Abstract
Conserving large mammals on small islands poses a great challenge, given their high resource demand within the limited space available. The endangered Togean Islands babirusa (Babyrousa togeanensis) is one of these species, with a distribution range limited to four small islands in the Togean Archipelago, Indonesia. Despite being listed as endangered, very little information is available on the distribution and ecology of this species. To address this critical knowledge gap, we here report the first field-based ecological study of the Togean Islands babirusa across its entire distribution range. Following a stratified random sampling procedure, we distributed camera traps at 103 stations across four islands to collect data on the species distribution from July-October 2022. We performed an occupancy modeling analysis to assess the species’ habitat use, with various habitat features estimated through remote sensing and field measurements as covariates. We found that forest and mangrove availability over a large area positively influenced babirusa habitat selection. Babirusas only made use of agricultural areas when large forest areas were available nearby. Our results highlight the benefits of redesigning the national park area to accommodate babirusa habitat requirements, specifically by reassigning the non-forested park areas (about 30% of the park area) to non-protected forests currently outside the park boundary (about 50% of total forested area). Our case study exemplifies key challenges associated with conserving large mammals on small islands and highlights the importance of following an adaptive management approach, which in this case implies shifting 30% of the current protected area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Indonesian island of Sulawesi, along with its satellite islands, is globally recognized for its high level of endemism, supporting six large mammal species listed as threatened by the IUCN (Myers et al. 2000; Michaux 2010; Broto and Mortelliti 2019). Conserving large mammals, particularly on small islands such as the Sulawesi’s satellites, presents a significant challenge: large species require large areas to fulfill their resource demands (Haskell et al. 2002). The challenge is further exacerbated by the high density of humans residing in these small islands, which creates human-wildlife conflicts due to limited space (Calado et al. 2014). Identifying their critical habitat needs is vital to the preservation of these species, especially given limited conservation funds and limited space (Ceballos et al. 2005).
One such species is the Togean Islands babirusa (Babyrousa togeanensis, order Artiodactyla, family Suidae; hereafter, Togean babirusa), an endangered species endemic to the Togean Archipelago, Central Sulawesi, with a distribution range of less than 600 km² (Macdonald et al. 2016). Despite being protected and listed as Appendix-1 CITES, only one research article is available on the species, predominantly based on interview surveys (Akbar et al. 2007; Broto and Mortelliti 2019). Consequently, descriptions of its ecology and behavior are mostly speculations based on other babirusa species (Meijaard and Groves 2002; Ito and Melletti 2017). Being confined to small isolated islands, this species faces multiple threats, including inbreeding depression, hunting, and habitat loss (Macdonald et al. 2016). Given the limited information available on this species, developing an effective conservation strategy for its survival is a daunting task. Our goal here is to address this critical knowledge gap by identifying key factors affecting the distribution and occupancy of this species.
The previous study conducted on this species (Akbar et al. 2007) suggests that the Togean babirusa is often seen in agricultural and coastal regions, indicating a potential difference in habitat preference compared to other babirusa species (Macdonald 2017; Sheherazade et al. 2017). However, it is unclear whether the species is more abundant or more frequently observed there (Ito and Melletti 2017). Babirusas can weigh over 100 kg, which suggests they may have a large home range size, perhaps allowing them to adapt to fragmented landscapes by using extensive areas (Saunders and McLeod 1999; Macdonald 2017; Pfeifer et al. 2017). This implies the Togean babirusa may be able to tolerate habitat modification by foraging in agricultural areas. However, the babirusa’s crop-raiding behavior has led locals to view them as pests and kill them in retaliation (Akbar et al. 2007). As animals choose habitats that offer the greatest benefits while minimizing associated costs (Mayor et al. 2009), the Togean babirusa’s assumed preference for agricultural areas needs further investigation due to the higher potential for conflict with people in these areas. To effectively manage the species, the degree to which the Togean babirusa can adapt to fragmented landscapes needs to be quantified.
The presence of the Togean Islands National Park in the archipelago is expected to safeguard the babirusa population, which is one of Indonesia’s conservation priority species (Regulation of Ministry of Forestry Indonesia No: P.55/Menhut-II/2013). However, the extent to which the designated national park adequately protects the babirusa is unknown. During its establishment, the national park’s design primarily focused on marine resources, with limited information on terrestrial biodiversity (Surjadi and Supriatna 1998; Indrawan et al. 2014). Although commercial logging ceased in 1996, the archipelago is still experiencing deforestation for small-scale agriculture, a significant cause of deforestation in Indonesia (Surjadi and Supriatna 1998; Austin et al. 2019). The continuing loss of forest habitat represents an additional threat to the long-term persistence of the Togean babirusa, the magnitude of which remains unknown.
In this study, we report the first field-based ecological assessment of the Togean babirusa. Specifically, we identified key habitat features affecting the species’ habitat selection and distribution across its entire range by combining camera trap-based field inventories, in situ habitat measurement, and remote sensing. We conducted an islands-wide occupancy study to test our hypothesis that babirusa habitat selection and distribution are shaped by natural vegetation coverage in the archipelago. Given the babirusa’s potential adaptability, we predict that the species can utilize various land cover types, including human-modified ones. However, due to the perceived risk associated with humans in agricultural areas, we further predict that the occupancy probability in such areas will be concentrated near forest patches where the babirusa can quickly find cover (Gaynor et al. 2019). The use of occupancy models also allowed us to account for the uncertainty in detecting the species. This was particularly important because animals commonly go undetected even when present, leading to an incorrect assessment of the species’ habitat selection (MacKenzie et al. 2017). Behavioral adaptation to human activity may further confound detection if individuals are being more cautious in areas with higher human frequentation, i.e., avoiding camera traps (Séquin et al. 2003; Meek et al. 2015). This study serves as the first comprehensive evaluation of the ecology of the Togean babirusa, an essential step to improving the species’ conservation. Based on our findings, we also provide additional insights on possible conservation strategies for this endangered species.
