Abstract
Species respond differently to landscape structures and environmental changes. In nature conservation, however, responses of a few indicator groups are often generalised to the ecosystem level. In this study, we analyse how birds and butterflies respond to identical landscape structures and environmental gradients across a habitat mosaic in southern Kenya. The study area represents natural coastal forest (forest interior and forest edge) as well as different agro-environments (such as orchards and pastures), which partly may also be suitable surrogate habitats for forest species. We assessed birds and butterflies during the same time along identical line transects, covering the dry and the rainy season. The obtained results indicate that both species groups depict habitat types in some aspects similarly but in others in somewhat different ways. Thus, strongest differences in community similarity were visible between forest interior and the open landscape like pastures for both taxa. The forest community strongly overlapped with orchards for birds but less so for butterflies. Thus, orchards in close geographic proximity to natural forests might be a possible surrogate habitat for certain forest bird species, but less so for more sedentary forest butterfly species. The temporal variation in species richness, abundances, and community structures was much stronger for butterflies compared to birds. Thus, seasonality in tropical ecosystems has to be considered especially when interpreting community structures of butterflies, but much less so in birds. In general, birds and butterflies proved to be suitable indicator groups to evaluate ecologically landscape structures in East Africa, with birds more representing the landscape level and butterflies more the fine-grained habitat scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transformation of natural habitats into anthropogenic ecosystems frequently produces a mosaic of different habitat types across landscapes. The creation of such habitat mosaics in many cases is often reflected by a similar community structure (Habel et al. 2018), but responses to habitat structures and environmental changes are taxon-specific (Barbaro & van Halder 2009). Thus, the development of distinct species communities in particular depend on taxon-specific characteristics and responses, such as habitat requirements and dispersal behaviour (Zellweger et al. 2017). In addition to species´ specific responses, natural environmental shifts such as seasonality may additionally influence the availability of resources, and subsequently the occurrence and behaviour of single species (Habel et al. 2018), and thus community structures.
In general, species occurring across entire landscapes and which can be found in a large number of different habitat types create rather panmictic distributions and thus rather weaken habitat-specific species community structures. In turn, community structures with many sedentary, locally restricted specialist species are often highly distinct from each other (Schmitt et al. 2021). Therefore, it is of crucial importance to get information, which taxonomic groups are suitable indicator groups to identify effects of the availability of ecological resources along environmental gradients (Lund and Rahbek 2002; Vessby et al. 2002; Schulze et al. 2004; Wolters et al. 2006, Larson et al. 2012).
Birds and butterflies are known to respond sensitively to changes in habitat structures and the availability of resources, and thus excellently suite as environmental indicator taxa (Bibby 1999, Bonebrake et al. 2010). Both taxonomic groups are particularly suitable for this type of analysis because, owing to their popularity, they are among the best-studied groups of animals in terms of habitat needs, behaviour and distribution patterns (Hagemeijer & Blair 1997, Kudrna 2011). However, birds and butterflies have different-sized home ranges, dependent on different resources, and differ with respect to habitat specialisation and spatial scale, with birds rather acting at the landscape level and butterflies at the habitat scale (Fleishman et al. 2003; Perfecto et al. 2003). Thus, birds often establish larger territories and hereby occupy various types of habitats across a landscape (Mac Nally et al. 2004), while many butterfly species are restricted to one specific habitat or even micro-habitat structures within habitats (Habel et al. 2022a, b).
Furthermore, dispersal behaviour of birds is comparatively high (Greenwood and Harvey 1982), and in most cases significantly higher than that of butterflies, which are frequently characterised by high site-fidelity (Wurz et al. 2022) (apart of some few migratory species, Williams 1930, Chowdhury et al. 2021). The two taxonomic groups also have remarkable differences in their use of resources, which again impact their sensitivity with respect to habitat disturbance (Ulrich et al. 2016, 2018). While most birds are insectivorous, carnivorous or frugivorous (Sekercioglu 2012), most butterfly species dependent on the foliage of specific plant species during their larval stage, and (mostly opportunistically) on flowers for nectar as adults (Habel et al. 2018) as well as on (rotten) fruits – particularly in tropical forest ecosystems (Hamer et al. 2005). Since we did not observe the butterflies with bait traps but along transects, the proportion of potential fruit-feeding species is rather underrepresented and thus the role of (rotten) fruits not explicitly investigated in our study.
