance matrix with 9999 permutations. The factors were as follows: Site (Fixed; KA, KO, MG); Infrastructure type (Fixed; MT, Pad, UT); Transect (Random; Nested in Site and Infrastructure type); and Quadrat location (Fixed; i – vi). The terms of interest to assess the impacts of linear infrastructure on arthropod communities were: the interaction between site, infrastructure type, and quadrat location; the interaction between infrastructure type and quadrat location; plus the interaction between site and infrastructure type. This was to account for the geographic separation between the different sites, so patterns of interest were from within each individual site. For the overall arthropod community data, the ant community, and the springtail community, the number of zOTUs (zOTU richness) was calculated and tested using univariate PERMANOVA with Euclidean distance and 9999 permutations using the same design as above. Arthropod, ant, and springtail communities were then transformed to presence-absence. For ease of interpretation, community composition was illustrated using nonmetric multidimensional scaling (nMDS) on Jaccard similarity on centroids of quadrat number located at each treatment and each site with a k value of 2. A PERMANOVA was then conducted with the design as above using Jaccard similarity and 9999 permutations.
Results
Habitat characteristics
Of the terms of interest that may indicate an impact of infrastructure on the habitat characteristics measured, a significant interaction was detected between the infrastructure type and quadrat (Table 1). We found no significant interaction among site, infrastructure type, and quadrat, nor between site and infrastructure type. The factors of site, infrastructure type, and quadrat were all significantly different. While all three infrastructure types were different to each other on the disturbance interior (Quadrat 1), the MT habitat characteristics retained differences from the other two infrastructure types until the remnant interior (Quadrat 6). These habitat characteristics were higher bare ground cover, lower leaf litter, lower woody debris levels, lower elevation, and lower soil depth at the MT sites (Supp Fig. S1). In comparison, for both the Pads and the UT, there were no significant differences in habitat characteristics from the disturbance edge (Quadrat 2) onwards into the remnant interior (Appendix Table 1). The patterns of change with quadrat position among infrastructure types (interaction) were reflected in the PCA plots (Fig. 2) and in the correlation tests (Table 2). There was a trajectory of change as the quadrats moved further from the disturbance interior (Quadrat 1) into the remnant bushland interior (Quadrat 6). This directional change was characterised by less bare ground cover and increased woody debris and soil depth moving away from the disturbance (Table 2).
DNA metabarcoding results
A total of 15,507,193 quality-filtered sequences were generated across the 217 samples. A total of 212 samples had the minimum sequencing depth required of 2,000 sequences per sample and 208 samples passed the minimum required quality filtering thresholds. Only 5 zOTUs were detected in the negative control samples (extraction negatives and the PCR negatives); however, none of these zOTUs were from the phylum Arthropoda and thus were removed from the data set. In total 2,250 zOTUs were generated. However, there were only 1,101 zOTUs that met filtering criteria, and these were used in this study. These zOTUs represented 4 classes, 19 orders and 84 families of arthropods (simplified in Appendix Table 2, for full taxonomic information of all zOTUs and the zOTU tables, see the spreadsheet provided as Appendix 2). Ants made up 21% of the total zOTUs from this data set (238 zOTUs) and springtails made up 14% (170 zOTUs).
zOTU richness
Significant differences in the zOTU richness of the overall arthropod, ant and springtail communities between sites were evident (Table 3), with KO having lower richness compared to the other sites (Fig. 3). Overall arthropod richness differed significantly with infrastructure type, while ant and springtail richness did not. For the terms of interest indicating a potential disturbance effect, a significant interaction among site, infrastructure, and quadrat was observed for the ant community (Table 3 A), but not for the overall arthropod community or the springtail community. This might be influenced by the KA site, which showed more variation in richness across the quadrats at the different treatments. No significant difference in zOTU richness was found across the infrastructure type and quadrat interaction for ants or springtails, but there was for the overall arthropod community. The overall arthropod community showed some trends towards higher richness at Quadrat 6 compared to Quadrat 1 for the KO site and in the MT and Pads for the MG site. However, this trend was reversed for the MG UT infrastructure. Significant interactions were observed at the site and infrastructure level for all three arthropod communities. These were predominantly driven by a higher zOTU richness at the MG site UT and MT infrastructure types compared to the other sites and infrastructure types (e.g., the MG Pads) (Fig. 3).
Community composition
The site and infrastructure type interaction were influential in differentiating the communities of overall arthropods, ants, and springtails (Fig. 4, Table 3B), and this was evident int the distinct separation of arthropod communities when examining them at a site-by-site level (Fig. 5). However, unlike the habitat characteristics, no significant interaction was detected between the infrastructure type and the quadrat location (Table 3B), and for all the arthropod communities, there was no consistent directional trend observed between the quadrat locations (Fig. 5). For the overall arthropod communities, at the KA site there was a distinct trajectory observed from the quadrats on the disturbance (1) to the quadrats in the remnant vegetation (6) for both the MT and Pad infrastructure type (Fig. 5A). However, at the other sites, this pattern was less clear, particularly at MG, suggesting a homogenization of the overall arthropod communities across the transect from which quadrats were sampled (100 m).
