Abstract
Animal pollinators underpin the functioning and persistence of ecosystems globally. However, the vital role of pollination is being progressively eroded by the worldwide decline of pollinator species caused by human-induced environmental degradation, resulting in rising costs to biodiversity, agriculture, and economy. Most studies quantifying pollinator diversity and declines have focused on insects, whereas vertebrate pollinators remain comparatively neglected. Here, we present the first comprehensive study quantifying the macroecological patterns of species richness and extinction risk of bird and mammal pollinators in India, a region of extremely high biodiversity and increasing anthropogenic pressure. Our results reveal that hotspots of mammal pollinator diversity are restricted to the south of the Western Ghats, whereas bird pollinator diversity hotspots are scattered throughout the country. Analyses of hotspots of threatened species (based on the IUCN Red List) show that only mammal pollinators are currently classified as threatened in India, whereas multiple hotspots of population declines were observed for birds, and primarily in the Southwest for mammal pollinators. Our analyses failed to identify a role for species traits as drivers of these patterns, whereas most pollinators appear to be threatened by agriculture, logging and hunting for food, and medicinal purposes. Pollinator endangerment has widescale ecological and economic implications such as reduced food production, plant extinction, loss of functional and genetic diversity, and economic damage. We suggest protection of vertebrate pollinators should be emphasised in active conservation agendas in India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The progressive accumulation of the human industrial footprint has degraded environments globally, triggering an alarming erosion of biodiversity from across the tree of life (Parmesan 2006; Davidson et al. 2009; Hoffmann et al. 2010; Stork 2010; Dirzo et al. 2014; Urban 2015; Ripple et al. 2016, 2017; Finn et al. 2023). Among species undergoing declines, pollinators have attracted increasing attention given the diverse and widespread costs expected to result from their loss (Biesmeijer et al. 2006; National Research Council 2007; Potts et al. 2010; vanEngelsdorp et al. 2010). Both invertebrates and vertebrates pollinate a variety of angiosperms, thus playing a crucial role in the functioning and persistence of ecosystems and the biodiversity they sustain (Proctor et al. 1996). Vertebrate pollination is a common phenomenon across the tropics, therefore, the dependency of plants on vertebrate pollinators is high in these regions (Fleming and Muchhala 2008; Fleming et al. 2009; Ratto et al. 2018). Vertebrates, particularly birds, pollinate approximately 5% of a region’s flora and it can be as much as 10% in island ecosystems (Anderson 2003; Kato and Kawakita 2004; Bernardello et al. 2006), and bats are believed to be the main pollinators of nearly 1000 economically important plants species in the tropics (Bumrungsri et al. 2008; Aziz et al. 2017; Sritongchuay et al. 2019; Tremlett et al. 2020). However, most research on the decline of animal pollinators has been focused on insect pollinators (Biesmeijer et al. 2006, Kleijn and Raemakers 2008, Potts et al. 2010, vanEngelsdorp et al. 2010, Roulston and Goodell 2011, Carvalheiro et al. 2013, Vanbergen and Initiative 2013, Ollerton et al. 2014, Scheper et al. 2014, Goulson et al. 2015, Powney et al. 2019), whereas the study of extinctions across vertebrate pollinators remains comparatively neglected. Therefore, some significant gaps about the diversity, spatial distribution and ‘hotspots’ of extinction risk among vertebrate pollinators are still to be closed by empirical research, especially at large taxonomic and geographic scales.
Vertebrates pollinate a wide variety of economically and ecologically important plants across tropical and subtropical regions (Law and Lean 1999; Yumoto 2000; Ollerton et al. 2011; Maas et al. 2013). From among these regions, the highly biodiverse subcontinent of India is known to host a range of vertebrate species that pollinate a wide variety of plant species of significant economic importance for the country (Atluri et al. 2000; Nathan et al. 2005; Sinu et al. 2011; Erancheri et al. 2013; Raju et al. 2013; Khanduri and Kumar 2017). Remarkably, although the territory of India covers ~ 2% of the world’s landmass, this country hosts ~ 7.5% of animal species globally (ZSI 2017; Venkataraman and Sivaperuman 2018), among which many are pollinators. Between 2014 and 2015, the commercial demand for herbal raw drugs in India was estimated at 512,000 metric tonnes, and the estimated export of these drugs, including extracts, at 134,500 metric tonnes (Goraya and Ved 2017). Vertebrate pollinators pollinate some of these plants.
Conservation planning and investment in India are based on large charismatic animals. Whilst this can be beneficial: these animals tend to have large ranges and therefore need large protected areas for their protection, small animals and other taxa are often neglected, regardless of their ecological importance (Treves and Karanth 2003; Smith et al. 2012; Macdonald et al. 2015; Srivathsa et al. 2020). Pollinators tend to be small, often inconspicuous animals, and are not considered in terms of conservation planning, despite their importance, both in agricultural and natural ecosystems.
The key roles that vertebrate pollinators play in the functioning and economy of ecosystems in India strongly contrast with the lack of large-scale data on their biodiversity, patterns of distribution and extinction risks. Therefore, to prevent pollinator biodiversity loss and maintain the pollination services (and their associated economic benefits) contributed by vertebrate pollinators that are not redundant with those provided by insects (i.e., the economic contribution from insect pollination to Indian agriculture, at an estimated US$ 22.52 billion during 2012–2013; Chaudhary and Chand 2017), we quantify the spatial organisation of pollinator species richness (i.e., biodiversity hotspots) to then identify patterns of spatial distribution of species classified as threatened and those undergoing population declines according to the IUCN Red List. This information is critical to enhance capacities for effective conservation and policy implementation. We expect that both bird and mammal pollinators would show different hotspots of species richness patterns across the geographical regions of India due to differences in their ability to move between habitats. We also predict that the risk of extinction and population declines of bird and mammal pollinators will increase with body size given the increased probability of hunting in association with the tendency of large-sized endotherms for low reproductive rates, which impacts on their chances of rapid demographic recovery (Purvis et al. 2000; Cardillo et al. 2005; Hutchings et al. 2012; Comeros-Raynal et al. 2016; Verde Arregoitia 2016; Ripple et al. 2017, Pincheira-Donoso et al. 2021). Similarly, we predict that species with small geographic range sizes and low-density populations are more predisposed to extinction risk since they are more vulnerable to local catastrophes and demographic stochasticity, as well as inbreeding events (Kattan 1992; Manne et al. 1999; Gaston and Fuller 2009; Pincheira-Donoso and Hodgson 2018; Chichorro et al. 2019; Shuai et al. 2021). Finally, we investigate the extinction risk of pollinators from anthropogenic activities due to habitat loss and their unsustainable exploitation for bushmeat in the markets.
Materials and methods
Pollinator species name collection
We collated from the primary literature a de novo database containing all species of vertebrate pollinators of India from mainstream search engines (e.g., Web of Science, Google Scholar). We also retrieved data from additional sources of literature available at the Biodiversity Heritage Library (biodiversitylibrary.org) using the author’s name and year of publication for Singh 1929, Ali 1931, McCann 1931, McCann 1933, and Davidar 1985 and emailed authors where papers could not be accessed. We used four groups of search terms in Web of Science and Google Scholar: (1) vertebrate, bird, avian, mammal, bat; (2) pollination, flowers, plant; (3) interaction, reproduction; and (4) India. Search methods included one word from each group, where relevant, and all possible four combinations were used to retrieve bird and mammal pollinator species (see Tables S1 & S2; accessed until 15th June 2022). Our initial search on the Web of Science yielded 1,178 articles. However, for Google Scholar, we adopted a methodology of selecting the first 20 pages of each keyword thus yielding 2,800 articles. Hence, we retrieved 3,978 articles through both databases and ten articles through other sources (Biodiversity Heritage Library and authors). We screened the titles and abstracts of all the articles for relevance. After eliminating spurious results, 294 appropriate studies were assessed for eligibility. We read the full text of all the remaining articles to determine their suitability to be included in the database, resulting in 57 eligible studies for the synthesis of the vertebrate pollinator database (see Fig. S1 for full PRISMA report). We reviewed literature written in English only, therefore, we might underrepresent the overall published studies if published in other Indian vernacular languages. These studies altogether provided 67 bird and 18 mammal pollinators, resulting in a total of 85 vertebrate pollinator species for the study (see Supplementary Material for a full list of retrieved papers used to compile the pollinator dataset).