As birds and butterflies are the most popular environmental indicator groups in vertebrates and invertebrates, respectively, we still need to improve our understanding of taxon-specific responses to habitat structures and environmental conditions, in particular in the tropics with still only few studies available (Habel et al. 2017). Although birds and butterflies serve as appropriate surrogate taxa to represent the ecological status of a landscape, there might also be significant differences in species´ responses on environmental conditions and subsequently differences in species community structures (e.g. Blair 1999, Nelson and Nelson 2001, Fleishman et al. 2003, Zellweger et al. 2017). For the tropics, there are only a few studies to date in which more than one taxonomic group has been surveyed for the same study area in order to produce a comparative study (e.g. Perfecto et al. 2003, Schulze et al. 2004, Larson et al. 2012).
In our study, we assessed birds and butterflies in parallel and along identical line transects across a habitat mosaic in southern Kenya. We observed these organisms along transects covering various natural and anthropogenic habitats, such as pristine coastal forest, forest edge, pastures, and orchards. We assessed species richness, abundance, community structure, and trait composition for both taxonomic groups. Data collection took place during the dry and the following rainy season. Based on these data, we address the following research questions:
-
1.
Do birds and butterflies respond similarly to an Afrotropical landscape mosaic?
-
2.
Can orchards serve as a surrogate habitat for typical forest species?
-
3.
How do Afrotropical birds and butterflies differ in their land use indicator quality?
-
4.
Does seasonality impact the community structures of Afrotropical birds and butterflies in a similar way?
Materials and methods
Study area and study set-up
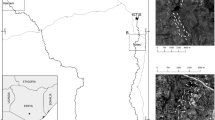
The study area is located in Kilifi County (southern Kenya), close to the Indian Ocean (Fig. 1). It represents a mosaic of natural coastal forest (the sacred Mijikenda forest homestead), forest edges, arable fields, pastures and orchards (with mango, cashew and coconut trees). The natural forest covers an area of 75 hectare (Fungomeli et al. 2020). The climate is hot (annual mean temperature of the coastal region is 26.6 °C, with average rainfalls of 1100 mm/a; Jaetzold et al. 2010). Rains are divided into two rainy seasons lasting from end-March to July (long rains) and from October to December (short rains) (Jaetzold et al. 2010).
We established 32 line transects (100 m each, with minimum distances of 100 m among each other to reduce potential effects from autocorrelation). Transects were set in four habitat types, i.e. the natural coastal forest, forest edge, pasture and orchard. Each habitat type was represented by eight transects. Each of the transect was visited eight times for birds and 20 times for butterflies. Data collection was conducted from February to May 2022, covering the dry and rainy season.
Collection of bird and butterfly data
Bird surveys were conducted using the point count technique according to Bibby et al. (1998) along the respective line transects. Point count stations were located in the centre of each 100 m transect established for the butterfly survey. During point counts, all birds heard and seen to a radius of about 50 m were recorded and noted. Point counts were undertaken during morning (6‒10 am) and during afternoon (4‒6 pm), for 10 min at each point. Birds flying from behind were not recorded to avoid double counts. This procedure was repeated 4 times during the dry season and 4 times during the rainy season. All raw data are given in Table A2 of Appendix A.
For butterflies, transect walks were conducted at slow pace. All butterflies observed within a 5 m buffer at each side (left, right, in front, above) of the transect line were determined and noted (species, individuals) (Pollard & Yates 1993, modified). Whenever possible, butterfly species were determined in the field. If this was not possible, individuals were netted, photographed (the under and upper wing side), and determined afterwards (based on Larsen 1991). In total, each transect was visited and sampled 20 times (ten times during the dry and ten times during the rainy season). All raw data are contained in Table A2 of Appendix A.
Habitat parameters
Habitat parameters that may affect the occurrence of birds and butterflies were assessed at the start, centre and end of each transect. We recorded the following parameters: Percentage of canopy cover, height of the tallest tree (m), percentage of shrub cover, mean shrub height (m), percentage of herb cover, mean herb height (cm), percentage of litter cover, and number of blossoms. Estimates were done based on a 10 m radius. Additionally, it was noted whether a creek (also if being dry) was present within a distance of 20 m from the transect line. The occurrence of blossoms was classified into five classes: 0 = no flower, 1 = 1‒9 flowers, 2 = 10‒48, 3 = 50‒99, 4 = ≥ 100. The number of blossoms was assessed 10 times during each of the two seasons, as flowers may strongly change within short time periods. From these counts, we calculated season-specific mean values for the respective line transect and season. All other parameters were assessed only three times (i.e. in the beginning (dry season), the middle (transitions between seasons) and the end (rainy season) of the study period). All raw data are contained in Table A3 of Appendix A.