For the springtail communities, only the site and infrastructure type interaction were found to be significantly different (Table 3B), and this was seen through the strong clustering of points on the NMDS (Fig. 4C), but the finer patterns observed in the overall communities were not observed when sites and infrastructure types were separated as all quadrat locations clustered closer together on the NMDS (Fig. 5C). For the ant communities at KA, the distinction between the infrastructure types was not as clear as for the overall arthropod community (Fig. 5B). However, at the other sites (KO and MG), there was a separation of the ant communities by infrastructure type (Fig. 5B). But this distinction between the quadrats did not show the same directionality as the overall arthropod community, i.e., a clear path from Quadrat 1 to Quadrat 6.
Discussion
Mining has a large global environmental footprint, but knowledge and understanding of its impact is still limited (Tang and Werner 2023), and this is particularly the case for exploration infrastructure (Raiter et al. 2017). In this we study, we investigated the effect of tracks, roads and drill pads on biophysical habitat characteristics and ground-dwelling arthropod communities. While changes in habitat were only visible to 10 m from the disturbance, impacts to arthropod communities could be detected up to 100 m into the remnant vegetation, and these responses were more complex. In general, we found similar patterns expressed in the compositional changes for arthropods overall and between our chosen indicator groups (ants and springtails), but they were not the same across all sites and infrastructure types (MT, UT, and Pads).
Habitat disturbance around linear infrastructure and impacts on arthropod communities
Habitat characteristics were altered by anthropogenic disturbance similarly across the different infrastructure types. This change was broadly characterised by decreased woody debris and shallower soil depth (i.e., increased compaction) closer to the actual disturbance. This pattern, consistent across sites and infrastructure types, aligns with previous research on linear infrastructure impacts on natural vegetation (Dániel-Ferreira et al. 2020; Goosem 2012). For some of our sites, this change in habitat characteristics extended out to 10 m from the disturbance, suggesting edge effects of at least this spatial scale. However, zOTU richness of arthropods could not capture this pattern, possibly because richness (number of zOTUs) does not necessarily respond to disturbance like other metrics such as community composition do (Fernandes et al. 2019). In general, a habitat can often only support a certain number of species. As the habitat changes over time or because of disturbances, the composition of species rather than species richness changes (Lengyel et al. 2016). Other traditional univariate diversity measures that consider abundance alongside the number of species (e.g., Shannon’s, (Maurer and McGill 2011)) may be more useful for measuring these differences; however, it is not possible to obtain robust species abundance data using metabarcoding techniques to calculate these metrics. Therefore, looking at both zOTU richness and composition together, alongside changes in habitat characteristics, is more effective at assessing community responses to change (Fernandes et al. 2019; van der Heyde et al. 2022a).
Using zOTU composition, we were able to detect finer scale effects between quadrats that indicated disturbance. For some site-infrastructure combinations, such as the MT at KA, there was a pattern of change in zOTU composition among quadrats from communities on the infrastructure through to those in remnant bushland, which indicated an edge effect extending 100 m into the remnant bushland. However, this pattern was inconsistent across all the MT at the three sites. This is not unexpected, as a previous study found that physical changes in the landscape caused by seismic lines were very visible, but they did not have a great impact on ant communities beyond one year post-disturbance (Watts et al. 2002). This was attributed to changes in vegetation within 10–100 m of the infrastructure resulting in only minor reorganisation of foraging patterns for ants, but not the overall community structure (Watts et al. 2002).
The overall habitat characteristics of quadrats on the Pads and UT remained the same as for MT. Even years after the initial disturbance, the infrastructure areas we studied were relatively clear, and the surrounding vegetation community structure had not been restored. This has been attributed to the specialisation of vegetation communities to particular soil chemical and physical characteristics, which remain altered following disturbance and limits the return of vegetation (Gibson et al. 2012; Yates et al. 2011). Rehabilitation of such sites around exploration infrastructure needs to be considered and planned for, just as other aspects of mining impacts are in the development of mine rehabilitation plans (Manero et al. 2020). At our study sites, exploration disturbances were either currently maintained (MT) or left to revegetate passively (UT and Pads), without any intervention methods such as recontouring or seeding (Meli et al. 2017). Tracks and roads are considered as part of mine infrastructure, and mine closure requirements now consider rehabilitation of tracks as part of the overall environmental rehabilitation and closure obligations (DMIRS, 2021; Young et al. 2019). However, Australia has an extensive legacy of unrestored exploration infrastructure (Mills 2022).