For birds, we classified as pollinators those species that regularly make contact with the stigma and anthers of flowers in the process of collecting nectar/pollen (Tandon et al. 2003), or identified as pollinators by assessing pollen load on the stigma or fruit production through pollination efficiency experiments (Schemske and Horvitz 1984), or animal agents reported to pollinate flowers that do not open naturally, and they require manipulation by visitors for opening unless flowers fall off without pollination (Davidar 1983).
For mammals, we classified them as pollinators based on the descriptions of foraging behaviour, such as regularly observed sucking or licking flowers’ nectar, carrying pollen load on fur, or fruit/seed production through pollination efficiency experiments (Regan et al. 2015).
To avoid duplication of species due to taxonomic variants, we used the Handbook of the Birds of the World and BirdLife International 2017 (Hoyo et al. 2014) and the IUCN Red List mammal taxonomy (IUCN 2018) as the taxonomic authority for bird and mammal scientific names.
Collecting species’ threat status and ecological data
We used the function rl_search from the package rredlist (Gearty and Chamberlain 2022) to retrieve the IUCN risk status and population trend data for each species. We manually collected information on anthropogenic threats, pet and food trades and their usage in various markets from the IUCN portal.
Assessing extinction risk and population trends of pollinators
To investigate threats to bird and mammal pollinators we categorized species into threatened or non-threatened as per the IUCN categories, where Vulnerable (VU), Endangered (EN) and Critically Endangered (CR) species were considered as threatened, and the remaining categories (Least Concern (LC) and Near Threatened (NT)) non-threatened. We then employed the IUCN ‘consensus approach’ explained below to calculate the proportion of threatened species (PropThreat) (Clausnitzer et al. 2009; Hoffmann et al. 2010; Böhm et al. 2013, Pincheira-Donoso et al. 2021, 2023). As per the formula: PropThreat = (CR + EN + VU)/(N – DD), where N is the total number of species in the sample per category and DD is the number of species in the Data Deficient category.
Additionally, we estimated the proportion of species with decreasing populations as: PropDecr = DecreaseN/(N − UnknownN) where DecreaseN is the number of decreasing species, UnknownN is the number of unknown-trend species, and N is the total number of species in all four population trends (Finn et al. 2023). We accounted for the unknown-trend species by assuming that no unknown-trend species are decreasing [lower margin; PropDecrL = DecreaseN/N] but all unknown-trend species are decreasing [upper margin; PropDecrU = (DecreaseN + UnknownN)/N]. We also estimated the proportion for species with stable and increasing population trends.
Spatial distribution of species richness
We collected occurrence records for all 67 bird and 18 mammal pollinator species identified using Global Biodiversity Information Facility (GBIF.org 2023), iNaturalist web portal (iNaturalist 2023), and India Biodiversity portal (Vattakaven et al. 2016). We additionally collected 136 occurrence records for 17 mammal pollinator species from various published literature sources (see Supplementary Material: pollinator occurrences reference list), as these species were not sufficiently represented in the main databases. Using the R package CoordinateCleaner (Zizka et al. 2019), we cleaned the inaccurate geographic records, such as records in the sea, species outliers, duplicates and invalid coordinates (Table S3).
Using the function convexHull from the package alphahull (Pateiro-Lopez et al. 2022) and spatialEco (Evans and Murphy 2023), we created alphahull polygons for each species of bird and mammal pollinator (Coordinate Reference System: Kalianpur 1975/UTM Zone 44N, EPSG: 24344; length unit in metre). For bird pollinators, we used an alpha-value of 0.1 for the species who had sufficient occurrence data (N = 65 out of 67). For mammals, we used an alpha-value of 0.5 (N = 16 out of 18). We used alpha-values of 2.7 and 0.4 for two bird pollinators (Dicrurus adsimilis and Rubigula melanictera), and an alpha-value of 2 for two mammal pollinators (Platacanthomyus lasiurus and Tophozous longimanus) based on the minimum occurrence points required to create polygons. We removed overlapping polygons of each species by using the function st_union from the sf package (Pebesma 2018), and each cleaned individual polygon was then combined for bird and mammal pollinator groups.
We imported India’s shapefile using the function st_read from the R package sf (Pebesma 2018) and created a 0.1 × 0.1-degree grid for India using the function st_make_grid. Using the function st_intersection from the above package, we intersected the grids with India map boundaries. Using the same function, we imported the species’ shapefiles and spatially joined them with the gridded India map using the function st_join from the sf package (Pebesma 2018). Then we counted the number of overlapping polygons (i.e., species) for each grid cell, resulting in a species richness count. We used the R package tmap (Tennekes 2018) to visualize the gridded species richness patterns. All distribution maps were reported on Kalianpur 1975/UTM Zone 44N, EPSG: 24,344 coordinate reference system. To create the maps of species richness, we organised species into a range of different groups: (i) all species (bird = 67, mammal = 18), (ii) species with decreasing population trends according to the IUCN Red List (bird = 16, mammal = 10), (iii) species classified as threatened by the IUCN Red List (bird = 0, mammal = 5), (iv) species for which population trends are unknown (bird = 9, mammals = 2), and (v) endemic species (bird = 4, mammal = 8).
Additionally, we investigated the hotspots of the proportion of species for which population is decreasing or threatened across India. For this, we transferred the variables of decreasing/threatened shapefile to all species shapefile (0.1-degree gridded shapefile obtained from our species richness mappings) by using the function match from the R software (R Core Team 2020). We then calculated the proportion for each grid cell by dividing the decreasing/threatened richness by all species’ richness using the same software. We used the package tmap (Tennekes 2018) to visualise the patterns.
Estimating variations in spatial distribution of species richness
To investigate spatial variation in spatial patterns between bird and mammal pollinators, we employed the proportions of grid cells with the presence of at least one pollinator species. We compared the proportions of bird and mammal pollinators presence (at least single species in a grid cell) with their total grid cells using a Chi-square test in R (function prop.test).
Spatial correlations between decreasing/threatened species and species with unknown population trend
To evaluate the risk of population decrease/threat in the species for which population trends are unknown, we assessed the spatial association between the richness of species that are decreasing (for birds) and/or threatened (for mammals) and the richness of species with unknown population trends using Tjøstheim's coefficient with the function cor.spatial (nonparametric) from the SpatialPack package (Vallejos et al. 2020). A positive correlation between spatial patterns of species with population decrease/threatened and species with unknown population trend indicates that those poorly evaluated species are likely to be at risk (assuming species experience similar anthropogenic threat). Similarly, using the function cor.test (nonparametric Spearman), we also analysed the latitudinal trend in bird and mammal pollinator richness.
Estimation of species range sizes
To explore extinction risk among Indian bird and mammal pollinators based on their range size, we imported the shapefiles (which we created for the spatial richness mapping) and intersected them across the geographic boundary of India using the function st_intersection from the R package sf (Pebesma 2018). We then calculated EOO (extension of occurrence) as the area (km2) of polygons of each species using the function st_area of the sf package (Pebesma 2018). We considered a species range size as the sum of all polygons of each species.
After estimating range sizes, species were categorised into range size quartiles (see Jetz and Rahbek 2002; Szabo et al. 2009; Geng et al. 2012), using the function quantile in R (R Core Team 2020). We consider the species’ ranges falling in the first quartile (< 25%) as narrow-ranged and species’ ranges falling above this quartile (> 25%) as broad-ranged species (the estimation and categorisation of narrow and broad-ranged species was based on the ranges obtained from data collected in Indian territory only, but the overall range size of species can be distinct from our estimation). As a result, the bird group contains 50 broad and 17 narrow-ranged species, and the mammal group contains 13 broad and 5 narrow-ranged species (25% of quartile range breaks for bird was 26,786.42 km2 and, mammal was 5030.54 km2; Table S4).
