Abstract
Species present in transformed landscapes utilise resources from either the transformed patches, natural patches, or both. Depending on the degree to which species are specialised, species-specific responses to landscape transformation is both varied and dynamic. Understanding species-specific responses across a landscape is therefore essential to make informed conservation decisions. We investigate how spillover of stenotopic, cultural, and ubiquitous insect species respond to the different edge combinations present in a timber plantation mosaic. We use a multi-taxon approach, sampling two distinct insect assemblages (ground and foliage) across four different biotopes in a grassland-forest-plantation landscape in the Midlands of KwaZulu-Natal, South Africa, which forms part of the Maputaland-Pondoland-Albany biodiversity hotspot. We show that in this landscape, ecological networks consisting of natural grassland and forest supported high insect diversity in both ground and foliage assemblages. The foliage assemblage displayed stronger responses to the different biotope combinations than the ground assemblage. There was remarkably little spillover of habitat specialist species of either the ground or foliage assemblages from grassland or forest into the plantation stands. The few species associated with plantation stands (cultural species) had consistent levels of spillover across different types of plantation blocks, but not into the natural areas. Natural biotopes in this landscape conserved many habitat specialists, whereas plantations maintained low levels of diversity. Thus, we recommend that conservation practitioners consider the fidelity and spillover of the species present in the local landscape before designing conservation plans in these dynamic production landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species in transformed landscapes (for example, where natural ecosystems such as grasslands are replaced with plantation forestry) may often use resources from the semi-natural and natural habitat patches which provide essential and alternative resources, such as overwintering sites, alternative host species, and energy sources (Rand et al. 2006). However, there is additional complexity as specialization among individual species drives their local distribution among transformed and natural areas according to their resource requirements (Dröse et al. 2019; Wimp and Murphy 2021). In contrast, for some habitat generalist species (ubiquitous species) present in both natural and transformed regions of the landscape, transformation may have little effect on their local distribution patterns. In addition, transformation can lead to increasing abundance of cultural species (species that readily occupy certain transformed areas) within the transformed regions of these landscapes, as cultural species show strong affinities towards production areas of transformed landscapes (Duelli and Obrist 2003; Plath et al. 2021).
Species spillover occurs when species present in one biotope spillover into surrounding biotopes (Brudvig et al. 2009). The directionality and magnitude of species spillover across biotopes depends on species adaptations, life history traits, and degree of specialization (Loreau et al. 2013) and varies according to shifts in resource concentration gradients (Rand et al. 2006; Loreau et al. 2013). Furthermore, areas of high concentrations of individuals can act as source populations, facilitating spillover into neighbouring biotopes (Tscharntke et al. 2005; Rand et al. 2006). However, when resources are poor, species will spillover to seek more optimal habitat quality with more suitable resources such as a suitable microclimate, food, or refuges (Reis and Sisk 2004; Rand et al. 2006). In doing so, species richness and abundance may decrease with distance from the edge as conditions become increasingly sub-optimal, especially in production areas (Brudvig et al. 2009).
The physical contrast between natural and transformed patches varies between landscapes. Contrast is highest where intensively managed monocultures are present alongside natural heterogenous areas, potentially leading to isolation of specialist species (Fischer et al. 2008; Kremen 2015). However, when there is less physical and biological contrast between agricultural patches and natural sites there is likely to be more movement and establishment of species either side of the boundary (Fischer et al. 2008). The responses of organisms to these different boundaries needs to be understood to make informed conservation management decisions (Grau et al. 2013; Kremen 2015).