Methods
Study sites
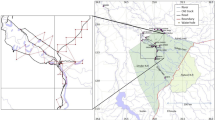
Our study was conducted in the Togean Archipelago in Central Sulawesi, Indonesia, specifically on four islands (Fig. 1): Batudaka (244 km2), Togean (177 km2), Talatako (96 km2), and Malenge (13 km2). These four islands are the only locations where the Togean babirusa is present (Ito and Melletti 2017); therefore, our study represents the entire range of this species. Portions of those four islands’ interior comprise the Togean Islands National Park, which represents about 30% of the land area. The entire archipelago is part of the recently declared UNESCO’s Togean Tojo Una-Una Biosphere Reserve (Purwanto and Nugroho 2022).
The placement of 103 camera trap stations in the study site, Togean Archipelago, Indonesia. We performed stratified random sampling to distribute the camera stations across four types of land cover: forests, agricultural areas, pioneer vegetation, and mangroves. Two camera traps were deployed on each station, spacing about 100 m. The land cover map was developed from Landsat-8 and Landsat-9 imagery acquired in 2022. The inset maps show the study site’s relative position to the Sulawesi mainland and Southeast Asia
The Togean Archipelago experiences a tropical marine climate, with an annual temperature range of 26–34 °C. The estimated monthly precipitation in the period June-October 2022 (which is when our study was conducted) ranges between 203 and 445 mm, indicating little seasonal variation during our fieldwork (Funk et al. 2015). The elevational gradient of our study sites, estimated from remote sensing, ranges from − 21 to 514 m asl, with an average of 65 m. The islands are primarily covered in tropical lowland forests in the interior, while mangroves can be found in some coastal regions. In areas designated for agriculture, coconut and clove plantations predominate (Surjadi and Supriatna 1998; pers. obs.). Based on recent satellite imagery, we estimated that the remaining forested areas of our study sites make up approximately 50% of the total land surface.
Distribution of the Togean babirusa
To collect data on the babirusa, we used camera traps at 103 stations throughout our study sites (Fig. 1). Specifically, we included 50 stations on Batudaka, 30 on Togean, 20 on Talatako, and three on Malenge Island. We distributed the camera stations following a stratified random sampling procedure across four land cover types: forests (53%), agricultural areas (24%), mangroves (12%), and pioneer vegetation (11%). The distance between the two nearest camera stations was 1785 ± CI 109 m.
In each station, we deployed two camera traps about 100 m apart. We placed the cameras (camera points) at locations where the chances of recording babirusas were high, such as in front of wallowing ponds or near animal tracks. The cameras were attached to tree trunks about 30–50 cm above ground level, but sometimes higher depending on the surrounding environment. We programmed each camera to remain active for 24 h a day and to take a 20-second video each time its sensor was triggered.
We used 50 Browning BTC-5HD-APX-20C and six Bushnell Essential E2 camera traps, allowing us to deploy cameras at 25–28 stations simultaneously. After 15–21 days, we retrieved the cameras, downloaded the data, and redeployed them at different stations. We sampled 103 stations using four camera trapping rotations from July to October 2022. At 95 stations, the same camera model (Browning) was used exclusively for both camera points, while six stations utilized a mix of Browning and Bushnell cameras. Due to technical issues, two stations were only equipped with a single camera trap (Browning).
All videos of babirusa recorded within a 24-hour period at one station were compiled and classified as detected (denoted as 1) if there was at least one video of the babirusa, or not detected (denoted as 0) if there was no recording of the species. This procedure produced a sequence of 0 and 1 for each station (detection history).
Habitat features
We used combinations of remote sensing and GIS to assess the habitat features of the study sites. Specifically, we examined land cover diversity, the proportion of specific land cover types, distance to human settlements, distance to roads, and slope (Bennett et al. 2006; Lindenmayer and Fischer 2006). Additionally, we also measured canopy openness in situ at the camera points.
To assess those first three variables, we generated a land cover map based on satellite imagery, then applied GIS to extract the value for each variable. First, we employed Google Earth Engine (GEE) to produce a cloud-free mosaic of Landsat-8 and Landsat-9 imagery of the study site in 2022. We provide the details of this procedure in Online Resource 1. Second, we employed ArcGIS Pro to perform land cover classification on the imagery using supervised classification with random forest algorithm (Sheykhmousa et al. 2020). The input variables for the classification were the visible bands (Blue, Green, Red), Near Infrared (NIR), Short-wave Infrared 1 and 2 (SWIR-1 and SWIR-2), Principal Component Analysis (PCA) of visible bands, NIR, SWIR-1, and SWIR-2, and spectral indices. The spectral indices were Normalized Difference Vegetation Index (NDVI), Normalized Difference Moisture Index (NDMI), Modified Normalized Difference Water Index (MNDWI), and Bare Soil Index (BSI). We assigned six categories for this classification schema: forest, pioneer vegetation, mangrove, agriculture, developed, and inland water (Fig. 1). Third, we used various geoprocessing tools in ArcGIS Pro to extract the variables’ value from the land cover map; the details are presented below.