Predicting extinction risk
To predict extinction risk based on intrinsic traits in pollinators, we used the phylogenetic generalised least squares regression model (PGLS) from the package caper (Orme et al. 2018) with a phylogenetic variance/covariance matrix to account for the evolutionary non-independency between species (for birds, phylogeny obtained from Jetz et al. (2012) and for mammals from Upham et al. (2019)). We used the IUCN risk status (threatened/not-threatened) and/or population trend (decreasing/not-decreasing) as the dependent variable, and body mass (adult body mass in grams), clutch (number of eggs laid in a single brood), or litter size (number of offspring at a birth; data from Amniote life-history database (Myhrvold et al. 2015), and other research databases: see Table S5) and range size (extension of occurrences in km2) as predictor variables. This approach allows us to predict the probability of being classified as threatened or registering a decreasing population trend. Using the function pgls, we fitted the models of PGLS to the testing data. We used a Pagel’s lambda transformation with λ optimised by maximum likelihood (ML) (Orme et al. 2018), to account for the strength of phylogenetic signal in the evolutionary correlations of pollinator species (Pagel 1999). We analysed the significance of each predictor variable individually and combined after log transforming the predictor variables.
We could not find sufficient data for further variables to analyse the impact on species threat or population trends. Thus, we decided to include the variables that have sufficient data points. We used a sample size of 58 bird species (from a total of N = 67) for population decreasing and 18 (N = 18) and 16 (N = 18) mammal species for threat and population decreasing analyses after removing data deficient species (we treat as data deficient the species that were labelled as “unknown” in other sections of the methods). Using the same model, we further tested the effect of body mass on the food trade of pollinators (based on the species being used in local, national, and international markets). We used a sample size of 67 bird and 18 mammal species for the analyses.
Results
Our final dataset consisted of 67 bird (6 orders, 27 families) and 18 mammal (4 orders, 6 families) species that met our criteria for them to be classified as pollinators in India. These species represented 6% and 4.2% of the total Indian bird and mammal species, respectively. No studies have reported reptiles as pollinators in India.
Macroecological patterns of species richness distribution
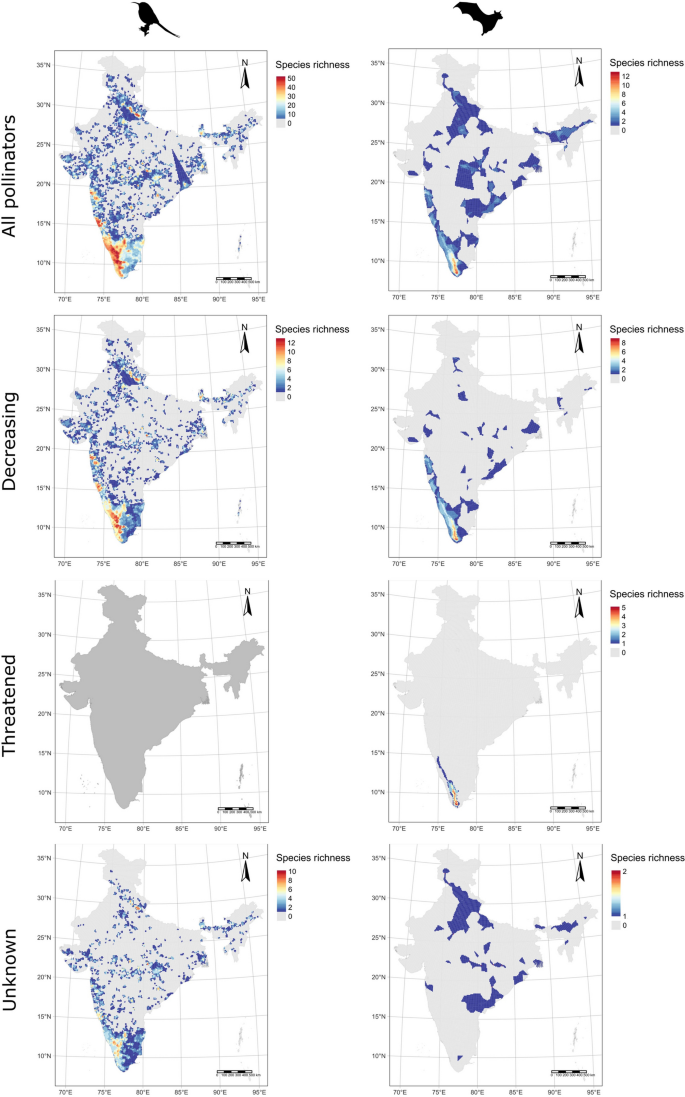
Our results showed that the distribution of bird and mammal pollinators varied spatially across India (Fig. 1a-b). However, bird pollinators have a broader distributional pattern (proportion test: Chi-squared = 1096.1, df = 1, p = 0.001; Table S6) relative to mammals (12,728/33,012 (38.6%) of grid cells in birds; 8,742/33,012 (26.5%) for mammals; Fig. S2). The hotspots of bird pollinators occurred along the Western Ghats and Himalayan biodiversity hotspots of India (Fig. 1a), whereas the hotspots of mammal pollinators largely occurred south of the Western Ghats (Fig. 1b). The lowest species richness of pollinators occurred in many regions of India, including the Indian and Pakistani controlled Kashmir region (Fig. 1a-b). The result also showed a scattered pattern of population decrease of pollinators across India. The hotspots of population decrease for bird pollinators were across the Western Ghats and Himalayan biodiversity hotspots (Fig. 1c). Whereas the hotspots of population decrease and threat of mammal pollinator diversity were in the Western Ghats biodiversity hotspot (Fig. 1d-f). In addition, we found that the proportion of species undergoing population declines and species classified as threatened overlapped with the Himalayan (for birds) and Western Ghats biodiversity hotspots (for mammals), respectively (Fig. S3a, S3b & S3c).
Species richness maps of pollinators for (a, b) all pollinators, (c, d) species with decreasing populations, (e, f) threatened and (g-h) population-unknown species for (a, c, e, g) birds and (b, d, f, h) mammal pollinators of India. No bird pollinators are currently threatened in India (e). The maps were created using a spatial resolution of 0.1-degree (approximately 10 km) longitudinal and latitudinal grids
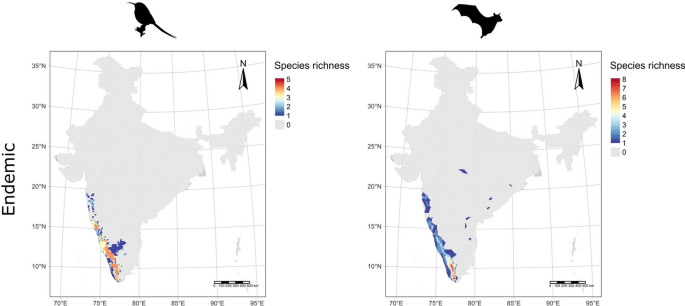
Our dataset showed that 9 bird and 2 mammalian pollinator species do not have population trend data. The distributional patterns of these species showed that the hotspots of bird pollinators occurred across the Western Ghats and Himalayan biodiversity hotspots and certain isolated parts of central India (Fig. 1g). However, the hotspot of mammal pollinators occurred in the Himalayan and eastern parts of India (Fig. 1h). Similarly, the Western Ghats biodiversity hotspot was the hottest hotspot for the Indian endemic bird and mammal pollinator diversity (Fig. 2a-b).
Spatial correlations between decreasing/threatened and species with unknown population trend
The spatial patterns of decreasing and/or threatened species richness were not correlated with those of unknown population trend for bird (Tjøstheim's coefficient = 0.0002 ± 0.00) and mammal pollinators (Tjøstheim's coefficient =—0.065 ± 0.0001). Further, we found a weak decline in all bird (r =—0.315, p = 0.001; Fig. S4a) and mammal (r =—0.183, p = 0.001; Fig. S4b) pollinators richness with increasing latitude.
Extinction risk
Our assessment of the extinction risk of Indian bird and mammal pollinators showed bird pollinators were less threatened than mammal pollinators (Table 1). Among mammal pollinators, the narrow-ranged pollinators were more threatened than the broad-ranged pollinators, however, this was statistically not significant (Table 1).
The species undergoing population decrease followed the same pattern as extinction risk, where many more mammal pollinators (63%) were significantly decreasing compared to bird pollinators (28%; Table 2). Similarly, many broad and narrow-ranged pollinators were decreasing in India. In birds and mammals, the population of narrow-ranged pollinators was more likely to be decreasing relative to broad-ranged pollinators, but for neither group was this statistically significant (Table 2).