We determine the spillover effects of stenotopic, cultural, and ubiquitous insect species, and how these groups respond to the different edge combinations present in a plantation forestry landscape mosaic. To do this we address the following objectives: (1) We assess the degree of spillover among transformed biotopes and natural biotopes. Transformed biotopes are defined as ‘mature closed canopy plantation stands’, ‘recently planted young plantations’, and ‘natural biotopes, composed of both natural grassland and indigenous forest’. We use a multi-taxon approach to cover both ground living and foliage assemblages and assess how species diversity may change between biotopes. (2) we then identify species showing fidelity to contrasting biotopes ranging from indigenous grassland (grassland stenotopic species), indigenous primary forest patches (forest stenotopic species), and timber production stands (cultural species), as well as species associated with a combination of biotopes (ubiquitous species). (3) We then determine how insect fidelity in each assemblage prevents or enhances spillover into adjacent biotopes. We hypothesize that grassland species, in both ground and foliage assemblages, will show higher spillover into open-canopy young plantation stands compared to mature closed-canopy plantation stands due to the lower structural contrast between the grasslands and these young plantations. We also expect species among the forest assemblages to show a higher degree of spillover than grassland species, into mature plantation blocks as these patches have lower structural contrast. We expect cultural species to spillover between plantation stands rather than into the neighbouring grasslands and forests as these species are likely adapted to survive in the transformed areas.
Methods
Study site and study design
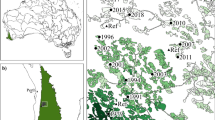
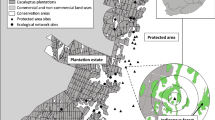
Baynesfield, Richmond and the Byrne Valley in KwaZulu-Natal (bounded by 29° 43’ 5.88” and 29° 51’ 29.52” S; and 30° 10’ 27.48” and 30° 22’ 28.56” E), South Africa, situated in the Maputaland-Pondoland-Albany biodiversity hotspot, was the study area. The region is characterised by a mosaic landscape of timber production areas interspersed with natural patches of primary Southern Mistbelt Forest (referred to as forests from now on) and interconnected tracts of Midlands Mistbelt Grasslands (referred to as grasslands from now on). These forest patches are naturally fragmented, located in fire refugia, at elevations between 1200 and 1400 m above sea level. Podocarpus trees dominate the climax tree community. However, logging of these forests for hardwood timber some decades ago has resulted in the remaining patches being highly fragmented, isolated, and covering an area less than their original extent. Currently, these forest patches are not actively managed. The grasslands are also located at higher elevations where the climate is cool and moist. Some important grass species include Andropogon appendiculatus, Aristida junciformis, Hyparrhenis hirta and Themeda triandra (Mucina and Rutherford 2006). Within the timber production landscape of South Africa these grassland and forest patches are often connected and ensure functional connectivity is maintained within these transformed landscapes. Furthermore, these remnant patches provide refuges and resources for indigenous plant, vertebrate, and invertebrate species (Samways and Pryke 2016).
Sites were located on a privately managed conservancy, as well as on three plantation estates (Baynesfield, Enon and Highlands). Four biotopes were identified for sampling: (1) forest (F), (2) grassland (G), (3) closed canopy plantation (CC) of mature Eucalyptus with trees > 7 years old, and (4) open canopy plantation (OC), where Eucalyptus trees were 2–5 years old, with an open canopy. These four biotopes were selected as they represented the dominant biotopes in the landscape, and all four biotopes were present on all timber estates, while only grasslands and forest patches were present in the conservancy (Fig. 1).
Biotope pairs were defined according to where two biotopes met at a discernible boundary. Five biotope pair combinations were identified, with sampling transects laid out perpendicularly across the boundary. In total, 36 transects, each consisting of a pair of biotopes: CC-G (n = 7); OC-G (n = 8); CC-F (n = 8); CC-OC (n = 7) and F-G (n = 6) were sampled. As biotope pairs correspond to transect types, biotope pairs will now be referred to as transect types. Spatial distribution of the grasslands, forests and Eucalyptus plantations limited the number of transects that were possible per transect type (Fig. 1). At least 300 m separated transects of the same type when situated alongside each other to maintain independent sampling. Transects were 120 m long, extending 60 m either side of the boundary between a biotope pair. Along each transect, six sampling stations were established, with increasing distance from the edge, at 15 m, 30 m, and 60 m into each biotope. In these timber production landscapes, 0 to 32 m from the edge encompasses the edge effect for a wide variety of arthropod taxa (Pryke and Samways 2012), therefore the different station distances selected capture samples from the edge (15 m), edge-core transition zone (30 m), and core biotope sites (60 m). Sampling stations were coded to represent the biotope in which samples were collected as well as indicate distance from the edge, for example CC60 means the station was situated in the closed canopy plantations 60 m from the edge.