Effectiveness of indicator groups to assess changes in arthropod communities
One of our study aims was to examine whether two indicator groups of arthropods, ants and springtails, reflected overall patterns in arthropods at our sites in response to exploration disturbances. While we observed similar patterns between the three sites and infrastructure types in terms of ant and springtail communities, the patterns were not identical across both groups. Furthermore, the effect of site, infrastructure type, and quadrat was not significant for the springtail community composition but was significant for the whole arthropod communities and for ants. Overall, the ant communities were more reflective of the differences in the total arthropod communities than were springtails, presumably reflecting the consistent responses of ants to disturbance (Hoffmann and Andersen 2003). This implies that while springtail communities may be useful in examining broader disturbances in the environment (Greenslade and Majer 1993), to examine the edge effects at a fine scale, ant communities are more informative.
Choosing an indicator taxon group can be an important consideration when designing metabarcoding studies. Ants and springtails are valuable indicator taxa in their own right (Greenslade 2007; Tiede et al. 2017). For example, ants are reported to have consistent responses to disturbance (Hoffmann and Andersen 2003) and hence make good indicators. Springtails can be highly spatially aggregated in the soil (Greenslade 2007), and are very sensitive to subtle changes in soil conditions (e.g., pH) (Sławski and Sławska 2000). So, they theoretically could be useful for understanding the fine-scale impacts of disturbance. However, springtail species composition is impacted by weather events or seasonal conditions (Yin et al. 2019), and patterns of disturbance may be confounded by these variables. From this, we conclude that comparing the performance of different taxa (whole arthropods, ants, and springtails) is important to determine the utility of these groups as indicators. It is also important to build reference libraries, improve the taxonomic resolution of assays, and improve the understanding of specific species ecology when focusing only on select indicator taxon groups in order to choose the appropriate indicator group. In comparison, capturing whole species assemblages in a tree of life fashion (Stat et al. 2017) may give more holistic understanding of changes in the environment, but would require the development of barcodes and functional understanding of species groups across a larger scale, at a much higher cost.
DNA metabarcoding as a tool to assess arthropod communities
In this study, we demonstrated the utility of DNA metabarcoding for assessing arthropod community responses to environmental changes at a fine scale. Our analysis revealed over 84 families of arthropods and identified differences between sites, infrastructure types, quadrats, and indicator groups. However, like any survey method, the effectiveness of DNA metabarcoding is influenced by the technological limitations, study design, and execution (van der Heyde et al. 2020). A significant challenge in DNA metabarcoding studies is the reliance on reference databases, particularly in understudied arthropod systems like ours (van der Heyde et al. 2020; van der Heyde et al. 2022a, b). While we detected 2,250 zOTUs, only 1,101 could be identified as being from the phylum Arthropoda (~ 49%), which is consistent with findings from other studies lacking custom reference databases (for example, Klimova et al. 2023; van der Heyde et al. 2022a, b; Wildermuth et al. 2023). Improving database representation is crucial for enhancing the resolution of zOTU identifications, which, coupled with a deeper understanding of species ecology, could facilitate the assignment of ecological function to taxonomic groups (Pawlowski et al. 2018).
Enhancing the representation of understudied taxonomic groups, such as Collembola, would reduce the number of sequences that are discarded because of unassignable identities (van der Heyde et al. 2022a). With less than 50% of our generated data usable, we may be only capturing half the disturbance impacts at these sites. However, the dynamic nature of global barcode databases, including initiatives like the International Barcode of Life project (www.ibol.org), offers hope for future analysis providing a more comprehensive understanding of disturbance effects on arthropod biodiversity. Despite relying on an incomplete arthropod barcode database, our findings align with those of previous studies using morphological identification to study arthropod responses to disturbance (Majer et al. 2020; Yu et al. 2006). Therefore, we have confidence that our observed trends reflect real patterns in arthropod communities at our study sites.
Visual observations of our samples strongly suggested that there were differences in the abundance of some taxa in samples collected from the disturbances and within the remnant vegetation. However, because reliable abundance is difficult to obtain from metabarcoding data (Elbrecht and Leese 2015), we were only able to make assessments based on the presence/absence of our arthropod zOTUs and were not able to examine abundance-related differences. However, abundance data is critical for calculating different diversity metrics and may allow for a more nuanced interpretation of community responses to disturbance at our sites. Approaches to obtain biomass or abundance estimates will be a valuable addition to metabarcoding surveys and there is scope for development in this space using various techniques; recent papers have shown success with using modelling frameworks (Shelton et al. 2022) and DNA spike-ins (Luo et al., 2023) to deduce relative abundance of species within samples.