We calculated the Shannon Diversity Index to evaluate land cover diversity (Nagendra 2002) within four buffer sizes: 1 km (– 3 km2), 2 km (– 12.5 km2), 3 km (– 28 km2), and 4 km (– 50km2). We selected these buffers to cover possible home range sizes of the species. We based these buffer sizes on the home range of adult wild boars (Sus scrofa) which ranges from 2 to 35 km2 (Saunders and McLeod 1999). We also calculated the proportion of forest, pioneer vegetation, mangrove, and agriculture within those four buffers, excluding water bodies, using the Focal Statistics tool in ArcGIS Pro. Distance to settlement and road were calculated using the Euclidean Distance tool. We only mapped the road networks accessible by a motorcycle by exploring them using handheld GPS. All human settlements were also verified through ground surveys. The slope variable was processed from the 30 m SRTM (Shuttle Radar Topography Mission) Digital Elevation Model.
To estimate canopy openness in situ, we used fisheye photographs taken at each camera trap point using an Olympus Tough TG-6 Digital Camera and Olympus FCON-T02 Fisheye Converter Lens. Photographs were taken 180 cm above the forest floor, facing vertically. We processed each photo using the Gap Light Analyzer software (Frazer et al. 1999), excluding outer rings beyond a 60° zenith angle to avoid including sloped terrain in the image. We averaged canopy openness values (expressed as a percent of open sky) of the two camera points for each station.
Occupancy modeling
We employed single-season single-species occupancy models (MacKenzie et al. 2002) to identify habitat features affecting the Togean babirusa’s habitat selection. We fitted the occupancy models to the babirusa’s detection history using unmarked package in R (Fiske and Chandler 2011; R Core Team 2022). In performing this analysis, we first identified factors affecting the detection probability of the babirusa. Then, we incorporated those factors as the detection probability covariates to model occupancy.
We used the following variables to construct the detection probability models: camera active days, camera model, island, canopy openness, slope, land cover diversity (all buffers), proportion of forest, pioneer vegetation, mangrove, and agriculture (all buffers), and distance to settlement and road (a complete list of the covariates is presented in Table S2, Online Resource 2). Prior to this analysis, we performed a Moran’s I test to assess spatial autocorrelation of babirusa’s detection among stations (Dormann et al. 2007; Bivand and Wong 2018). Since we did not detect spatial autocorrelation in our data (Fig. S1, Online Resource 3), a spatial auto-covariate was not included in the models.
We modeled the detection probability following a forward stepwise approach. We first generated single predictor models using each of the above variables, then evaluated the models based on their ΔAIC. We selected variables in the models ranked within 2 ΔAIC and paired them to create additive models. If only one model ranked within 2 ΔAIC, we created additive models by pairing the variable in that model with variables from other models with 2 ΔAIC above the null (model without a predictor). When evaluating the variables, we only selected and paired variables that were not correlated (r < 0.6) and were not the same feature at different spatial scales (e.g., the proportion of forest within 2 km vs. 3 km). After re-evaluating model ΔAIC scores, we retained the top-ranked detection probability model and used it to model the occupancy, following the same selection process. To construct the occupancy models, we used the same predictors for modeling detection, except for variables exclusive in affecting detectability (i.e., camera active days, camera model, island, and canopy openness). In addition, we also fitted a model with interaction effects if the top additive model consisted of more than one covariate. We mapped the babirusa distribution following the selected occupancy model using raster package in R (Hijmans 2023).
Results
We detected babirusas at 73 camera stations (70%), of which 49 were in the forests, 11 in pioneer vegetation, three in mangroves, and ten in agricultural areas. Among islands, babirusas were detected at 30 stations on Batudaka, 27 on Togean, 13 on Talatako, and three on Malenge. Overall, we obtained 1565 identifiable camera trap videos of the babirusa, comprising 489 daily detections.
Our top model shows that the detection probability of the babirusa was affected by several factors, including island, canopy openness, and the proportion of pioneer vegetation within a 4 km radius (Table 1). The detectability of the babirusa varied across different islands, particularly the detection in Batudaka Island was lower than the other three (Fig. 2a). Detectability also varied following the gradient of pioneer vegetation coverage, where detection rates were higher in areas with a lower proportion of pioneer vegetation within 4 km (β = -3.898 ± SE 1.459; Fig. 2b). Lastly, the chance to detect the babirusa was higher with lower canopy openness (β = -0.103 ± SE 0.026; Fig. 2c).
Prediction of Togean babirusa’s detection probability from the top-ranked single-season occupancy model. a: the detection probability on Batudaka Island was lower than the other three. The line bars indicate 95% confidence intervals. Panel a was predicted using the mean proportion of pioneer vegetation within 4 km (0.12) and canopy openness (9.02). b: the detectability decreases following the increase of pioneer vegetation within 4 km. The canopy openness was set constant at its mean value. c: the detection probability decreases as the canopy openness increases. The proportion of pioneer vegetation within 4 km was set constant at its mean value. Grey ribbons in panels b and c indicate 95% confidence intervals. Panel b and c were predicted for the Togean Island
The top-ranked model includes the proportion of forests within a 4 km radius and the proportion of mangroves within a 4 km radius as predictors for occupancy. The increase in forest availability led to an increase in occupancy (β = 9.45 ± SE 2.6; Fig. 3a). Similarly, an increase in mangrove availability also increased occupancy (β = 8.41 ± SE 4.92; Fig. 3b). The variation in occupancy change along the gradient of forest availability was greater than that along the gradient of mangrove availability. All models within 2 ΔAIC included the proportion of forest within a 4 km radius as a covariate (Table 1). We present the Togean babirusa distribution throughout the entire study site, predicted using the top-ranked occupancy model, in Fig. 4a.