Invertebrate sampling
Sampling was conducted during March-April 2017 to coincide with the austral summer (i.e., the wettest and hottest time of the year) when adult insect activity is the highest for most species. Four different sampling techniques were used, two specific methods for each target assemblage. Active searches and pitfall traps were used to sample the ground active insect assemblages and sweep netting and vacuum suction samples were conducted to sample the foliage dwelling assemblages. Based on the recommendations of Gerlach et al. (2013), ants and beetles were chosen to represent the ground assemblage, while bugs, grasshoppers, flies, wasps, and bees were selected to represent the foliage assemblage. These taxa were chosen to represent the ground and foliage assemblages while remaining taxonomically and functionally complementary to one another.
To obtain ground assemblage samples at each station along a transect, four pitfall traps (70 mm diameter) were dug in flush with the soil and half filled with a 50:50 mixture of ethylene glycol and water, with a drop of detergent added to break the surface tension (Samways et al. 2010). Traps were placed in a line parallel to the biotope boundary and spaced 1 m apart. Each transect was represented by 24 samples. Traps were left out for one week, collected, and samples washed and stored in 75% ethanol until identification. For analyses, the four traps per station were pooled. In addition, the soil surface and leaf litter of a randomly selected 1 m2 quadrat were searched actively by two collectors for 10 min at each station. All specimens visible to the naked eye were collected and stored in 75% ethanol until identification and added to the overall data set. Sampled individuals (beetle and ant) were identified to morphospecies level, an acceptable method to use in biodiversity-rich areas where taxonomic knowledge of the individual species is lacking (Gerlach et al. 2013). Each morphospecies identified was then later identified to family level using the relevant keys in Scholtz & Holm (1985), the most comprehensive set of keys for the insects in the region.
Foliage assemblage samples were collected at each sampling station along a transect using a sweep net and a vacuum sampler. Samples of both methods were collected along a secondary transect that ran parallel to the biotope edge to ensure that the sampling distance was consistently at 15 m, 30 m, and 60 m respectively. All samples were collected from vegetation during dry weather conditions. The grassland samples were from the grass layer (i.e., not from any taller vegetation present), while in the forests and plantations only the shrubs and smaller plants forming the forest floor cover were sampled. Sweep net (net diameter 50 cm) and suction samples (using a Stihl SH56 petrol powered leaf shredder and fitted with an oval nozzle 14 cm x 10 cm wide, combined with a fine grain mesh collection net) were collected in opposite directions to each other to prevent one method from interfering with the success of the other. Sampling consisted of 200 sweeps with the sweep net (at each step the net was passed through the vegetation in front of the collector, sweeps therefore alternated direction with each step), and 100 insertions of the suction sampler into the vegetation in front of the collector. Specimens were stored in 75% ethanol until identification at a later stage. Flies (Diptera), bugs (Hemiptera), grasshoppers (Orthoptera) and bees and wasps (Hymenoptera) were identified to morphospecies from both the sweep net samples and vacuum samples that comprised the foliage assemblage. Keys in Scholz & Holme (1985) were used to identify morphospecies to family level.
A reference collection for both assemblage types is housed in the entomology museum of the Department of Conservation Ecology and Entomology, Stellenbosch University, South Africa.
Statistical analyses
To ensure sample completeness before further analysis, species accumulation curves based on Chao entropy values were calculated for the four sampled biotopes in both assemblage types. iNEXT (Hsieh et al. 2016) in R (R Core Team 2020) was used. To test for spatial autocorrelation in the data, a Mantel test was performed in R using ade4 (Dray and Dufour 2007), a species-site matrix and the central coordinates of each transect were used. The Mantel test indicated that spatial autocorrelation was present in the ground active data (Mantel test = 0.13, p = 0.002), although there was no spatial autocorrelation in the foliage data (Mantel test = 0.06, p = 0.06). To account for the influence of the spatial distribution of sampling sites (Bolker et al. 2009), random spatial variables were included in all models conducted on both the ground and foliage data sets.