Seasonal variation impacts the composition of arthropod (Santorufo et al. 2014) and plant communities and their interactions (CaraDonna et al. 2017). Therefore, the resolution of our findings may have been improved by surveying arthropod communities over multiple time points. Thus, repeated surveys and other monitoring methodologies (e.g., indicator species studies) may be required to determine if the observed patterns are one-off occurrences or a result of broader ecological trends.
Furthermore, metabarcoding of arthropod communities in this way is subject to the same considerations as traditional methods. Study design may have also played a role in the patterns observed. Here, we used multiple 100 m transects across infrastructure types at one site, and we detected differences in arthropod communities at this scale. An interesting comparison would be to expand these transects out to greater distances into remnant bushland in order to determine broader landscape scale differences that might result from habitat modification within the same site. Increasing the replicates within a quadrat may also be beneficial in adding support to these results.
Conclusions
Mining exploration infrastructure significantly influenced both the physical habitat and arthropod communities. These disturbances resulted in similarly altered physical habitat characteristics across infrastructure types at all sites. There were detectable edge effects on the habitat characteristics, but these impacts did not extend far into remnant bushland (< 10 m). For arthropod communities, however, the effect of disturbance extended into the remnant bushland for 100 m, showing similar patterns for whole communities, and for ants, but not for springtails. Our study has highlighted the utility of DNA metabarcoding as a tool to identify changes in arthropod communities impacted by mining and showcased the differences between whole arthropod communities versus common indicator species groups. We recommend continued monitoring of arthropod communities in our study system, especially over different spatial scales, seasons, and time points, to develop a more holistic understanding of their responses to disturbance. Our data emphasise the need to consider different types of disturbances associated with mining activity when assessing the impact of new mining projects, including exploration infrastructure, as they may all have the potential to impact surrounding faunal communities and associated ecosystem processes to a significant degree.
A) Study site showing location of three mine sites and the locations of the different infrastructure types at each site, where MT is Maintained Track, Pad is Drill Pad, and UT is Unmaintained track B) Stylised image of experimental design and pitfall trap placement along each transect across the ecotone between the disturbance (maintained track, unmaintained track, or drill pad) and remnant vegetation. The grey dashed lines indicate prospective topographic lines
Principal component analysis (PCA) plot of the habitat characteristics at each site showing the three different infrastructure types where MT is Maintained Track, Pad is Drill Pad, and UT is Unmaintained track. Trajectories are overlaid starting from Quadrat i (1) on the disturbance to Quadrat vi (6) approximately 100 m into the remnant vegetation. Vectors overlaid of the correlation between each habitat characteristic and the principal component axes. The circle is a unit circle (radius = 1.0), used to illustrate the nature and strength of the correlation
zOTU richness at the different quadrat locations (Quadrat Number i-vi) at the different sites and infrastructure types for the (A) overall arthropod communities, (B) Formicidae communities, and (C) Collembola communities. Sites are notated as KA for Karara, KO for Koolanooka, and MG for Mount Gibson. Different infrastructure types and sites are highlighted where MT is Maintained Track, Pad is Drill Pad, and UT is Unmaintained track
Non-metric multidimensional scaling (NMDS) plots showing (A) overall arthropod communities, (B) Formicidae communities, and (C) Collembola communities at the three sites. Sites are notated as KA for Karara, KO for Koolanooka, and MG for Mount Gibson. Different infrastructure types and sites are highlighted where MT is Maintained Track, Pad is Drill Pad, and UT is Unmaintained track
Non-metric multidimensional scaling (NMDS) plots showing the (A) arthropod communities, (B) Formicidae communities, and (C) Collembola communities at the three sites. Different infrastructure types are colour coded where MT is Maintained Track, Pad is Drill Pad, and UT is Unmaintained track. Trajectories are overlaid starting from Quadrat i (1) to Quadrat vi (6)
Data availability
Sample information, zOTU Tables, taxonomy information and de-convoluted unique sequences are available at: https://doi.org/10.5281/zenodo.6395458.