Prediction of Togean babirusa’s occupancy probability from the top-ranked single-season occupancy model. a: occupancy probability increased with forest availability; the proportion of mangrove was set constant at its mean value (0.105). b: babirusa occupancy increased with the proportion of mangroves; the proportion of forest was set constant at its mean value (0.526). Grey ribbons indicate 95% confidence intervals
a: Predicted Togean babirusa occupancy throughout its distribution range, modeled using the top-ranked single season occupancy model. The color gradient indicates the occupancy probability of the species, with red representing low probability and blue representing high probability. b: Potential areas to redesign the Togean Islands National Park. Red color indicates agriculture and developed areas inside the national park boundary (about 30% of the total park area). Blue color indicates large forest patches not protected by the national park (about 50% of the total forested area), potential areas to reassign non-forested park areas
Discussion
We here report the results of the first field-based ecological study of the Togean babirusa, which encompasses its entire distribution range. We found that forest and mangrove coverage positively influence the occurrence of the species. The babirusa can take advantage of agricultural areas, but this is only possible when they are in close proximity to large forested areas. Through our analyses, we found that about 50% of the remaining forests, the essential habitat for the babirusa, are not protected by the national park. These results suggest the need to evaluate and reassess the existing conservation strategies in place for the species. Our case study exemplifies a key challenge related to managing large mammals on small islands (i.e., limited space and resources), but also shows that field-based surveys can be used to identify key conservation priorities, which in this case suggests the need to redesign a portion of the current protected area.
Factors affecting babirusa’s habitat selection and distribution
Our top-ranked model shows that availability of forested areas is the most important factor affecting the distribution of the babirusa, as shown in Fig. 3a, where the occupancy increases from below 0.2 to over 0.9 in response to increasing forest availability. Among buffer sizes tested, the 4 km radius was consistently included in top ranking models (Table 1), suggesting that babirusa requires extensive forest coverage at a large scale. Our findings contradict the previous assumption that the Togean babirusas favor non-forest areas. The observation bias of the previous interview survey (Akbar et al. 2007) likely caused this discrepancy, as locals spent more time in agricultural areas, and spotting the animal in the forest was also difficult. Our findings on the importance of forested areas corroborate those of other pig species, including Sulawesi warty pig (Sus celebensis), bearded pig (S. barbatus), and the two other babirusa species (Froese et al. 2015; Macdonald 2017; Sheherazade et al. 2017; Ke and Luskin 2019).
The Togean babirusa reliance on forests can be attributed to three factors. First, forests provide a variety of food sources known to be eaten by babirusas, including fruits, leaves, roots, invertebrates, and small vertebrates (Akbar et al. 2007; Macdonald et al. 2016). Second, forests provide sites and materials for nesting, a critical behavior among the Suidae family, especially for females during parturition (Fernández-Llario 2004). In line with this, we encountered at least six babirusa nests exclusively in the forest during our fieldwork. Third, forests provide a suitable and safe environment for wallowing. The sweat glands of the pig family are largely unresponsive to overheating, which makes wallowing a vital behavior for thermoregulation, in addition to protection from parasites (Bracke 2011; Rademaker et al. 2016).
Our study shows that mangroves are also an important habitat for the babirusa. Unlike babirusas in the Sulawesi mainland that rely on saltlicks (Clayton and MacDonald 1999), Togean babirusa can easily obtain salt from mangrove areas. Mangroves also provide a habitat for various invertebrates, such as clams, snails, and worms, a supplementary diet the babirusa can access during low tide (Kathiresan and Bingham 2001; Medeiros et al. 2019). The muddy substrate of mangroves also serves as ideal wallowing sites, as evidenced by the wallowing ponds we discovered within the mangrove environment (Online Resource 4). Moreover, the dense vegetation of mangroves offers the babirusa enough cover to access these benefits while staying hidden from humans.
Despite the benefits, mangroves alone are insufficient to sustain a high occupancy of babirusa. Figure 3b shows that increasing mangrove availability does not result in a significant rise in occupancy. When forest availability remains constant, occupancy levels are already above 50%, even without mangroves. In contrast, Fig. 3a shows that occupancy remains low when forest availability is low, even when mangrove is available (Fig. S2, Online Resource 3). Three reasons contribute to this pattern (Kathiresan and Bingham 2001): first, mangroves grow exclusively within intertidal zones, limiting babirusa access to these habitats to only a few hours during low tide. Second, the dietary options within mangroves are less diverse than in the forests, serving primarily as supplements. Lastly, mangroves lack a source of freshwater.
Agricultural areas can provide supplementary resources but will not sustain the babirusa population due to high competition with humans (Brady et al. 2011; Driscoll et al. 2013). Indeed, our camera traps recorded babirusas in agricultural areas, but our models suggest this area will be occupied only when a significant proportion of forest is also available nearby. This dynamic is illustrated in Fig. 4 where some non-forested national park areas (Fig. 4b) have a high babirusa’s occupancy (Fig. 4a) because a large amount of forests surround the areas. Studies on crop raiding by wild boar and other ungulates show similar spatial patterns (Nyhus et al. 2000; Linkie et al. 2007; Costa et al. 2021). Although offering alternative diets, agricultural areas are hostile because of the high mortality risk from hunting (Love et al. 2017). Our camera traps recorded many babirusas with severed legs from snaring (Online Resource 5), indicating a significant human-wildlife conflict as farmers actively seek to kill the babirusa for pest control.