Traits
All birds observed were classified into feeding guilds (insectivore, frugivore, carnivore, nectarinivore, granivore, omnivore; Kissling et al. 2007). Butterfly species were classified into five distribution classes (1 = Kenyan endemic, 2 = endemic to East Africa, 3 = restricted to eastern and southern Africa (including the Arabian Peninsula), 4 = restricted to sub-Saharan Africa (including western Africa), 5 = distributed across the African continent and beyond). They were also classified depending on habitat specialisation (habitat generalists = species present in all four habitat types during the rainy season; habitat specialists = species found in only one single habitat type). This working definition allowed us to compare butterfly species composition across habitat types and seasons. Raw data are given in Table A4 of Appendix A.
Statistics
To assess the differences in bird and butterfly community composition among habitat types and seasons, we used variance based principal components (PCA), two way Permanova and ANOVA, and contingency table analysis. We estimated species richness using the first order Jackknife.
A general linear fixed effects model (glm) with robust parameter estimation served to link bird and butterfly richness to butterfly abundances and habitat characteristics (metric predictors), and habitat type and season (categorical predictors). Due to collinearity of the habitat parameters, we reduced dimensionality by PCA, and the first two eigenvectors (explaining 68.7% and 26.9% of variance) entered the linear model as habitat characteristics. Additionally, we visualised the distribution of species across habitat types using principal coordinates analysis (Sørensen dissimilarity) and unconstrained seriation.
Principal coordinates analysis (Sørensen dissimilarity, Eigenvector scales) of dry (a, b, c) and rainy (d, e, f) seasons separated pastures (dark yellow: butterflies, light yellow: birds) from forest interior (dark red: butterflies, light red: birds) while forest edges (dark blue: butterflies, light blue: birds) and orchards (dark green: butterflies, light green: birds) clustered together
Results
In total, we recorded 1899 birds representing 89 species (Table 1, Appendix A). During the dry season, the forest interior, and during the rainy season, orchards showed highest bird species richness (observed and estimated richness, Table 1), although two-way ANOVA did not point to significant differences in bird species richness among seasons and habitat types (Table 2). Similarly, the glm did not indicate that bird species richness and abundances significantly differed among seasons and habitat types (Table 3). Additionally, the model did not find evidence that habitat characteristics and numbers of butterflies affected bird richness (Table 3). Particularly, abundances of insectivorous birds were not significantly correlated with butterfly abundances (Fig. B1).
Frugivore, granivore and insectivore bird species richness did not markedly differ among seasons (Table 1). We did not detect granivore species in the forest interior, and only two species along the forest edge (Table 1). In both seasons, pastures were inhabited by a comparatively low number of frugivorous birds (Table 1). Bird abundances significantly differed among habitats (Table 2), and we recorded the highest number of individuals on pastures (Table 1). Granivores reached highest abundances in orchards and on pastures (Table 1), while insectivores were most abundant on pastures (Table 1).
We recorded 6258 butterflies representing 94 species (Table 1). During both, dry and rainy season, orchards were most species rich (Table 1). The lowest numbers of individuals and species were recorded in the forest interior. Two way ANOVA and glm pointed to significant difference in butterfly richness and abundances among seasons and habitats (Tables 2 and 3).
For birds and butterflies, analyses of community composition pointed to significant differences among habitats and seasons. Only ten birds (i.e. 11%) and five butterfly species (i.e. 5%) occurred in all habitat types during both seasons, but 26 bird and 18 butterfly species in all four habitat types in one of the seasons (Table B1). Pastures and forest interior strongly differed in their bird and butterfly composition, while orchards and forest edges overlapped (Table 2; Fig. 2, Figs. B2, B3, B4, B5). Orchards and forest were more strongly differentiated in the dry than in the rainy season (Fig. 2). Ward and k-means cluster analyses identified specific pasture and forest interior butterfly communities during the rainy and less during the dry season (Fig. B5, Table B2). However, only 14 of the 36 forest associated butterfly species (38.9%) were indeed restricted to the forest interior or edge (Fig. B5).