Susceptibility to extinction risk as a function of species traits
No model supported a significant effect of any predictor on threat levels or population decrease when taken individually (see Supplementary Material: PGLS models) or combined (Tables S7, S8 & S9).
Extrinsic drivers of extinction risk
In addition to the agriculture expansion and other threats, most Indian mammal pollinators were significantly more threatened by biological resource use such as logging, hunting, poaching, catching and snaring than bird pollinators (Table S10). Our results showed that both bird and mammal pollinators are used in various industries, including food, where both groups were used equally (Table 3). In addition, analyses suggest that the likelihood for a species to be used as a food resource increases with increasing body size in bird and mammal pollinators (Table S11 & S12). We noticed that significantly more bird species were used in national pet markets relative to mammals. In contrast, significantly more mammalian pollinators were used for local medicinal purposes (Table S13).
Further, we identified that mammal pollinators were more widely hunted (Fisher’s Exact Test: odds ratio = 115.96, p = 0.001) across India than bird pollinators, where only a single bird species was hunted in accordance with the IUCN data (see Supplementary Material: Pollinator Dataset). The larger mammal pollinators were highly vulnerable for hunting (R2 = 0.312, F = 7.25, p = 0.02; Table S14).
Discussion
Using a novel dataset, our study presents the first comprehensive analysis of the broad scale patterns of vertebrate pollinator distribution and risk of extinction throughout India. Our analyses reveal that bird pollinators aggregate in geographically large biodiversity hotspots, compared to the smaller extent of mammal pollinator hotspots. We show that only mammal pollinators are currently classified as threatened with extinction in India, but we acknowledge that an important proportion of both bird and mammal pollinators are showing a population decrease. We identified the biodiversity hotspots, especially the Western Ghats biodiversity hotspot, as a centre of richness, declining, and threatened biodiversity for bird and mammal pollinators. Contrary to our initial expectation, our analyses failed to identify a role for species traits as drivers of extinction risk. Instead, most pollinators appear to be threatened by anthropogenic activities, such as hunting and logging.
Macroecological patterns of species richness distribution
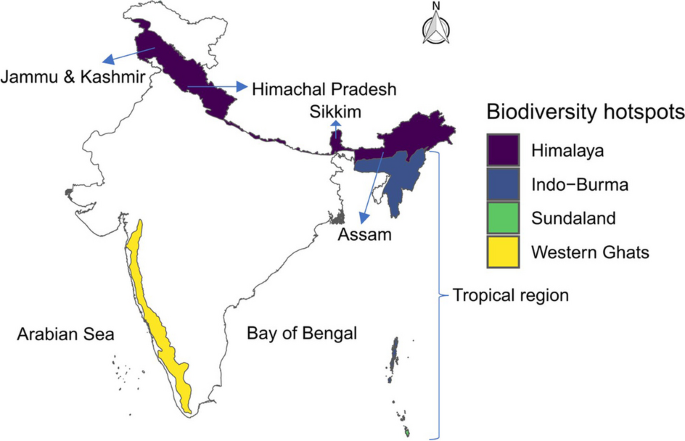
We found that the 67 species of bird pollinators we recorded in India were more widespread than the 18 mammalian pollinator species, with hotspots of species richness across the Western Ghats and Himalayan regions. In contrast, the mammalian pollinator hotspot was only in the southern region of the Western Ghats. Whilst no bird pollinators were threatened with extinction (according to the IUCN), several mammalian pollinators were threatened, and these also predominated in the southern Western Ghats. However, a significant proportion/numbers of species in both groups showed decreasing populations, for the mammals, again in the southern Western Ghats, but for birds, decreasing species peaked across the Western Ghats biodiversity hotspot and in Himachal Pradesh, Sikkim and Assam regions of the Himalayan biodiversity hotspot (Fig. 3). For all pollinators, the regions of India and Pakistan controlled Kashmir have the lowest species richness, but this is most likely due to deficient data from this region for geopolitical reasons (Bhatnagar et al. 2009; Singh et al. 2020).
Hotspots of pollinator richness
The higher species richness of both pollinator groups in the Western Ghats hotspot is potentially due to its tropical climate, a greater topographical variation, a higher diversity of plant species that support the survival of pollinator species by providing higher resource diversity, and its Gondwana origin (Ramesh et al. 1997, Myers et al. 2000, Briggs 2003, Gimaret-Carpentier et al. 2003, Pascal et al. 2004, Steffan-Dewenter et al. 2006, Davidar et al. 2007, Gunawardene et al. 2007, Prasad et al. 2009, Majewska and Altizer 2020, Kral-O’Brien et al. 2021, Wei et al. 2021). A recent global meta-analysis reported that plant dependence on vertebrate pollinators for fruit or seed production was greater in the tropical regions than at higher latitudes and, specifically, the bat-pollinated plants were more dependent on their vertebrate pollinators (Ratto et al. 2018). The higher diversity of mammalian pollinators, especially bats, is likely a result of mutual dependency of plants and their pollinators in this region, because studies found that the birds and bats are effective pollinators in terms of pollen deposition and long-distance pollens dispersal in tropical regions (Stiles 1978). Specifically, many bat pollinators and their flowers occur in tropical forest habitats, therefore, they may be more reliable pollinators for plant species and hence plant fitness in this region (Fleming et al. 2009; Muchhala et al. 2009).
Latitudinal pattern in species’ spatial distribution
Species richness in both bird and mammal pollinators showed a tendency to decline towards higher latitudes. This was consistent with previous findings reported in India for plants (Behera and Roy 2019; Page and Shanker 2020) and, more generally, with the widespread observation species diversity increases towards the tropics, known as the latitudinal diversity gradient (Pianka 1966; Willig et al. 2003; Hillebrand 2004; Mittelbach et al. 2007). It is believed that the tropical regions often provide year-round food supply relative to temperate regions (where plant species experience a period of dormancy due to cold season), hence allowing both specialised and opportunistic pollinators to survive (Cronk and Ojeda 2008).
Spatial distribution of population decreases and threats in pollinators
Besides examining the general patterns of species richness, we also considered where species were most threatened and where populations were decreasing. Our results show that the population sizes of both bird and mammal pollinators are decreasing across India. Approximately 28% (range 24 to 37%) of bird pollinator species, whose populations have been assessed by the IUCN, are decreasing. For mammal pollinators, it is 63% (range 56 to 67%). This peak of bird and mammal pollinator species with decreasing populations in the Himalaya, Indo-Burma and Western Ghats biodiversity hotspots indicates widespread bird and mammal exploitation and anthropogenic activities, including forest transformation to agricultural land in this region (Chatterjee et al. 2006; Kumara and Sinha 2009; Velho et al. 2012; Behera et al. 2014; Wordley et al. 2017; Ghosh-Harihar et al. 2019; Murugan et al. 2020). A high intensity of pollinators’ population decrease will trigger many ecological vulnerabilities in these hotspots as they host exceptional biodiversity (Chatterjee et al. 2006; Joppa et al. 2013; Huang et al. 2017). Globally, several studies have investigated the potential consequences of decreasing pollinator population on plants and ecosystem services. For example, with a meta-analysis using 126 experiments, Ratto et al. (2018) showed that the exclusion of bird pollinators caused, on average, a reduction of 46% in fruit/seed production, but this was 83% for bat pollinators. In the Himalayan biodiversity hotspot, studies showed that birds pollinate 10 Rhododendron (Ericaceae) species (Huang et al. 2017; Basnett et al. 2019), where the exclusion of birds caused decreased seed production in plants (Zhang et al. 2012; Huang et al. 2017), highlighting that the decline of pollinators would have major consequences for ecosystem functioning. However, more research to understand the effect of pollinators' population decrease and the consequences of reduced fruit/seed set on recruitment in future generations of plants is important in India, particularly in the tropical forests where many birds and mammals are actively involved in pollination and seed dispersal, respectively (Davidar 1983; Devy and Davidar 2003; Raju et al. 2005; Raju 2005; Raghuram et al. 2011).