Factors affecting detectability
Our occupancy analysis shows that babirusa detectability varied across islands, with the lowest value being on Batudaka Island (Fig. 2a). Batudaka appeared to have the lowest detection rate because only 30 out of 50 of its camera stations detected babirusa, the lowest proportion among islands. Batudaka is also the most developed island, characterized by higher human population density, better road networks, and more intensive agricultural practices. Furthermore, there is a small community on Batudaka that hunts babirusas for meat, in addition to pest control (Akbar et al. 2007). Therefore, this high anthropogenic pressure could lower the detectability of the babirusa because they become more cautious around camera traps (see Online Resource 6 as an example), as this equipment is associated with human presence (Séquin et al. 2003; Meek et al. 2014, 2015). The apparent high detection probability in Malenge Island was probably due to its small size, which makes camera station density higher. However, we acknowledge that Malenge had only three stations, resulting in a wide confidence interval for our estimated detection probability.
The proportion of pioneer vegetation and canopy openness also affected the detectability of the babirusa. In the Togean Archipelago, pioneer vegetation is characterized by dense understory that could limit babirusa movement, leading to lower detection in such areas. This pattern aligns with the observation of other species, such as barking deer (Muntiacus muntjac), Javan banteng (Bos javanicus), giant armadillo (Priodontes maximus), and mule deer (Odocoileus hemionus), which avoid dense understory to facilitate faster movement (Pudyatmoko 2019; Teixeira-Santos et al. 2020; Ganz et al. 2022). Furthermore, understory vegetation could also limit the camera trap’s field of view, reducing its ability to detect the animal. Babirusa detection was higher under denser forest canopies (i.e., lower canopy openness), presumably because the animals avoided open spaces associated with predation risk (Long et al. 2009), in this case, harassment from humans.
Conservation implications
Because forests are an important habitat component for the Togean babirusa, forest loss is likely the most significant threat to the species: our model suggests that if forest cover were reduced to 20%, the babirusa occupancy would drop below 20% (Fig. 3a). The presence of the Togean Islands National Park was expected to protect the Togean babirusa habitat, especially because the archipelago is experiencing deforestation for small-scale agriculture. However, as seen in Fig. 1, the design of the Togean Islands National Park does not seem to cover the remaining forest effectively. About 30% of the national park areas include agricultural and developed areas within park boundaries, and around 50% of large forest areas are not included in the national park (Fig. 4b). These non-national park forests do not have a legal protection status, making them vulnerable to deforestation.
To improve the conservation strategies for this endangered babirusa, we recommend redesigning the national park area to account for its habitat requirements. We show that maintaining at least 50% forest coverage over a large area will support high babirusa occupancy (Fig. 3a). The fact that this number is comparable to the current amount of forest remaining emphasizes the urgency to redesign the park zonation. Specifically, we suggest reassigning the 30% of the non-forested national park areas to unprotected forests currently outside the park boundary. By doing so, the total national park area will remain the same, allowing the national park agency to perform their task under the current operational budget and human resources more efficiently, i.e., by reallocating their patrolling budget from non-forests to forests. We strongly suggest including local communities in deciding which part of these unprotected forests to include in the park territory, ensuring their access to the forest is accommodated, which will also be fundamental to gaining their support (Bickford et al. 2007). We highlighted the areas covered by unprotected forests in Fig. 4b to show potential candidate areas suitable to transfer the protection status of the non-forested park.
However, protecting the forest alone will not protect the entire babirusa population, as they would likely still explore agricultural areas near the forests, which is the main cause of human-wildlife conflict. In this situation, we emphasize the importance of sociocultural approaches to facilitate conservation beyond the formal park area to promote human-wildlife coexistence (Ceballos et al. 2005; König et al. 2020). This is a challenging approach, considering the majority of locals depend on agricultural practices for their livelihood (Surjadi and Supriatna 1998). Developing strategies to promote this coexistence demands an understanding of the sociocultural aspect of the community (Dickman 2010), which will require an in-depth analysis. Currently, strategies that The Togean Islands National Park agency implements are a participatory approach and entrepreneurship training with several local communities (Indrawan et al. 2014), which should be expanded throughout the archipelago.
We acknowledge the complexity of designing a national park, especially in light of cultural and socio-economic factors (Dudley 2008), an important topic that will require further investigation. Nevertheless, within the scope of our study, we provide evidence-based suggestions that highlight priority areas that could guide the national park redesign. The Togean babirusa may potentially serve as an umbrella species as it relies on the high availability of the native vegetation and is distributed across the four major islands, representing a significant portion of the archipelago. Therefore, protecting the babirusa may potentially safeguard a substantial amount of habitat required by other species.