Pairwise analyses of species overlap showed a higher overlap of bird communities in the rainy than the dry season (Table 4). We obtained the highest overlap between forest interior and orchards in the rainy season (59%), particularly for insectivores (87%). During the dry season, this overlap was less than 50% (Table 4). Butterfly communities overlapped less than birds among habitats and seasons (Table 4). During the dry season, overlap in butterfly species composition was always less than 50%, while in the rainy season overlap > 50% occurred for orchards and pastures (58%) as well as orchard and forest edge (59%), in accordance with the results of the PCA analysis (Fig. 2).
The separate analysis of habitat generalists and specialists confirmed that 19 bird generalist species (Table B3) occurred in all four habitat types during the dry season, and 74% of them did so during the rainy season (Table B4). In the rainy season, particularly forest edges, orchards and pastures had a high (> 85%) pairwise overlap while the forest interior was slightly more different in species composition (< 81%) (Table B4). Specialist species were indeed more habitat specific and most species rich in the forest interior and on pastures (Table B3). Pairwise compositional overlap among habitat types was low in the dry season (< 20%, Table B4), while forest interior and orchards show a higher degree of overlap in the rainy season (35%, Table B4).
Per definition, 79% of all butterfly species were scored as specialists during the rainy season, while their percentage dropped to 45% during the dry season (Table B3). In both seasons, specialist butterflies were most species rich in orchards (dry: 51 and rainy: 57 species) and comparatively species poor in the forest interior (25 and 22 species, Table B3). As in birds, forest edges, orchards and pastures showed the highest overlap in specialist butterfly species (Table B4).
Discussion
Similarity in community structures
The diversification of communities of both taxonomic groups had pronounced similarities among habitats and seasons. Thus, we obtained distinct species communities in the different habitat types assessed. For both taxa, differences in community structures were strongest between forest interior and open pastures, while communities along forest edges and in orchards were more similar and largely overlapping. Community structures were more habitat-specific for both taxa in the dry season, and particularly strong for insectivorous birds. However, overlaps were in general more pronounced in birds than in butterflies.
The development of such small-scale, habitat-specific communities largely depends on two main factors: the degree of habitat and resource specialisation and as well as the dispersal of the respective species. Thus, the strong differentiation obtained in our study between forest interior and pasture communities might be the result of diverging abiotic and biotic conditions and resources provided by these different habitat types, and the species relying on them (see Mulwa et al. 2021). Open land species in both taxonomic groups show clear ecological as well as behavioural differences if compared with forest species, and the flight performance of birds and butterflies differs among these two habitat types (Le Roy et al. 2019). Thus, open land butterfly species for example have rather elongated wings, while forest butterflies are characterized by broader wings (Shreeve et al. 2009). These are adaptations to different flight types optimised to perform perfectly in the respective ecosystems.
Our results underline that the natural coastal forests of East Africa are an essential and unique ecosystem for both, birds and butterflies. Assumedly, birds and butterflies develop successfully even inside of small forest remnants (Soi et al. 2018). Birds breed and raise their brood there, but as adults, they expand their territories significantly and also colonise the adjacent habitats, such as orchards. Similarly, some butterfly species develop exclusively in the forest interior, such as members of the lichen-feeding genera Baliochila and Teriomima (see also Lehmann & Kioko 2003, Lehmann 2005). Such species rely on specific larval food plants as well as on specific microclimatic conditions (Veddeler et al. 2005). After successful larval development inside the forest, more mobile adults such as Graphium species frequently colonise adjacent, open and sunnier habitats (Habel et al. 2018), to benefit from nectar and ripe fruits (see Habel et al. 2018), and fly back into the forest for oviposition.
The intermediate position of community structures in transitional habitats, such as forest edges, call for an intermixing of forest and open land species there, for both taxa. However, the typical edge structure pattern with higher species-richness at forest edges than in the adjoining areas (cf. Lacasella et al. 2015, Habel et al. 2018, Tõnisalu and Väli 2022) was only weakly expressed in butterflies but not birds, as also observed in other taxa (cf. Łuczaj et al. 1997; Heliölä et al. 2001, Wermelinger et al. 2007). This minor difference might be explained by the higher mobility of birds and the often more opportunistic use of habitats allowing their dispersal further away from their typical habitat.