According to the IUCN, the only group of pollinators in India currently facing extinction is mammal pollinators, with a peak hotspot of threat in the Western Ghats, indicating the imperilled status of this region. This threat is mainly caused by habitat loss from high human population densities and associated issues like agricultural expansion and developmental activities (Prasad 1998; Cincotta et al. 2000; Jha et al. 2000; Giriraj et al. 2008; Reddy et al. 2016; Ghosh-Harihar et al. 2019). A high proportion of threatened species (28%) may have severe consequences for the provision of ecosystem services (Dobson et al. 2006; Whelan et al. 2015; Pyšek et al. 2017). For example, In New Zealand, Anderson et al. (2011) found that functional extinction of bird pollinators reduced pollination, seed production and plant density, reducing seed production by 84% and juvenile plants by 55% per adult. Interestingly, the five threatened mammal pollinators (1 bat, 2 monkeys, 2 rodents) are geographically restricted to the Western Ghats region, and some of them have very low reproductive potential, mostly one or two offspring per litter (Ross and Jones 1999; Brunet-Rossinni and Austad 2004; Dhawale and Sinha 2022). Therefore, the reduction in pollinator species can have negative consequences on dependent plant species. Importantly, the geographically restricted species often exhibit distinct traits that may put ecosystems at risk if they are wiped out (Mouillot et al. 2013; Loiseau et al. 2020).
The level of threat in this region is a major biodiversity concern because there is a high specialisation of plant and vertebrate pollinators in this region. Devy and Davidar (2003) found that about 75% of the tree species in this region are specialised to just 2–3 species of pollinators and several plants have a single pollinator species (for example, Helicia nilagirica by Platacanthomys lasiurus, and Syzygium mundagam by Cynopterus sphinx). Such increased specialisation, as we note for many plant species (for example, 31 plant species with a single pollinator species; see Tables S15 & S16; Fig. S5), raises the risk of extinction, and higher rates of specialised pollinator loss will have a cascading effect on dependent species (Dunn et al. 2009), because many plants have lost the ability to pollinate themselves and are entirely dependent on these pollinators to thrive (Cox and Elmqvist 2000). A recent simulation study from Indian Himalayan biodiversity hotspot suggested that the plant-pollinator network collapses faster when the most connected pollinators are removed, rather than the most connected plants (Rather et al. 2023), emphasising the importance of pollinators for plants survival. We encourage more research to understand this trend across India at various spatial–temporal levels.
Spatial correlations between decreasing/threatened and species with unknown population trend
We did not find a spatial correlation between the decreasing/threatened and unknown population trend of bird and mammal pollinators, suggesting less likelihood of population decrease/threat in their ranges. However, these relationships are likely to change as the scale of analysis changes (Keil et al. 2011; Viladomat et al. 2014).
Drivers of extinction risk
Our result did not support the fact that body mass, clutch, litter, or range size predicted threat and decline of species, in contrast to what has been found previously (Cardillo 2003; Cardillo et al. 2005; Hutchings et al. 2012; Comeros-Raynal et al. 2016; Ripple et al. 2017). However, biological resource use (e.g., logging, hunting) was the biggest threat for both birds and mammals, with agriculture also being an important threat for mammals. In terms of trade, most mammals were used as food, whilst birds were mostly collected as pets. It is therefore likely to be the level of human impact that bird and mammal pollinators are exposed to that makes them vulnerable independently from other intrinsic characteristics of the species (Purvis et al. 2000; Cardillo et al. 2005). This suggests strict policies on hunting and land use should be implemented and practiced in hotspot regions.
Conclusion
Our study on the pattern of distribution and extinction risk of Indian pollinators brings unique information on this group. Both bird and mammal pollinators show different biodiversity patterns across India with hotspots of species richness overlapping the Himalayan (for birds) and Western Ghats (for birds and mammals) biodiversity hotspots. The decline and threat of pollinators across these regions show the impact of anthropogenic disturbances. Our assessment using the IUCN data shows that 1 in 4 mammal pollinators is currently threatened with extinction in India, but the population trend shows that 1 in 4 bird and 1 in 2 mammal pollinators are currently decreasing. However, the insufficient population data for some species is a conservation concern and future research should be focused on them. We failed to find a relationship between species traits and threat/decrease. Instead, we showed that human activities threaten vertebrate pollinators mostly through habitat loss and hunting. We encourage more research to evaluate the relationship between these traits and threats with a large species pool. We believe that community education and marketing campaigns are essential along with the policy on hunting and land use to mitigate the extinction of vertebrate pollinators in India.
Data availability
The datasets generated during the current study can be accessed in the Zenodo repository: https://doi.org/10.5281/zenodo.10926527.
References
Ali S (1931) The role of sunbirds and flowerpeckers in the propagation and distribution of the tree-parasite, Loranthus longiflorus Dest., in the Konkan (W. India). J Bombay Nat Hist Soc 35:144–149
Anderson SH (2003) The relative importance of birds and insects as pollinators of the New Zealand flora. N Z J Ecol 27:83–94
Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J (2011) Cascading effects of bird functional extinction reduce pollination and plant density. Science 331:1068–1071
Atluri J, Rao SP, Reddi CS (2000) Pollination ecology of Helicteres isora Linn. (Sterculiaceae). Curr Sci 78:713–718
Aziz SA, Clements GR, McConkey KR, Sritongchuay T, Pathil S, Abu Yazid MNH, Campos-Arceiz A, Forget PM, Bumrungsri S (2017) Pollination by the locally endangered island flying fox (Pteropus hypomelanus) enhances fruit production of the economically important durian (Durio zibethinus). Ecol Evol 7:8670–8684
Basnett S, Ganesan R, Devy SM (2019) Floral traits determine pollinator visitation in Rhododendron species across an elevation gradient in the Sikkim Himalaya. Alp Bot 129:81–94
Behera M, Patidar N, Chitale V, Behera N, Gupta D, Matin S, Tare V, Panda S, Sen D (2014) Increase in agricultural patch contiguity over the past three decades in Ganga River Basin, India. Curr Sci 107:502–511
Behera MD, Roy PS (2019) Pattern of distribution of angiosperm plant richness along latitudinal and longitudinal gradients of India. Biodivers Conserv 28:2035–2048
Bernardello G, Anderson GJ, Stuessy TF, Crawford DJ (2006) The angiosperm flora of the Archipelago Juan Fernandez (Chile): origin and dispersal. Botany 84:1266–1281
Bhatnagar YV, Ahmad R, Kyarong SS, Ranjitsinh M, Seth C, Lone IA, Easa P, Kaul R, Raghunath R (2009) Endangered markhor Capra falconeri in India: through war and insurgency. Oryx 43:407–411
Biesmeijer JC, Roberts SP, Reemer M, Ohlemuller R, Edwards M, Peeters T, Schaffers A, Potts SG, Kleukers R, Thomas C (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354
Böhm M, Collen B, Baillie JE, Bowles P, Chanson J, Cox N, Hammerson G, Hoffmann M, Livingstone SR, Ram M (2013) The conservation status of the world’s reptiles. Biol Cons 157:372–385
Briggs JC (2003) The biogeographic and tectonic history of India. J Biogeogr 30:381–388