References
Andersen AN (2019) Responses of ant communities to disturbance: five principles for understanding the disturbance dynamics of a globally dominant faunal group. J Anim Ecol 88(3):350–362
Anderson M, Gorley R, Clarke KP (2008) PERMANOVA + for PRIMER: guide to software and statistical methods. PRIMER-E Ltd
Bohmann K, Elbrecht V, Carøe C, Bista I, Leese F, Bunce M, Yu DW, Seymour M, Dumbrell AJ, Creer S (2022) Strategies for sample labelling and library preparation in DNA metabarcoding studies. Mol Ecol Resour 22(4):1231–1246. https://doi.org/10.1111/1755-0998.13512
Bolger DT, Suarez AV, Crooks KR, Morrison SA, Case TJ (2000) Arthropods in urban habitat fragments in Southern California: Area, age, and edge effects. Ecol Appl 10(4):1230–1248
Boyer F, Mercier C, Bonin A, Le Bras Y, Taberlet P, Coissac E (2016) Obitools: a unix-inspired software package for DNA metabarcoding. https://doi.org/10.1111/1755-0998.12428. Molecular Ecology Resources
Canter L, Ross B (2010) State of practice of cumulative effects assessment and management: the good, the bad and the ugly. Impact Assess Project Appraisal 28(4):261–268
CaraDonna PJ, Petry WK, Brennan RM, Cunningham JL, Bronstein JL, Waser NM, Sanders NJ (2017) Interaction rewiring and the rapid turnover of plant–pollinator networks. Ecol Lett 20(3):385–394. https://doi.org/10.1111/ele.12740
Casimiro MS, Sansevero JBB, Queiroz JM (2019) What can ants tell us about ecological restoration? A global meta-analysis. Ecol Ind 102:593–598
Clarke KR, Gorley R (2015) Getting started with PRIMER v7. Marine Laboratory, Plymouth
Cross SL, Cross AT, Tomlinson S, Clark-Ioannou SM, Nevill PG, Bateman PW (2021) Mitigation and management plans should consider all anthropogenic disturbances to fauna. Global Ecol Conserv, 26, e01500
Dániel-Ferreira J, Bommarco R, Wissman J, Öckinger E (2020) Linear infrastructure habitats increase landscape-scale diversity of plants but not of flower-visiting insects. Sci Rep 10(1):1–11
DMIRS, Regulation I, Safety (2021) Mine Closure Completion Guideline – for demonstrating completion of mine closure in accordance with an approved mine Closure Plan. Department of Mines, Industry Regulation and Safety, Government of Western Australia,
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461
Elbrecht V, Leese F (2015) Can DNA-based ecosystem assessments quantify species abundance? Testing primer bias and biomass—sequence relationships with an innovative metabarcoding protocol. PLoS ONE, 10(7), e0130324
Ellis EC, Goldewijk K, Siebert K, Lightman S, D., Ramankutty N (2010) Anthropogenic transformation of the biomes, 1700 to 2000. Global Ecol Biogeography: J Macroecology 19(5):589–606
Ewers RM, Didham RK (2006) Continuous response functions for quantifying the strength of edge effects. J Appl Ecol 43(3):527–536
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34(1):487–515
Fernandes K, van der Heyde M, Coghlan M, Wardell-Johnson G, Bunce M, Harris R, Nevill P (2019) Invertebrate DNA metabarcoding reveals changes in communities across mine site restoration chronosequences. Restor Ecol 215:403
Frøslev TG, Kjøller R, Bruun HH, Ejrnæs R, Brunbjerg AK, Pietroni C, Hansen AJ (2017) Algorithm for post-clustering curation of DNA amplicon data yields reliable biodiversity estimates. Nat Commun 8(1):1188
Gibson N, Meissner R, Markey AS, Thompson WA (2012) Patterns of plant diversity in ironstone ranges in arid south western Australia. J Arid Environ 77:25–31
Goosem M (2007) Fragmentation impacts caused by roads through rainforests. Curr Sci 93(11):1587–1595
Goosem M (2012) Mitigating the impacts of rainforest roads in Queensland’s Wet tropics: effective or are further evaluations and new mitigation strategies required? Ecol Manage Restor 13(3):254–258
Greenslade P (2007) The potential of Collembola to act as indicators of landscape stress in Australia. Aust J Exp Agric 47(4):424–434
Greenslade P, Majer JD (1993) Recolonization by Collembola of rehabilitated bauxite mines in Western Australia. Aust J Ecol 18(4):385–394
Guijón R, Henríquez F, Naranjo JA (2011) Geological, geographical and legal considerations for the conservation of unique iron oxide and sulphur flows at El Laco and Lastarria volcanic complexes, Central Andes, Northern Chile. Geoheritage 3(4):299–315
Heino J (2014) Taxonomic surrogacy, numerical resolution and responses of stream macroinvertebrate communities to ecological gradients: are the inferences transferable among regions? Ecol Ind 36:186–194
Hodkinson ID, Jackson JK (2005) Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ Manage 35(5):649–666