The high diversity and abundance in the studied orchards might be due to the fact that they may act as surrogate habitat for some forest species, in particular birds. Additionally, the high heterogeneity of this habitat representing features of open and forested areas allows for a mixture of diverse elements, in particular for butterflies. Furthermore, orchards provide coveted shade at midday for numerous open-land species (Lugo 1997; Dean et al. 1999), thus making them even more attractive. However, against the background of a dynamic dispersal pattern depending on life-cycles, the interpretation of species richness and abundances and the ecological value of potential surrogate habitats should be done with caution (Ghazoul 2002). Consequently, high species richness and abundance in orchards do not necessarily indicate that this habitat type is a true surrogate ecosystem for typical forest species. In our case, this needs to be questioned, in particular for butterflies.
Divergence in diversity and abundance
Our results show that species richness and abundance of birds remained comparatively constant across seasons, while butterfly numbers fluctuate significantly and peak during the rainy season. Such seasonal fluctuations of insects are well-known also in the tropics and can be quite pronounced. The comparatively limited seasonal variability of birds might be explained by the contrasting reproduction biology of birds and butterflies as well as differences in longevity and taxon-specific mortality rates. Furthermore, many insects directly dependent on fresh leaves (larvae in butterflies) and nectar of flowering plants (imagoes in butterflies) (Wäckers et al. 2007) with strong seasonal fluctuations. Thus, their generation cycles are seasonal, and the abundance of continuously occurring species strongly increases for most insect species during the rainy season (da Silva et al. 2011, Habel et al. 2018, Schmitt et al. 2021).
We found highest bird species richness in the forest and adjoining orchards without significant changes along the two seasons. In contrast, most butterfly species were observed in orchards and the fewest in the forest interior. This difference might be real or an artefact of visibility in butterflies. Other than in birds, which can be recorded via their songs and which are often sufficiently large to be spotted in the canopy from the ground, the occurrences of important canopy Lepidoptera are difficult to record and are visible only at small clearings in the forest. In moth, particularly species-rich communities were reported for the canopy layer, strongly diverging from the understory (Schulze et al. 2001; Beck et al. 2002; Brehm 2007, Maicher et al. 2020, Rabl et al. 2020). Thus, diversity and abundances of these ecological indicators exhibit deviating spatial and temporal pattern what might be explained by taxon specific differences (Perfecto et al. 2003, Larson et al. 2012) and hence might be a more general feature.
The conservation value of natural coastal forests
Our results show that most habitat specialist bird species were observed inside the forest; the habitat generalists exist in the other, more disturbed anthropogenic habitats. A similar picture emerges for butterflies: Although the forest has a comparatively low total number of recorded species, the typical and ecologically demanding taxa are found in this habitat (but some of these species were also found in orchards, most likely as visitors). These results prove that especially the ecologically demanding species need natural habitats with little disturbance, while the generalists are found in anthropogenic, disturbed ecosystems. Even if a natural ecosystem has a comparatively low total number of species, precisely this ecosystem is the only suitable habitat for such ecologically demanding species. Similar results underlining the pivotal importance of the conservation of tropical forests were obtained in other regions of the tropics and for other groups of organisms (Axmacher et al. 2004; Brehm and Fiedler 2005; Fiedler et al. 2007; Struebig et al. 2013; Medellín et al. 2000). Therefore, the protection and conservation of these ecologically sensitive indicator species should be given priority in practice-oriented nature and species conservation.
Conclusions
Although both species groups to some extend show similar trends and pattern, birds and butterflies also reveal remarkable differences. Birds do not respond strongly on seasonal effects and remain largely constant throughout the year in terms of total species numbers, abundance and community structures, while butterflies show remarkable fluctuations. Birds as well as butterflies form distinct community structures for specific habitat types, but the spatial restriction of typical forest specialists is less pronounced in birds than in butterflies. In this context, when interpreting our data, we have to consider the entire life cycles and their differences in both groups. Thus, an orchard may represent a surrogate habitat for some adult forest species, in particular for the more mobile forest birds, but the entire development of such species still strongly relies on the largely intact natural forest, hence underlining its high conservation value.