Brunet-Rossinni AK, Austad SN (2004) Ageing studies on bats: a review. Biogerontology 5:211–222
Bumrungsri S, Harbit A, Benzie C, Carmouche K, Sridith K, Racey P (2008) The pollination ecology of two species of Parkia (Mimosaceae) in southern Thailand. J Trop Ecol 24:467–475
Cardillo M (2003) Biological determinants of extinction risk: why are smaller species less vulnerable? In animal conservation forum, vol 6. Cambridge University Press, pp 63–69
Cardillo M, Mace GM, Jones KE, Bielby J, Bininda-Emonds OR, Sechrest W, Orme CDL, Purvis A (2005) Multiple causes of high extinction risk in large mammal species. Science 309:1239–1241
Carvalheiro LG, Kunin WE, Keil P, Aguirre-Gutiérrez J, Ellis WN, Fox R, Groom Q, Hennekens S, Van Landuyt W, Maes D (2013) Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol Lett 16:870–878
Chatterjee S, Saikia A, Dutta P, Ghosh D, Pangging G, Goswami AK (2006) Biodiversity significance of North east India for the study n natural resources, water and environment nexus or development and growth in northeastern India (Background Paper No: 13). WWF-India, Technical report, New Delhi, pp 1–71
Chaudhary O, Chand R (2017) Economic benefits of animal pollination to Indian agriculture. Indian J Agric Sci 87:1117–1138
Chichorro F, Juslén A, Cardoso P (2019) A review of the relation between species traits and extinction risk. Biol Cons 237:220–229
Cincotta RP, Wisnewski J, Engelman R (2000) Human population in the biodiversity hotspots. Nature 404:990–992
Clausnitzer V, Kalkman VJ, Ram M, Collen B, Baillie JE, Bedjanič M, Darwall WR, Dijkstra K-DB, Dow R, Hawking J (2009) Odonata enter the biodiversity crisis debate: the first global assessment of an insect group. Biol Cons 142:1864–1869
Comeros-Raynal MT, Polidoro BA, Broatch J, Mann BQ, Gorman C, Buxton CD, Goodpaster AM, Iwatsuki Y, MacDonald TC, Pollard D (2016) Key predictors of extinction risk in sea breams and porgies (Family: Sparidae). Biol Cons 202:88–98
Cox PA, Elmqvist T (2000) Pollinator extinction in the Pacific Islands. Conserv Biol 14:1237–1239
Cronk Q, Ojeda I (2008) Bird-pollinated flowers in an evolutionary and molecular context. J Exp Bot 59:715–727
Davidar P (1983) Similarity between flowers and fruits in some flowerpecker pollinated mistletoes. Biotropica 15:32–37
Davidar P (1985) Ecological interactions between mistletoes and their avian pollinators in south India. J Bombay Nat Hist Soc 82:45–60
Davidar P, Arjunan M, Mammen PC, Garrigues J, Puyravaud JP, Roessingh K (2007) Forest degradation in the Western Ghats biodiversity hotspot: resource collection, livelihood concerns and sustainability. Curr Sci 93:1573–1578
Davidson AD, Hamilton MJ, Boyer AG, Brown JH, Ceballos G (2009) Multiple ecological pathways to extinction in mammals. Proc Natl Acad Sci 106:10702-10705
Devy MS, Davidar P (2003) Pollination systems of trees in Kakachi, a mid-elevation wet evergreen forest in Western Ghats, India. Am J Bot 90:650–657
Dhawale AK, Sinha A (2022) Gemini calling! First occurrence of successful twinning in wild, endangered lion-tailed macaques Macaca silenus in the Anamalai hills of the Western Ghats, India. bioRxiv:2022.2008.2003.502728. https://doi.org/10.1101/2022.08.03.502728
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B (2014) Defaunation in the Anthropocene. Science 345:401–406
Dobson A, Lodge D, Alder J, Cumming GS, Keymer J, McGlade J, Mooney H, Rusak JA, Sala O, Wolters V (2006) Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87:1915–1924
Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS (2009) The sixth mass coextinction: are most endangered species parasites and mutualists? Proc R Soc B: Biol Sci 276:3037-3045
Erancheri P, Sunojkumar P, Dilsha Das M (2013) Bat pollination in medicinally important Cochlospermum religiosum. Ann Plant Sci 2:292–295
Evans JS, Murphy M (2023) spatialEco. R package version 2.0–2. https://github.com/jeffreyevans/spatialEco. Accessed Dec 2023
Finn C, Grattarola F, Pincheira-Donoso D (2023) More losers than winners: investigating Anthropocene defaunation through the diversity of population trends. Biol Rev 98:1732–1748
Fleming TH, Geiselman C, Kress WJ (2009) The evolution of bat pollination: a phylogenetic perspective. Ann Bot 104:1017–1043
Fleming TH, Muchhala N (2008) Nectar-feeding bird and bat niches in two worlds: pantropical comparisons of vertebrate pollination systems. J Biogeogr 35:764–780
Gaston KJ, Fuller RA (2009) The sizes of species’ geographic ranges. J Appl Ecol 46:1–9
GBIF.org (2023) GBIF occurrence download https://doi.org/10.15468/dd.3grghf
Gearty W, Chamberlain S (2022) rredlist:‘IUCN’Red List client. R package version 0.7. https://CRAN.R-project.org/package=rredlist, https://github.com/ropensci/rredlist (devel), https://docs.ropensci.org/rredlist (docs)
Geng Y, Wang Z, Liang C, Fang J, Baumann F, Kühn P, Scholten T, He JS (2012) Effect of geographical range size on plant functional traits and the relationships between plant, soil and climate in Chinese grasslands. Glob Ecol Biogeogr 21:416–427
Ghosh-Harihar M, An R, Athreya R, Borthakur U, Chanchani P, Chetry D, Datta A, Harihar A, Karanth KK, Mariyam D (2019) Protected areas and biodiversity conservation in India. Biol Cons 237:114–124
Gimaret-Carpentier C, Dray S, Pascal JP (2003) Broad-scale biodiversity pattern of the endemic tree flora of the Western Ghats (India) using canonical correlation analysis of herbarium records. Ecography 26:429–444
Giriraj A, Irfan-Ullah M, Murthy MSR, Beierkuhnlein C (2008) Modelling spatial and temporal forest cover change patterns (1973–2020): A case study from South Western Ghats (India). Sensors 8:6132–6153
Goraya G, Ved D (2017) Medicinal plants in India: an assessment of their demand and supply. National Medicinal Plants Board, Ministry of AYUSH, Government of India, New Delhi and Indian Council of Forestry Research and Education, Dehradun. ISBN 978-81-211-0628-3
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957
Gunawardene NR, Daniels A, Gunatilleke I, Gunatilleke C, Karunakaran P, Nayak KG, Prasad S, Puyravaud P, Ramesh B, Subramanian K (2007) A brief overview of the Western Ghats-Sri Lanka biodiversity hotspot. Curr Sci 00113891:93
Hillebrand H (2004) On the generality of the latitudinal diversity gradient. Am Nat 163:192–211
Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SH, Carpenter KE, Chanson J, Collen B, Cox NA (2010) The impact of conservation on the status of the world’s vertebrates. Science 330:1503–1509
Hoyo JD, Collar NJ, Christie DA, Elliott A, Fishpool LD, Allen R (2014) Handbook of the birds of the world and birdlife international illustrated checklist of the birds of the world. Lynx Editions BirdLife International, Barcelona, Spain and Cambridge, UK
Huang Z-H, Song Y-P, Huang S-Q (2017) Evidence for passerine bird pollination in Rhododendron species. AoB plants 9:plx062
Hutchings JA, Myers RA, García VB, Lucifora LO, Kuparinen A (2012) Life-history correlates of extinction risk and recovery potential. Ecol Appl 22:1061–1067
iNaturalist (2023) Available from https://www.inaturalist.org. Accessed December 2023
IUCN (2018) The IUCN red list of threatened species. Version 2018‐1. International union for conservation of nature and natural resources gland. Available from https://www.iucnredlist.org/. Accessed Aug 2022
Jetz W, Rahbek C (2002) Geographic range size and determinants of avian species richness. Science 297:1548–1551
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 491:444–448
Jha C, Dutt C, Bawa K (2000) Deforestation and land use changes in Western Ghats, India. Curr Sci 79:231–238
Joppa L, Visconti P, Jenkins C, Pimm S (2013) Achieving the convention on biological diversity’s goals for plant conservation. Science 341:1100–1103
Kato M, Kawakita A (2004) Plant-pollinator interactions in New Caledonia influenced by introduced honey bees. Am J Bot 91:1814–1827