Large mammals that inhabit small islands are highly vulnerable to habitat loss (Heinen et al. 2018). These include other Suidae species such as Sus verrucosus blouchi (Rademaker et al. 2016), S. cebifrons (Meijaard et al. 2017), S. ahoenobarbus (Meijaard and Widmann 2017), and Babyrousa babyrussa (Sheherazade et al. 2017). Unlike animals in the mainland that can disperse long distances (Lindenmayer and Fischer 2006), small island populations lack such options. Although our model species, the Togean babirusa, is known to have a wide ranging diet, its habitat use is highly influenced by the availability of natural vegetation, emphasizing the need to protect the remaining forests on these small islands (Ceballos et al. 2005). However, given the small spatial scale, overlapping space use between large mammals and humans is unavoidable, and the protected area alone may be insufficient for large mammals’ protection. Therefore, besides the species, conservation strategies should also involve and target local communities to promote human-wildlife coexistence (Dickman 2010; Nyhus 2016). Also, conservation efforts in small islands should go beyond the designated protected area and consider the entire islands as a whole entity (Fernandes et al. 2015), i.e., by performing monitoring throughout the islands, regardless of the area’s protection status.
Our study represents the first in-depth ecological assessment of the Togean babirusa, offering valuable insights for species management. Contrary to previous work, we discovered a strong association between the babirusa and forest coverage, highlighting the urgency of preserving the remaining native vegetation in the Togean Archipelago. We emphasize the importance of regularly monitoring the species’ occupancy to evaluate the effectiveness of conservation strategies over time. Furthermore, we encourage further research to determine the extent of human-wildlife conflict, as it may be a significant factor contributing to babirusa mortality. Our case study exemplifies key challenges associated with conserving large species on small islands and highlights the critical importance of following an adaptive management approach, which in this case implies shifting 30% of the current protected area.
Data availability
The datasets and R script generated and analyzed in this study are available from the corresponding author on reasonable request.
References
Akbar S, Indrawan M, Yasin MP et al (2007) Status and conservation of Babyrousa babyrussa in the Togean Islands, based on direct observations and questionnaire surveys (intermittently, 1990–2001). Suiform Sound 7:16–25
Austin KG, Schwantes A, Gu Y, Kasibhatla PS (2019) What causes deforestation in Indonesia? Environ Res Lett 14:024007. https://doi.org/10.1088/1748-9326/aaf6db
Bennett AF, Radford JQ, Haslem A (2006) Properties of land mosaics: implications for nature conservation in agricultural environments. Biol Conserv 133:250–264. https://doi.org/10.1016/j.biocon.2006.06.008
Bickford D, Supriatna J, Andayani N et al (2007) Indonesia’s protected areas need more protection: suggestions from island examples. Biodivers Hum Livelihoods Prot Areas Case Stud Malay Archipel 53–77. https://doi.org/10.1017/CBO9780511542169.005
Bivand RS, Wong DWS (2018) Comparing implementations of global and local indicators of spatial association. Test 27:716–748. https://doi.org/10.1007/s11749-018-0599-x
Bracke MBM (2011) Review of wallowing in pigs: description of the behaviour and its motivational basis. Appl Anim Behav Sci 132:1–13. https://doi.org/10.1016/j.applanim.2011.01.002
Brady MJ, McAlpine CA, Possingham HP et al (2011) Matrix is important for mammals in landscapes with small amounts of native forest habitat. Landsc Ecol 26:617–628. https://doi.org/10.1007/s10980-011-9602-6
Broto B, Mortelliti A (2019) The status of research on the mammals of Sulawesi, Indonesia. Mamm Rev 49:78–93. https://doi.org/10.1111/mam.12141
Calado H, Fonseca C, Vergílio M et al (2014) Small islands Conservation and protected areas. Rev Gestão Costeira Integr 14:167–174. https://doi.org/10.5894/rgci523
Ceballos G, Ehrlich PR, Soberón J et al (2005) Global Mammal Conservation: what must we manage? Science 309:603–607. https://doi.org/10.1126/science.1114015
Clayton L, MacDonald DW (1999) Social Organization of the Babirusa (Babyrousa babyrussa) and their use of Salt licks in Sulawesi, Indonesia. J Mammal 80:1147–1157. https://doi.org/10.2307/1383165
Costa HCM, Benchimol M, Peres CA (2021) Wild ungulate responses to anthropogenic land use: a comparative pantropical analysis. Mamm Rev 51:528–539. https://doi.org/10.1111/mam.12252
Dickman AJ (2010) Complexities of conflict: the importance of considering social factors for effectively resolving human – wildlife conflict. Anim Conserv 13:458–466. https://doi.org/10.1111/j.1469-1795.2010.00368.x
Dormann CF, McPherson JM, Araújo MB et al (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628. https://doi.org/10.1111/j.2007.0906-7590.05171.x
Driscoll DA, Banks SC, Barton PS et al (2013) Conceptual domain of the matrix in fragmented landscapes. Trends Ecol Evol 28:605–613. https://doi.org/10.1016/j.tree.2013.06.010
Dudley N (ed) (2008) Guidelines for applying protected Area Management categories. IUCN, Gland, Switzerland