Data availability
All raw data are provided electronically as supplementary material.
References
Axmacher JC, Tünte H, Schrumpf M, Müller-Hohenstein K, Lyaruu HV, Fiedler K (2004) Diverging diversity patterns of vascular plants and geometrid moths during forest regeneration on Mt Kilimanjaro, Tanzania. J Biogeogr 31:895–904
Barbaro L, van Halder I (2009) Linking bird, carabid beetle and butterfly life-history traits to habitat fragmentation in mosaic landscapes. Ecography 32:321–333
Beck JAN, Schulze CH, Linsenmair KE, Fiedler K (2002) From forest to farmland: diversity of geometrid moths along two habitat gradients on Borneo. J Trop Ecol 18:33–51
Bibby C, Jones M, Marsden S (1998) Expedition eld techniques: bird surveys. Royal Geographical Society, London, United Kingdom.
Bibby CJ (1999) Making the most of birds as environmental indicators. Ostrich 70:81–88
Blair RB (1999) Birds and butterflies along an urban gradient: surrogate taxa for assessing biodiversity? Ecol Appl 9:164–170
Bonebrake TC, Ponisio LC, Boggs CL, Ehrlich PR (2010) More than just indicators: a review of tropical butterfly ecology and conservation. Biol Conserv 143:1831–1841
Brehm G (2007) Contrasting patterns of vertical stratification in two moth families in a Costa Rican lowland rain forest. Basic Appl Ecol 8:44–54
Brehm G, Fiedler K (2005) Diversity and community structure of geometrid moths of disturbed habitat in a montane area in the Ecuadorian Andes. J Res Lepidoptera 38:1–14
Chowdhury S, Fuller RA, Dingle H, Chapman JW, Zalucki MP (2021) Migration in butterflies: a global overview. Biol Rev 96:1462–1483
da Silva NAP, Frizzas MR, Oliveira CM (2011) Seasonality in insect abundance in the Cerrado of Goiás State, Brazil. Revista Brasileira de Entomologia 55, 1.
Dean WRJ, Milton SJ, Jeltsch F (1999) Large trees, fertile islands, and birds in arid savanna. J Arid Environ 41:61–78
Fiedler K, Hilt N, Brehm G, Schulze CH (2007) Moths at tropical forest margins—how mega-diverse insect assemblages respond to forest disturbance and recovery. Linking ecologicconomic and social constraints of land use and conservation, Stability of tropical rainforest margins, pp 37–58
Fleishman E, Betrus CJ, Blair RB (2003) Effectsof spatial scale and taxonomic group on partitioning of butterfly and birddiversity in the Great Basin, USA. LandscapeEcology 18:675–685
Fungomeli M, Cianciaruso M, Zannini P, Githitho A, Frascaroli F, Fulanda B, Kibet S, Wiemers M, Mbuvi MT, Matiku P, Chiarucci A (2020) Woody plant species diversity of the coastal forests ofKenya: filling in knowledge gaps in a biodiversity hotspot. Plant Biosystems - An International Journal Dealing with allAspects of Plant Biology 154:973–982
Ghazoul J (2002) Impact of logging on the richness and diversity of forest butterflies in a tropical dry forest in Thailand. Biodivers Conserv 11:521–541
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal of birds. Annu Rev Ecol Syst 13:1–21
Habel JC, Lens L, Eggermont H, Githiru M, Mulwa RK, Suleiman Shauri H, Lewinsohn TM, Weisser WW, Schmitt T (2017) More topics from the tropics: additional thoughts to Mammides. — Biodivers Conserv 26:237–241
Habel JC, Seibold S, Ulrich W, Schmitt T (2018) Seasonality overrides differences in butterfly species composition between natural and anthropogenic forest habitats. Anim Conserv 21:405–413
Habel JC, Angerer V, Gros P, Teucher M, Eberle J (2022a) The relevance of transition habitats for butterfly conservation. Biodivers Conserv 31:1577–1590
Habel JC, Teucher M, Gros P, Gfrerer V, Eberle J (2022b) The importance of dynamic open-canopy woodlands for the conservation of a specialist butterfly species. Landscape Ecol 37:2121–2129
Hagemeijer WJM, Blair MJ (1997) The EBCC atlas of European breeding birds: their distribution and abundance. T& A D Poyser, London
Hamer KC, Hill JK, Benedick S, Mustaffa N, Chey VK, Maryati M (2005) Diversity and ecology of carrion- and fruit-feeding butterflies in bornean rain forest. J Trop Ecol 22:25–33
Heliölä J, Koivula M, Niemelä J (2001) Distribution of carabid beetles (Coleoptera, Carabidae) across a boreal forest–clearcut ecotone. Conserv Biol 15:370–377
Jaetzold R, Schmidt H, Hornetz B, Shisanya CA (2010) Farm Management Handbook of Kenya. Ministry of Agriculture/ Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ).