Hoffmann BD, Andersen AN (2003) Responses of ants to disturbance in Australia, with particular reference to functional groups. Austral Ecol 28(4):444–464
Hooke RL, Martín-Duque JF, Pedraza J (2012) Land transformation by humans: a review. GSA Today: Publication Geol Soc Am 22(12):4–10
Ji Y, Ashton L, Pedley SM, Edwards DP, Tang Y, Nakamura A, Kitching R, Dolman PM, Woodcock P, Edwards FA, Larsen TH, Hsu WW, Benedick S, Hamer KC, Wilcove DS, Bruce C, Wang X, Levi T, Lott M, Yu DW (2013) Reliable, verifiable and efficient monitoring of biodiversity via metabarcoding. Ecol Lett 16(10):1245–1257
Jordana R, Greenslade P (2020) Biogeographical and ecological insights from Australasian faunas: the megadiverse collembolan genus. Entomobrya (Entomobryidae) Zootaxa 4770(1):1–104
Kerr JT, Sugar A, Packer L (2000) Indicator Taxa, rapid biodiversity assessment, and nestedness in an endangered ecosystem. Conserv Biol 14(6):1726–1734
Khosravi R, Hemami M-R (2019) Identifying landscape species for ecological planning. Ecol Ind 99:140–148
Klimova A, Rodríguez-Estrella R, Meng G, Gutiérrez‐Rivera JN, Jimenez‐Jimenez ML, Liu S (2023) Metabarcoding reveals seasonal and spatial patterns of arthropod community assemblages in two contrasting habitats: Desert and oasis of the Baja California Peninsula, Mexico. Divers Distrib 29(3):438–461
Kwok ABC, Wardle GM, Greenville AC, Dickman CR (2016) Long-term patterns of invertebrate abundance and relationships to environmental factors in arid Australia. Austral Ecol 41(5):480–491
Laurance WF, Goosem M, Laurance SGW (2009) Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol 24(12):659–669
Lengyel S, Déri E, Magura T (2016) Species richness responses to structural or compositional habitat diversity between and within grassland patches: a multi-taxon approach. PLoS ONE, 11(2), e0149662
Luo M, Ji Y, Warton D, Yu DW (2023) Extracting abundance information from DNA-based data. Mol Ecol Resour 23(1):174–189
Lynggaard C, Yu DW, Oliveira G, Caldeira CF, Ramos SJ, Ellegaard MR, Gilbert MTP, Gastauer M, Bohmann K (2020) DNA-based arthropod diversity assessment in amazonian iron mine lands show ecological succession towards undisturbed reference sites. Front Ecol Evol 8:426
Majer JD, Nichols OO (1998) Long-term recolonization patterns of ants in western Australian rehabilitated bauxite mines with reference to their use as indicators of restoration success. J Appl Ecol 35(1):161–182
Majer JD, de Sousa-Majer MJ, Heterick BE (2020) Partial clearing of a road corridor leads to homogenisation of the invertebrate fauna. Pac Conserv Biology. https://doi.org/10.1071/PC20039
Manero A, Kragt M, Standish R, Miller B, Jasper D, Boggs G, Young R (2020) A framework for developing completion criteria for mine closure and rehabilitation. J Environ Manage 273:111078
Maurer BA, McGill BJ (2011) Measurement of species diversity. Biol Diversity: Front Meas Assess, 55–65
McGeoch MA (1998) The selection, testing and application of terrestrial insects as bioindicators. Biol Rev Camb Philos Soc 73(2):181–201
McKenney BA, Kiesecker JM (2010) Policy development for biodiversity offsets: a review of offset frameworks. Environ Manage 45(1):165–176
Meier R, Wong W, Srivathsan A, Foo M (2016) $1 DNA barcodes for reconstructing complex phenomes and finding rare species in specimen-rich samples. Cladistics 32(1):100–110
Meissner R, Caruso Y (2008) Flora and vegetation of banded iron formations of the Yilgarn Craton: Koolanooka and Perenjori Hills. Conserv Sci Western Australia 7(1):73–88
Meli P, Holl KD, Benayas R, Jones JM, Jones HP, Montoya PC, D., Mateos M, D (2017) A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLoS ONE, 12(2), e0171368
Mills LN (2022) Getting closure? Mining rehabilitation reform in Queensland and Western Australia. The Extractive Industries and Society, p 101097
Moir ML, Brennan KEC, Koch JM, Majer JD, Fletcher MJ (2005) Restoration of a forest ecosystem: the effects of vegetation and dispersal capabilities on the reassembly of plant-dwelling arthropods. For Ecol Manag 217(2):294–306
Mousavi-Derazmahalleh M, Stott A, Lines R, Peverley G, Nester G, Simpson T, Zawierta M, De La Pierre M, Bunce M, Christophersen CT (2021) eDNAFlow, an automated, reproducible and scalable workflow for analysis of environmental DNA (eDNA) sequences exploiting Nextflow and Singularity. https://doi.org/10.1111/1755-0998.13356. Molecular Ecology Resources
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10(2):58–62
Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens HH, Szoecs E, Wagner H (2019) vegan: Community Ecology Package (Version 2.5-6) [Computer software]. https://cran.ism.ac.jp/web/packages/vegan/vegan.pdf
Oliver I, Beattie AJ (1996) Designing a cost-effective invertebrate survey: a test of methods for rapid assessment of biodiversity. Ecol Appl 6(2):594–607
Pawlowski J, Kelly-Quinn M, Altermatt F, Apothéloz-Perret-Gentil L, Beja P, Boggero A, Kahlert M (2018) The future of biotic indices in the ecogenomic era: integrating (e) DNA metabarcoding in biological assessment of aquatic ecosystems. Sci Total Environ 637:1295–1310
Pik AJ, Oliver I, Beattie AJ (1999) Taxonomic sufficiency in ecological studies of terrestrial invertebrates. Aust J Ecol 24(5):555–562
Pohlman CL, Turton SM, Goosem M (2009) Temporal variation in microclimatic edge effects near powerlines, highways and streams in Australian tropical rainforest. Agric for Meteorol 149(1):84–95
Prendergast JR, Quinn RM, Lawton JH, Eversham BC, Gibbons DW (1993) Rare species, the coincidence of diversity hotspots and conservation strategies. Nature 365(6444):335–337
Prodan A, Tremaroli V, Brolin H, Zwinderman AH, Nieuwdorp M, Levin E (2020) Comparing bioinformatic pipelines for microbial 16S rRNA amplicon sequencing. PLoS ONE, 15(1), e0227434
R Core Team (2019) R: A Language and Environment for Statistical Computing (Version 3.6.1) [Computer software]. R Foundation for Statistical Computing. https://www.R-project.org/
Raiter KG, Possingham HP, Prober SM, Hobbs RJ (2014) Under the radar: mitigating enigmatic ecological impacts. Trends Ecol Evol 29(11):635–644
Raiter KG, Prober SM, Hobbs RJ, Possingham HP (2017) Lines in the sand: quantifying the cumulative development footprint in the world’s largest remaining temperate woodland. Landscape Ecol 32(10):1969–1986
Ratnasingham S, Hebert PDN (2007) BOLD: the Barcode of Life Data System (www.barcodinglife.org). Mol Ecol Notes. https://doi.org/10.1111/j.1471-8286.2006.01678.x
Redondo-Vega JM, Gómez-Villar A, Santos-González J, González-Gutiérrez RB, Álvarez-Martínez J (2017) Changes in land use due to mining in the north-western mountains of Spain during the previous 50 years. CATENA 149:844–856
Ries L, Sisk TD (2004) A predictive model of edge effects. Ecology 85(11):2917–2926
Roy AH, Rosemond AD, Leigh DS, Paul MJ, Wallace JB (2003) Habitat-specific responses of stream insects to land cover disturbance: biological consequences and monitoring implications. J North Am Benthological Soc 22(2):292–307
Santorufo L, Van Gestel CA, Maisto G (2014) Sampling season affects conclusions on soil arthropod community structure responses to metal pollution in Mediterranean urban soils. Geoderma 226:47–53
Santos BA, Peres CA, Oliveira MA, Grillo A, Alves-Costa CP, Tabarelli M (2008) Drastic erosion in functional attributes of tree assemblages in Atlantic forest fragments of northeastern Brazil. Biol Conserv 141(1):249–260
Shelton AO, Gold ZJ, Jensen AJ, D′ Agnese E, Allan A, Van Cise E, Kelly A, R. P (2022) Toward quantitative metabarcoding. Ecology, 104(2), e3906
Siddig AAH, Ellison AM, Ochs A, Villar-Leeman C, Lau MK (2016) How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in ecological indicators. Ecol Ind 60:223–230
Silva THSE, Karunarathna NB, Karunaratne WA, I. P (2017) Competence of litter ants for rapid biodiversity assessments. Lundiana: International Journal of Biodiversity, 2017, 1–10
Sławski M, Sławska M (2000) The forest edge as a border between forest and meadow. Vegetation and Collembola communities. Pedobiologia 44(3–4):442–450
Stat M, Huggett MJ, Bernasconi R, DiBattista JD, Berry TE, Newman SJ, Harvey ES, Bunce M (2017) Ecosystem biomonitoring with eDNA: metabarcoding across the tree of life in a tropical marine environment. Sci Rep 7(1):1–11
Stephens C (2018) Exploration infrastructure impacts on Banded Iron Formation (BIF) ranges in the Midwest, Western Australia [Honours Dissertation, Curtin University]. Curtin eSpace
Swart RC, Pryke JS, Roets F (2018) Arthropod assemblages deep in natural forests show different responses to surrounding land use. Biodivers Conserv 27(3):583–606
Tang L, Werner TT (2023) Global mining footprint mapped from high-resolution satellite imagery. Commun Earth Environ 4(1):134
Terlizzi A, Bevilacqua S, Fraschetti S, Boero F (2003) Taxonomic sufficiency and the increasing insufficiency of taxonomic expertise. Mar Pollut Bull 46(5):556–561
Therivel R, Ross B (2007) Cumulative effects assessment: does scale matter? Environ Impact Assess Rev 27(5):365–385
Tibbett M (2015) Mining in ecologically sensitive landscapes. CSIRO Publishing