Fernandes JP, Guiomar N, Gil A (2015) Strategies for conservation planning and management of terrestrial ecosystems in small islands (exemplified for the macaronesian islands). Environ Sci Policy 51:1–22. https://doi.org/10.1016/j.envsci.2015.03.006
Fernández-Llario P (2004) Environmental correlates of nest site selection by wild boar Sus scrofa. Acta Theriol 49:383–392. https://doi.org/10.1007/BF03192536
Fiske IJ, Chandler RB (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23. https://doi.org/10.18637/jss.v043.i10
Frazer GW, Canham CD, Lertzman KP (1999) Gap light Analyzer (GLA), Version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, Millbrook, New York
Froese GZL, Contasti AL, Mustari AH, Brodie JF (2015) Disturbance impacts on large rain-forest vertebrates differ with edge type and regional context in Sulawesi, Indonesia. J Trop Ecol 31:509–517. https://doi.org/10.1017/S0266467415000450
Funk C, Peterson P, Landsfeld M et al (2015) The climate hazards infrared precipitation with stations - a new environmental record for monitoring extremes. Sci Data 2:1–21. https://doi.org/10.1038/sdata.2015.66
Ganz TR, DeVivo MT, Kertson BN et al (2022) Interactive effects of wildfires, season and predator activity shape mule deer movements. J Anim Ecol 91:2273–2288. https://doi.org/10.1111/1365-2656.13810
Gaynor KM, Brown JS, Middleton AD et al (2019) Landscapes of fear: spatial patterns of risk perception and response. Trends Ecol Evol 34:355–368. https://doi.org/10.1016/j.tree.2019.01.004
Haskell JP, Ritchie ME, Olff H (2002) Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature 418:527–530. https://doi.org/10.1038/nature00840
Heinen JH, van Loon EE, Hansen DM, Kissling WD (2018) Extinction-driven changes in frugivore communities on oceanic islands. Ecography 41:1245–1255. https://doi.org/10.1111/ecog.03462
Hijmans RJ (2023) raster: Geographic Data Analysis and Modeling
Indrawan M, Lowe C, Sundjaya et al (2014) Co-management and the creation of national parks in Indonesia: positive lessons learned from the Togean Islands National Park. J Environ Plan Manag 57:1183–1199. https://doi.org/10.1080/09640568.2013.788834
Ito M, Melletti M (2017) Togian Babirusa Babyrousa togeanensis (Sody, 1949). In: Melletti M, Meijaard E (eds) Ecology, Conservation and Management of Wild pigs and peccaries. Cambridge University Press, Cambridge, pp 76–84
Kathiresan K, Bingham BL (2001) Biology of mangroves and mangrove Ecosystems. In: Advances in Marine Biology. pp 81–251
Ke A, Luskin MS (2019) Integrating disparate occurrence reports to map data-poor species ranges and occupancy: a case study of the vulnerable bearded pig Sus barbatus. Oryx 53:377–387. https://doi.org/10.1017/S0030605317000382
König HJ, Kiffner C, Kramer-Schadt S et al (2020) Human–wildlife coexistence in a changing world. Conserv Biol 34:786–794. https://doi.org/10.1111/cobi.13513
Lindenmayer D, Fischer J (2006) Habitat Fragmentation and Landscape Change: an ecological and Conservation Synthesis. Islandpress, Washington
Linkie M, Dinata Y, Nofrianto A, Leader-Williams N (2007) Patterns and perceptions of wildlife crop raiding in and around Kerinci Seblat National Park, Sumatra. Anim Conserv 10:127–135. https://doi.org/10.1111/j.1469-1795.2006.00083.x
Long RA, Kie JG, Terry Bowyer R, Hurley MA (2009) Resource selection and movements by female mule deer Odocoileus hemionus: effects of reproductive stage. Wildl Biol 15:288–298. https://doi.org/10.2981/09-003
Love K, Kurz DJ, Vaughan IP et al (2017) Bearded pig (Sus barbatus) utilisation of a fragmented forest-oil palm landscape in Sabah, Malaysian Borneo. Wildl Res 44:603–612. https://doi.org/10.1071/WR16189
Macdonald A (2017) Sulawesi Babirusa Babyrousa celebensis (Deninger, 1909). In: Melletti M, Meijaard E (eds) Ecology, Conservation and Management of Wild pigs and peccaries. Cambridge University Press, Cambridge, pp 59–69
Macdonald A, Leus K, Masaaki I, Burton J (2016) Babyrousa togeanensis. In: IUCN Red List Threat. Species 2016 e.T136472A44143172. https://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T136472A44143172.en. Accessed 1 Oct 2020
MacKenzie DI, Nichols JD, Lachman GB et al (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. https://doi.org/10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2
MacKenzie DI, Nichols JD, Royle JA et al (2017) Occupancy estimation and modeling: inferring patterns and Dynamics of species occurrence: Second Edition. Occup Estim Model inferring patterns Dyn species Occur Second. 1–641. https://doi.org/10.1016/C2012-0-01164-7
Mayor SJ, Schneider DC, Schaefer JA, Mahoney SP (2009) Habitat selection at multiple scales. Ecoscience 16:238–247. https://doi.org/10.2980/16-2-3238
Medeiros K, Bastos M, Jones G, Bezerra B (2019) Behavior, Diet, and Habitat Use by blonde Capuchin monkeys (Sapajus flavius) in a Coastal Area Prone to flooding: direct observations and Camera Trapping. Int J Primatol 40:511–531. https://doi.org/10.1007/s10764-019-00103-z
Meek PD, Ballard G-A, Fleming PJS et al (2014) Camera traps can be heard and seen by animals. PLoS ONE 9:e110832. https://doi.org/10.1371/journal.pone.0110832
Meek PD, Ballard GA, Fleming PJS (2015) The pitfalls of wildlife camera trapping as a survey tool in Australia. Aust Mammal 37:13–22. https://doi.org/10.1071/AM14023