Kissling WD, Rahbek C, Böhning-Gaese K (2007) Food plant diversity as broadscale determinant of avian frugivore richness. Proc Royal Soc London B 274:799–808
Kudrna O (2011) Distribution atlas of butterflies in Europe. Gesellschaft für Schmetterlingsschutz e.V, Halle
Lacasella F, Gratton C, De Felici S, Isaia M, Zapparoli M, Marta S, Sbordoni V (2015) Asymmetrical responses of forest and beyond edge arthropod communities across a forest–grassland ecotone. Biodivers Conserv 24:447–465
Larsen TB (1991) The butterflies of Kenya and their natural history. Oxford University Press, Oxford
Larsen FW, Bladt J, Balmford A, Rahbek C (2012) Birds as biodiversity surrogates: will supplementing birds with other taxa improve effectiveness? J Appl Ecol 49:349–356
Le Roy C, Debat V, Llaurens V (2019) Adaptive evolution of butterfly wing shape: from morphology to behaviour. Biol Rev 94:1261–1281
Lehmann I (2005) Lepidoptera diversity, floristic composition and structure of three Kaya forests on the South coast of Kenya. J East Afr Nat History 94:121–163
Lehmann I, Kioko E (2003) The final report on butterflies and moths and their habitats. Three Kaya forests of the Kenya Coast. Kwale District” Presented to The Ministry Of Education, Science And Technology, Nairobi, Kenya
Łuczaj Ł, Sadowska B (1997) Edge effect in different groups of organisms: vascular plant, bryophyte and fungi species richness across a forest-grassland border. Folia Geobotanica & Phytotaxonomica 32:343–353
Lugo AE (1997) The apparent paradox of reestablishing species richness on degraded lands with tree monocultures. For Ecol Manag 99:9–19
Lund MP, Rahbek C (2002) Cross-taxon congruence in complementarity and conservation of temperate biodiversity. Anim Conserv 5:163–171
Mac Nally R, Fleishman E, Bulluck LP, Betrus CJ (2004) Comparative influence of spatial scale on beta diversity within regional assemblages of birds and butterflies. J Biogeogr 31:917–929
Maicher V, Sáfián S, Murkwe M, Delabye S, Przybyłowicz Ł, Potocký P, Tropek R (2020) Seasonal shifts of biodiversity patterns and species’ elevation ranges of butterflies and moths along a complete rainforest elevational gradient on Mount Cameroon. J Biogeogr 47:342–354
Medellín RA, Equihua M, Amin MA (2000) Bat diversity and abundance as indicators of disturbance in neotropical rainforests. Conserv Biol 14:1666–1675
Mulwa M, Teucher M, Ulrich W, Habel JC (2021) Bird communities in a degraded forest biodiversity hotspot of East Africa. Biodivers Conserv 30:2305–2318
Nelson GS, Nelson SM (2001) Bird and butterfly communities associated with two types of urban riparian areas. Urban Ecosyst 5:95–108
Perfecto I, Mas A, Dietsch T, Vandermeer J (2003) Conservation of biodiversity in coffee agroecosystems: a tri-taxa comparison in southern Mexico. Biodivers Conserv 12:1239–1252
Pollard E, Yates TJ (1993) Monitoringbutterflies for ecology and conservation–the British butterfly monitoringscheme. Chapman & Hall, London
Rabl D, Gottsberger B, Brehm G, Hofhansl F, Fiedler K (2020) Moth assemblages in Costa Rica rain forest mirror small-scale topographic heterogeneity. Biotropica 52:288–301
Schmitt T, Ulrich W, Delic A, Teucher M, Habel JC (2021) Seasonality and landscape characteristics impact species community structure and temporal dynamics of east African butterflies. Sci Rep 11:15103
Schulze CH, Linsenmair KE, Fiedler K (2001) Understorey versus canopy: patterns of vertical stratification and diversity among Lepidoptera in a Bornean rain forest. In Tropical Forest Canopies: Ecology and Management: Proceedings of ESF Conference, Oxford University, 12–16 December 1998 (pp. 133–152). Springer, Netherlands
Schulze CH, Waltert M, Kessler PJ, Pitopang R, Veddeler D, Mühlenberg M, Tscharntke T (2004) Biodiversity indicator groups of tropical land-use systems: comparing plants, birds, and insects. Ecol Appl 14:1321–1333