Kattan GH (1992) Rarity and vulnerability: the birds of the Cordillera Central of Colombia. Conserv Biol 6:64–70
Keil P, Biesmeijer JC, Barendregt A, Reemer M, Kunin WE (2011) Biodiversity change is scale-dependent: an example from Dutch and UK hoverflies (Diptera, Syrphidae). Ecography 34:392–401
Khanduri VP, Kumar KS (2017) Reproductive effort and success in Bombax ceiba L. in a tropical forest of Mizoram, Indo-Burma region of North-East India. Braz J Bot 40:157–166
Kleijn D, Raemakers I (2008) A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology 89:1811–1823
Kral-O’Brien KC, O’Brien PL, Hovick TJ, Harmon JP (2021) Meta-analysis: Higher plant richness supports higher pollinator richness across many land use types. Ann Entomol Soc Am 114:267–275
Kumara HN, Sinha A (2009) Decline of the endangered lion-tailed macaque Macaca silenus in the Western Ghats, India. Oryx 43:292–298
Law BS, Lean M (1999) Common blossom bats (Syconycteris australis) as pollinators in fragmented Australian tropical rainforest. Biol Cons 91:201–212
Loiseau N, Mouquet N, Casajus N, Grenié M, Guéguen M, Maitner B, Mouillot D, Ostling A, Renaud J, Tucker C (2020) Global distribution and conservation status of ecologically rare mammal and bird species. Nat Commun 11:5071
Maas B, Clough Y, Tscharntke T (2013) Bats and birds increase crop yield in tropical agroforestry landscapes. Ecol Lett 16:1480–1487
Macdonald E, Burnham D, Hinks A, Dickman A, Malhi Y, Macdonald D (2015) Conservation inequality and the charismatic cat: Felis felicis. Glob Ecol Conserv 3:851–866
Majewska AA, Altizer S (2020) Planting gardens to support insect pollinators. Conserv Biol 34:15–25
Manne LL, Brooks TM, Pimm SL (1999) Relative risk of extinction of passerine birds on continents and islands. Nature 399:258–261
McCann C (1931) On the fertilisation of the flowers of the sausage tree (Kigelia pinnata) by bats. J Bombay Nat Hist Soc 35:467–471
McCann C (1933) The flying fox (P. giganteus) and the palm squirrel (F. tristiatus) as agents of pollinization in (Grevillea robusta A. Cunn.) the silky oak. J Bombay Nat Hist Soc 36:761–764
Mittelbach GG, Schemske DW, Cornell HV, Allen AP, Brown JM, Bush MB, Harrison SP, Hurlbert AH, Knowlton N, Lessios HA (2007) Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol Lett 10:315–331
Mouillot D, Bellwood DR, Baraloto C, Chave J, Galzin R, Harmelin-Vivien M, Kulbicki M, Lavergne S, Lavorel S, Mouquet N (2013) Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol 11:e1001569
Muchhala N, Caiza A, Vizuete JC, Thomson JD (2009) A generalized pollination system in the tropics: bats, birds and Aphelandra acanthus. Ann Bot 103:1481–1487
Murugan CM, Bhavan PS, Mahandran V, Nathan PT (2020) Hunting bats for bushmeat despite Nipah concerns in Idukki, Kerala, India. Ecotropica 22:202006–202006
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Myhrvold NP, Baldridge E, Chan B, Sivam D, Freeman DL, Ernest SM (2015) An amniote life-history database to perform comparative analyses with birds, mammals, and reptiles: Ecological Archives E096–269. Ecology 96:3109–3109
Nathan PT, Raghuram H, Elangovan V, Karuppudurai T, Marimuthu G (2005) Bat pollination of kapok tree, Ceiba pentandra. Curr Sci 88:1679–1681
National Research Council (2007) Status of pollinators in North America. Status Pollinators North Am: Natl Acad Press 10:11761
Ollerton J, Erenler H, Edwards M, Crockett R (2014) Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346:1360–1362
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W (2018) The caper package: comparative analysis of phylogenetics and evolution in R. R package version 1.0.1. 5:1-36. https://CRAN.R-project.org/package=caper. Accessed Jan 2024
Page NV, Shanker K (2020) Climatic stability drives latitudinal trends in range size and richness of woody plants in the Western Ghats, India. Plos One 15:e0235733
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Pascal J, Ramesh B, Franceschi DD (2004) Wet evergreen forest types of the southern Western Ghats, India. Trop Ecol 45:281–292
Pateiro-Lopez B, Rodriguez-Casal A, Pateiro-Lopez MB (2022) alphahull: Generalization of the Convex Hull of a Sample of Points in the Plane. R package version 2.5. https://CRAN.R-project.org/package=alphahull. Accessed Jan 2023
Pebesma E (2018) Simple features for R [R package sf version 0.6–0]. https://CRAN.R-project.org/package=sf. Accessed Oct 2022
Pianka ER (1966) Latitudinal gradients in species diversity: a review of concepts. Am Nat 100:33–46
Pincheira-Donoso D, Hodgson DJ (2018) No evidence that extinction risk increases in the largest and smallest vertebrates. Proc Natl Acad Sci 115:E5845-E5846
Pincheira-Donoso D, Harvey LP, Cotter SC, Stark G, Meiri S, Hodgson DJ (2021) The global macroecology of brood size in amphibians reveals a predisposition of low-fecundity species to extinction. Glob Ecol Biogeogr 30:1299–1310
Pincheira-Donoso D, Harvey LP, Johnson JV, Hudson D, Finn C, Goodyear LE, Guirguis J, Hyland EM, Hodgson DJ (2023) Genome size does not influence extinction risk in the world’s amphibians. Funct Ecol 37:190–200
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353
Powney GD, Carvell C, Edwards M, Morris RK, Roy HE, Woodcock BA, Isaac NJ (2019) Widespread losses of pollinating insects in Britain. Nat Commun 10:1018
Prasad SN (1998) Conservation planning for the Western Ghats of Kerala: II. Assessment of habitat loss and degradation. Curr Sci 75:228–235
Prasad V, Farooqui A, Tripathi S, Garg R, Thakur B (2009) Evidence of late Palaeocene-early Eocene equatorial rain forest refugia in southern Western Ghats, India. J Biosci 34:777–797
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Harper Collins Publishers, New York, NY
Purvis A, Gittleman JL, Cowlishaw G, Mace GM (2000) Predicting extinction risk in declining species. Proc R Soc London Ser B: Biol Sci 267:1947–1952
Pyšek P, Blackburn TM, García-Berthou E, Perglová I, Rabitsch W (2017) Displacement and local extinction of native and endemic species. Impact Biol Invasions Ecosyst Serv 12:157–175
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed Aug 2022
Raghuram H, Singaravelan N, Nathan PT, Emmanuvel K, Rajan K, Marimuthu G (2011) Foraging ecology of pteropodid bats: pollination and seed dispersal. Zupan JL, and Mlakar (Eds.), SL, Bats: biology, behavior and conservation. Nova Science Publishers Inc, New York, NY
Raju AJ, Rao SP, Rangaiah K (2005) Pollination by bats and birds in the obligate outcrosser Bombax ceiba L. (Bombacaceae), a tropical dry season flowering tree species in the Eastern Ghats forests of India. Ornithol Sci 4:81–87
Raju AJS (2005) Passerine bird pollination and seed dispersal in Woodfordia floribunda Salisb. (Lythraceae), a common low altitude woody shrub in the Eastern Ghats forests of India. Ornithol Sci 4:103–108
Raju AJS, Lakshmi PV, Ramana KV (2013) Entomophily, ornithophily and anemochory in the highly self-incompatible Boswellia ovalifoliolata Bal. & Henry (Burseraceae), an endemic and endangered medicinal tree species. Biol Sci-PJSIR 56:1–10
Ramesh B, Menon S, Bawa KS (1997) A vegetation based approach to biodiversity gap analysis in the Agastyamalai region, Western Ghats, India. Ambio 26:529–536
Rather ZA, Ollerton J, Parey SH, Ara S, Watts S, Paray MA, Khuroo AA (2023) Plant-pollinator meta-network of the Kashmir Himalaya: Structure, modularity, integration of alien species and extinction simulation. Flora 298:152197
Ratto F, Simmons BI, Spake R, Zamora-Gutierrez V, MacDonald MA, Merriman JC, Tremlett CJ, Poppy GM, Peh KSH, Dicks LV (2018) Global importance of vertebrate pollinators for plant reproductive success: a meta-analysis. Front Ecol Environ 16:82–90
Reddy CS, Jha CS, Dadhwal VK (2016) Assessment and monitoring of long-term forest cover changes (1920–2013) in Western Ghats biodiversity hotspot. J Earth Syst Sci 125:103–114