Meijaard E, Groves CP (2002) Proposal for taxonomic changes within the genus Babyrousa. IUCN/SSC Pigs Peccaries Hippos Spec Gr Newsl 2:9–10
Meijaard E, Widmann P (2017) Sus ahoenobarbus. In: IUCN Red List Threat. Species 2017 e.T21177A44140029. https://www.iucnredlist.org/species/21177/44140029. Accessed 11 Jun 2023
Meijaard E, Oliver WRT, Leus K (2017) Sus cebifrons. In: IUCN Red List Threat. Species 2017 e.T21175A44139575. https://www.iucnredlist.org/species/21175/44139575. Accessed 11 Jun 2023
Michaux B (2010) Biogeology of Wallacea: geotectonic models, areas of endemism, and natural biogeographical units. Biol J Linn Soc 101:193–212. https://doi.org/10.1111/j.1095-8312.2010.01473.x
Myers N, Mittermeier RA, Mittermeier CG et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nagendra H (2002) Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl Geogr 22:175–186. https://doi.org/10.1016/S0143-6228(02)00002-4
Nyhus PJ (2016) Human–Wildlife Conflict and Coexistence. Annu Rev Environ Resour 41:143–171. https://doi.org/10.1146/annurev-environ-110615-085634
Nyhus PJ, Tilson R, Sumianto (2000) Crop-raiding elephants and conservation implications at Way Kambas National Park, Sumatra, Indonesia. Oryx 34:262–274. https://doi.org/10.1046/j.1365-3008.2000.00132.x
Pfeifer M, Lefebvre V, Peres CA et al (2017) Creation of forest edges has a global impact on forest vertebrates. Nature 551:187–191. https://doi.org/10.1038/nature24457
Pudyatmoko S (2019) Spatiotemporal inter-predator and predator–prey interactions of mammalian species in a tropical savanna and deciduous forest in Indonesia. Mammal Res 64:191–202. https://doi.org/10.1007/s13364-018-0391-z
Purwanto Y, Nugroho H (2022) Indonesian Contributions to UNESCO’s Man and the Biosphere Programme (MAB): Summary Report for the Year 2019–2021
R Core Team (2022) R: a Language and. Environment for Statistical Computing
Rademaker M, Meijaard E, Semiadi G et al (2016) First ecological study of the Bawean Warty Pig (Sus Blouchi), one of the Rarest pigs on Earth. PLoS ONE 11:e0151732. https://doi.org/10.1371/journal.pone.0151732
Saunders G, McLeod S (1999) Predicting home range size from the body mass or population densities of feral pigs, Sus scrofa (Artiodactyla: Suidae). Austral Ecol 24:538–543. https://doi.org/10.1046/j.1442-9993.1999.01001.x
Séquin ES, Jaeger MM, Brussard PF, Barrett RH (2003) Wariness of coyotes to camera traps relative to social status and territory boundaries. Can J Zool 81:2015–2025. https://doi.org/10.1139/z03-204
Sheherazade HE, Indrawan M (2017) Moluccan Babirusa Babyrousa babyrussa (Linnaeus, 1758). In: Melletti M, Meijaard E (eds) Ecology, Conservation and Management of Wild pigs and peccaries. Cambridge University Press, Cambridge, p 7075
Sheykhmousa M, Mahdianpari M, Ghanbari H et al (2020) Support Vector Machine Versus Random Forest for Remote sensing image classification: a Meta-analysis and systematic review. IEEE J Sel Top Appl Earth Obs Remote Sens 13:6308–6325. https://doi.org/10.1109/JSTARS.2020.3026724
Surjadi P, Supriatna J (1998) Bridging Community Needs and Government Planning in the Togean Islands, Central Sulawesi, Indonesia. ITMEMS 1998 Proc 280–286
Teixeira-Santos J, Ribeiro AC, da Wiig C Ø, et al (2020) Environmental factors influencing the abundance of four species of threatened mammals in degraded habitats in the eastern Brazilian Amazon. PLoS ONE 15:e0229459. https://doi.org/10.1371/journal.pone.0229459
Acknowledgements
We thank the Togean Islands National Park Agency for facilitating our project activities and the Alliance for Tompotika Conservation (AlTo) for providing field support. This study was conducted under SIMAKSI permit issued by the Togean Islands National Park Agency No: SI.02/T.23/TU/KSA/06/2022 and IACUC (Institutional Animal Care and Use Committee) protocol A2021-12-04. We thank all of our field team, particularly Pandji Ariyo Kresno, Suardi, and Ikal. We also thank all local leaders for allowing us to deploy camera-traps within their village territory.
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. This study was supported by American Indonesian Cultural & Educational Foundation (AICEF); Zoological Society for the Conservation of Species and Populations (ZGAP; Project code: 1.Babyrousa togeanensis.IDN.2021); IUCN Save Our Species (Project code: 2022 C-1); The Mohamed bin Zayed Species Conservation Fund (Project number: 212527935); The Rufford Foundation (Project ID: 36883-1); and Graduate Student Government of the University of Maine. The Ph.D. study of author ASJ was supported by the Fulbright Scholarship.
Author information
Authors and Affiliations
Contributions
ASJ, BWB, and AM conceptualized the idea and designed methodology; ASJ and BWB collected the data; ASJ and KL performed remote sensing; ASJ, GFD, SF, and AM analyzed the data; ASJ and AM led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Andreas Schuldt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jati, A.S., Broto, B.W., Dri, G.F. et al. Conserving large mammals on small islands: A case study on one of the world’s most understudied pigs, the Togean islands babirusa. Biodivers Conserv 33, 1207–1223 (2024). https://doi.org/10.1007/s10531-024-02800-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-024-02800-5