Sekercioglu CH (2012) Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J Ornithol 153:153–161
Shreeve T, Konvička M, Hv Dyck (2009). In: Settele J (ed) Functional significance of butterfly wing morphology variation. Cambridge University Press, Cambridge
Soi B (2018) Status of restricted-range bird species of east African Coastal forests in five south Coast Forest of Kenya. J Agric Ecol Res Int 16:1–10
Struebig MJ, Turner A, Giles E, Lasmana F, Tollington S, Bernard H, Bell D (2013) Quantifying the biodiversity value of repeatedly logged rainforests: gradient and comparative approaches from Borneo. Adv Ecol Res 48:183–224
Tõnisalu G, Väli Ü (2022) Edge effect in rodent populations at the border between agricultural landscapes and forests. Eur J Wildl Res 68:34
Ulrich W, Lens L, Tobias JA, Habel JC (2016) Contrasting patterns of species richness and functional diversity in bird communities of east African cloud forest fragments. PLoS ONE 11:e0163338
Ulrich W, Banks-Leite C, De Coster G, Habel JC, Matheve H, Newmark WD, Lens L (2018) Environmentally and behaviourally mediated co-occurrence of functional traits in bird communities of tropical forest fragments. Oikos 127:274–284
Veddeler D, Schulze CH, Steffan-Dewenter I, Buchori D, Tscharntke T (2005) The contribution of tropical secondary forest fragments to the conservation of fruit-feeding butterflies: effects of isolation and age. Biodivers Conserv 14:3577–3592
Vessby K, Söderström BO, Glimskär A, Svensson B (2002) Species-richness correlations of six different taxa in Swedish seminatural grasslands. Conserv Biol 16:430–439
Wäckers FL, Romeis J, van Rijn P (2007) Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Ann Rev Entomol 52:301–323
Wermelinger B, Flückiger PF, Obrist MK, Duelli P (2007) Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges. J Appl Entomol 131:104–114
Williams CB (1930) The Migration of butterflies. Oliver & Boyd, Edinburgh & London, p 473
Wolters V, Bengtsson J, Zaitsev AS (2006) Relationship among the species richness of different taxa. Ecology 87:1886–1895
Wurz A, Grass I, Lees DC, Rakotomalala AANA, Sáfián S, Martin DA, Tscharntke T (2022) Land-use change differentially affects endemic, forest and open-land butterflies in Madagascar. Insect Conserv Divers 15:606–620
Zellweger F, Roth T, Bugmann H, Bollmann K (2017) Beta diversity of plants, birds and butterflies is closely associated with climate and habitat structure. Glob Ecol Biogeogr 26:898–906
Acknowledgements
We thank Alex Syingi of National Museums of Kenya (NMK), Mr. Kubo and Mr Dubi both of Kaya Kambe for their assistance in the field. We thank the German Academic Exchange Service (DAAD) in the framework of the Biocult project for financial support. We thank an anonymous and very constructive reviewer for critical and fruitful comments.
Funding
Open access funding provided by Paris Lodron University of Salzburg. Funding was received by the German Academic Exchange Service, DAAD.
Author information
Authors and Affiliations
Contributions
JCH, TS and MT elaborated the study design, MM, TS and LW collected the data, WU did statistical analyses, all contributed while writing this article. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
There exist no competing interests.
Ethical approval
Not applicable.
Additional information
Communicated by David Hawksworth.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Habel, J.C., Mulwa, M., Wagner, L. et al. Diverging responses to natural and anthropogenic habitats in aftrotropical birds and butterflies. Biodivers Conserv 33, 971–985 (2024). https://doi.org/10.1007/s10531-023-02762-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-023-02762-0