Regan EC, Santini L, Ingwall-King L, Hoffmann M, Rondinini C, Symes A, Taylor J, Butchart SH (2015) Global trends in the status of bird and mammal pollinators. Conserv Lett 8:397–403
Ripple WJ, Chapron G, López-Bao JV, Durant SM, Macdonald DW, Lindsey PA, Bennett EL, Beschta RL, Bruskotter JT, Campos-Arceiz A (2016) Saving the world’s terrestrial megafauna. Bioscience 66:807–812
Ripple WJ, Wolf C, Newsome TM, Hoffmann M, Wirsing AJ, McCauley DJ (2017) Extinction risk is most acute for the world’s largest and smallest vertebrates. Proc Natl Acad Sci 114:10678-10683
Ross C, Jones KE (1999) Socioecology and the evolution of primate reproductive rates. Comp Primate Socioecol 22:73–110
Roulston TH, Goodell K (2011) The role of resources and risks in regulating wild bee populations. Ann Rev Entomol 56:293–312
Schemske DW, Horvitz CC (1984) Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science 225:519–521
Scheper J, Reemer M, van Kats R, Ozinga WA, van der Linden GT, Schaminée JH, Siepel H, Kleijn D (2014) Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc Natl Acad Sci 111:17552-17557
Shuai L, Chen C, Liu W, Xu W, Wang Y, Zeng Z, Zhang Z, Zhao L, Wang Y (2021) Ecological correlates of extinction risk in Chinese terrestrial mammals. Divers Distrib 27:1294–1307
Singh PB, Mainali K, Jiang Z, Thapa A, Subedi N, Awan MN, Ilyas O, Luitel H, Zhou Z, Hu H (2020) Projected distribution and climate refugia of endangered Kashmir musk deer Moschus cupreus in greater Himalaya, South Asia. Sci Rep 10:1511
Singh T (1929) A preliminary note on the pollination of the coral tree (Erythrina indica Lamk.). J Bombay Nat Hist Soc 33:460–462
Sinu PA, Kuriakose G, Shivanna K (2011) Is the bumblebee (Bombus haemorrhoidalis) the only pollinator of large cardamom in central Himalayas, India? Apidologie 42:690–695
Smith RJ, Veríssimo D, Isaac NJ, Jones KE (2012) Identifying Cinderella species: uncovering mammals with conservation flagship appeal. Conserv Lett 5:205–212
Sritongchuay T, Hughes AC, Bumrungsri S (2019) The role of bats in pollination networks is influenced by landscape structure. Glob Ecol Conserv 20:e00702
Srivathsa A, Majgaonkar I, Sharma S, Singh P, Punjabi GA, Chawla MM, Banerjee A (2020) Opportunities for prioritizing and expanding conservation enterprise in India using a guild of carnivores as flagships. Environ Res Lett 15:064009
Steffan-Dewenter I, Klein AM, Gaebele V, Alfert T, Tscharntke T (2006) Bee diversity and plant-pollinator interactions in fragmented landscapes. In: Waser NM, Ollerton J (eds) Specialization and generalization in plant-pollinator interactions. University of Chicago Press
Stiles FG (1978) Ecological and evolutionary implications of bird pollination. Am Zool 18:715–727
Stork NE (2010) Re-assessing current extinction rates. Biodivers Conserv 19:357–371
Szabo ND, Algar AC, Kerr JT (2009) Reconciling topographic and climatic effects on widespread and range-restricted species richness. Glob Ecol Biogeogr 18:735–744
Tandon R, Shivanna K, Mohan Ram H (2003) Reproductive biology of Butea monosperma (Fabaceae). Ann Bot 92:715–723
Tennekes M (2018) tmap: thematic maps in R. J Stat Softw 84:1–39
Tremlett CJ, Moore M, Chapman MA, Zamora-Gutierrez V, Peh KSH (2020) Pollination by bats enhances both quality and yield of a major cash crop in Mexico. J Appl Ecol 57:450–459
Treves A, Karanth KU (2003) Human-carnivore conflict and perspectives on carnivore management worldwide. Conserv Biol 17:1491–1499
Upham NS, Esselstyn JA, Jetz W (2019) Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol 17:e3000494
Urban MC (2015) Accelerating extinction risk from climate change. Science 348:571–573
Vanbergen AJ, Initiative TIP (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ 11:251–259
vanEngelsdorp D, Hayes J Jr, Underwood RM, Pettis JS (2010) A survey of honey bee colony losses in the United States, fall 2008 to spring 2009. J Apic Res 49:7–14
Vattakaven T, George RM, Balasubramanian D, Réjou-Méchain M, Muthusankar G, Ramesh BR, Prabhakar R (2016) India Biodiversity Portal: An integrated, interactive and participatory biodiversity informatics platform. Biodivers Data J 4:e10279. https://doi.org/10.3897/BDJ.3894.e10279
Velho N, Karanth KK, Laurance WF (2012) Hunting: A serious and understudied threat in India, a globally significant conservation region. Biol Cons 148:210–215
Vallejos R, Osorio F, Bevilacqua M (2020) Spatial relationships between two georeferenced variables: with applications in R. Springer Nature, Berlin/Heidelberg, Germany. https://doi.org/10.1007/978-3-030-56681-4
Venkataraman K, Sivaperuman C (2018) Biodiversity hotspots in India. Indian Hotspots: Vertebr Faunal Divers Conserv Manag 1:1–27
Verde Arregoitia LD (2016) Biases, gaps, and opportunities in mammalian extinction risk research. Mammal Rev 46:17–29
Viladomat J, Mazumder R, McInturff A, McCauley DJ, Hastie T (2014) Assessing the significance of global and local correlations under spatial autocorrelation: a nonparametric approach. Biometrics 70:409–418
Wei N, Kaczorowski RL, Arceo-Gómez G, O’Neill EM, Hayes RA, Ashman T-L (2021) Pollinators contribute to the maintenance of flowering plant diversity. Nature 597:688–692
Whelan CJ, Şekercioğlu ÇH, Wenny DG (2015) Why birds matter: from economic ornithology to ecosystem services. J Ornithol 156:227–238
Willig MR, Kaufman DM, Stevens RD (2003) Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu Rev Ecol Evol Syst 34:273–309
Wordley CF, Sankaran M, Mudappa D, Altringham JD (2017) Bats in the Ghats: Agricultural intensification reduces functional diversity and increases trait filtering in a biodiversity hotspot in India. Biol Cons 210:48–55
Yumoto T (2000) Bird-pollination of three Durio species (Bombacaceae) in a tropical rainforest in Sarawak, Malaysia. Am J Bot 87:1181–1188
Zhang FP, Cai XH, Wang H, Ren ZX, Larson-Rabin Z, Li DZ (2012) Dark purple nectar as a foraging signal in a bird-pollinated Himalayan plant. New Phytol 193:188–195
Zizka A, Silvestro D, Andermann T, Azevedo J, Duarte Ritter C, Edler D, Farooq H, Herdean A, Ariza M, Scharn R (2019) CoordinateCleaner: standardized cleaning of occurrence records from biological collection databases. Methods Ecol Evol 10:744–751
ZSI (2017) Animal discoveries of 2016. New species and new records. Zoological Survey of India, Kolkata. Director, Zoological Survey of India (eds): Survey of India, pp 1–95
Acknowledgements
We thank those who have conducted research in pollination biology for making data openly available for data extraction. We express our sincere gratitude to the Ministry of Social Justice and Empowerment, Government of India for financial support through National Overseas Scholarship for this study awarded to RK. We extend our thanks to the University of Lincoln for facilities and technical support for the completion of this study. We also thank the associate editor and the anonymous referee for the constructive comments to improve our manuscript.
Funding
This work was supported by the Ministry of Social Justice and Empowerment, Government of India. Author Ratheesh Kallivalappil has received research support through the National Overseas Scholarship for PhD education.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature search and data collection were performed by Ratheesh Kallivalappil. The analysis was performed by Ratheesh Kallivalappil, Florencia Grattarola, and Sheena C. Cotter. The first draft of the manuscript was written by Ratheesh Kallivalappil, Florencia Grattarola, Dilkushi de Alwis Pitts, Sheena C. Cotter and Daniel Pincheira-Donoso and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Jens Dauber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kallivalappil, R., Grattarola, F., de Alwis Pitts, D. et al. Species diversity and extinction risk of vertebrate pollinators in India. Biodivers Conserv 33, 2109–2130 (2024). https://doi.org/10.1007/s10531-024-02848-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-024-02848-3