Tiede Y, Schlautmann J, Donoso DA, Wallis CIB, Bendix J, Brandl R, Farwig N (2017) Ants as indicators of environmental change and ecosystem processes. Ecol Ind 83:527–537
Vamos EE, Elbrecht V, Leese F (2017) Short COI markers for freshwater macroinvertebrate metabarcoding. Metabarcoding Metagenomics, 1, e14625
van der Heyde M, Bunce M, Wardell-Johnson G, Fernandes K, White NE, Nevill P (2020) Testing multiple substrates for terrestrial biodiversity monitoring using environmental DNA metabarcoding. Mol Ecol Resour 20(3). https://doi.org/10.1111/1755-0998.13148
van der Heyde M, Bunce M, Dixon KW, Fernandes K, Majer J, Wardell-Johnson G, White NE, Nevill P (2022a) Evaluating restoration trajectories using DNA metabarcoding of ground-dwelling and airborne invertebrates and associated plant communities. Mol Ecol 31:2172–2188. https://doi.org/10.1111/mec.16375
van der Heyde M, Bunce M, Nevill P (2022b) Key factors to consider in the use of environmental DNA metabarcoding to monitor terrestrial ecological restoration. Sci Total Environ 848:157617
van Jaarsveld AS, Freitag S, Chown SL, Muller C, Koch S, Hull H, Scholtz CH (1998) Biodiversity assessment and conservation strategies. Science 279(5359):2106–2108
Watts C, McArthur A, Oakey H, Verbyla A (2002) The impact of seismic lines on ant communities in the Cooper Basin and potential use of ants as bioindicators of ecological recovery rates. South Australian Museum and BiometricsSA. https://sarigbasis.pir.sa.gov.au/WebtopEw/ws/samref/sarig1/image/DDD/RB200200035.pdf
Wildermuth B, Seifert CL, Husemann M, Schuldt A (2023) Metabarcoding reveals that mixed forests mitigate negative effects of non-native trees on canopy arthropod diversity. Ecol Appl, 33(8), e2921
Yates CJ, Gibson N, Pettit NE, Dillon R, Palmer R (2011) The ecological relationships and demography of restricted ironstone endemic plant species: implications for conservation. Aust J Bot 59(7):692–700
Yin R, Gruss I, Eisenhauer N, Kardol P, Thakur MP, Schmidt A, Xu Z, Siebert J, Zhang C, Wu GL, Schädler M (2019) Land use modulates the effects of climate change on density but not community composition of Collembola. Soil Biol Biochem 138:107598. https://doi.org/10.1016/j.soilbio.2019.107598
Young RE, Manero A, Miller BP, Kragt ME, Standish RJ, Jasper DA, Boggs GS (2019) A framework for developing mine-site completion criteria in Western Australia. The Western Australian Biodiversity Science Institute, Perth, Western Australia
Yu DW, Ji Y, Emerson BC, Wang X, Ye C, Yang C, Ding Z (2012) Biodiversity soup: metabarcoding of arthropods for rapid biodiversity assessment and biomonitoring. Methods Ecol Evol 3(4):613–623
Yu XD, Luo TH, Yang J, Zhou HZ (2006) Distribution of ground-dwelling beetles (Coleoptera) across a forest‐clearcut ecotone in Wolong Natural Reserve, southwestern China. Insect Sci 13(2):127–135
Acknowledgements
This project was supported by the Australian Research Council Industrial Transformation Training Centre for Mine Site Restoration. Computational resources were provided by the Pawsey Supercomputing Centre, funding from the Australian Government, and the Government of Western Australia. KF was partially supported by funding from the Food Agility CRC. We would like to thank Marti J Anderson and Adam H Smith for their helpful advice on statistical analysis for this study. We also thank the mining companies, Sinosteel Midwest Corporation Ltd, Karara Mining Ltd, and Mount Gibson Iron Ltd for access to sites for sampling.
Funding
This project was supported by the Australian Research Council Industrial Transformation Training Centre for Mine Site Restoration. Computational resources were provided by the Pawsey Supercomputing Centre, funding from the Australian Government, and the Government of Western Australia. KF was partially supported by funding from the Food Agility CRC.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
SC-I, and PN conceptualised the study. SC-I collected the samples and processed them. KF conducted the experimentation and analysis. BJS assisted with statistical analysis. KF wrote the original draft of the manuscript. All authors contributed to review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Akihiro Nakamura.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernandes, K., Clark-Ioannou, S., Saunders, B.J. et al. Mining exploration infrastructure affects biophysical habitat characteristics and ground-dwelling arthropod communities. Biodivers Conserv (2024). https://doi.org/10.1007/s10531-024-02865-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10531-024-02865-2