As the study area was in a biodiversity hotspot and the focal taxa the hyper-diverse insects, it is unlikely that sampling will ever reach completeness. So, to standardise effort and make the samples more comparable, Hill numbers and specifically Shannon’s entropy (q = 1) were used (Chao et al. 2014). Shannon’s entropy was calculated using hillR (Li 2018). To address the first objective to determine the degree of spillover between biotopes, generalised linear mixed effects models (GLMMs) were then used to compare the distribution of species diversity (Shannon’s entropy) between the six sampling stations of the five sampled transect types to assess patterns across the different types of edges. lme4 (Bates et al. 2015) was used. This was done for both the surface and foliage assemblages, resulting in 10 models, each based on the subset data per transect type for the two assemblage types. For all models sampling station was the fixed effect and transect number (the identity of each sampled transect) was the random effect. A gamma distribution was used in the GLMMs conducted with Laplace approximations, Quantile-quantile plots were used to compare the fit of the different distribution families in MASS (Venables and Ripley 2002) and car (Fox and Weisberg 2019). Tukey post hoc comparisons, in the package multcomp (Hothorn et al. 2008), were used to identify sampling stations whose species diversity differed significantly.
To address the second objective and to determine which species showed biotope fidelity, multi-level pattern analysis was used, which focuses on any significant association between species distributions and various biotopes, identifying species indicative of various biotopes as well as biotope combinations. The package indicspecies (De Cáceres and Legendre 2009) was used. Species of interest were those stenotopic species associated with the forests (F) or grasslands (G) (Table 1). Cultural species associated were those associated with either or both plantation types (Table 1). Ubiquitous species were those species whose associations were a combination of two or more biotopes (Table 1). As ubiquitous species are included, the inclusion of the F-G and CC-OC transects allows for direct comparison of specialist (stenotopic and cultural species) and generalist species (ubiquitous) across not only natural-transformed edges, but edges between both the natural biotopes and the transformed biotopes respectively.
Then, finally, to determine how insect fidelity impacts the spillover of species into neighbouring biotopes, species richness of each fidelity group (stenotopic, cultural, and ubiquitous) at each station was calculated (van Schalkwyk et al. 2020). From here, the data were subset according to transect type. For each fidelity group, appropriate transects were then selected to ensure that one or two of the biotopes comprising a transect aligned to the fidelity of the subsample of specimens. A total of nine transect-fidelity group pairings were identified and used in the analyses for both the ground and foliage assemblages, resulting in 18 unique biotope-fidelity iterations. Ten individual models were run for the ubiquitous species (five ground, five foliage) according to the fidelity definition, all transect types were relevant to the ubiquitous species. GLMMs with Poisson distributions and Laplace approximations were run to investigate the spillover of species fidelity groupings along the transects by comparing species richness at each station.
Results
Overall, the ground assemblage was made up of 12 974 individuals from 209 species, and the foliage assemblage had 9685 individuals from 673 species (Table 2). Although more abundant, the ground assemblage was less diverse in terms of species richness than the foliage assemblage (Supplementary material 1 summarises the species associations and stations at which each species was sampled). Observed species richness in the four sampled biotopes in both assemblages was consistently lower than the estimated diversity (Ground assemblage: CC species richness = 66 vs. Chao estimate 92.87 ± 15.44; F species richness = 81 vs. Chao estimate 132.17 ± 25.31; G species richness = 152 vs. Chao estimate 248.01 ± 32.9 and OC species richness = 82 vs. Chao estimate 197.49 ± 54.99. Foliage assemblage: CC species richness = 205 vs. Chao estimate 429.56 ± 64.38; F species richness = 261 vs. Chao estimate 373.77 ± 31.08; G species richness = 429 vs. Chao estimate 692.7 ± 52.74 and OC species richness = 175 vs. Chao estimate 310.58 ± 43.19). However, the diversity indices, when plotted on the accumulation curves for the sampled biotopes in each assemblage approached the extrapolated asymptotes (Supplementary material 2).
Coarse spillover effects were observed in both assemblage types, when considering diversity at the level of sampling station along each transect. In turn, significant differences were observed among the foliage assemblages along the CC-G, OC-G and F-CC transects where the transformed biotopes were compared to natural biotopes, while species diversity was significantly higher at the natural sampling stations. Where natural vs. natural (F-G transects) or non-natural vs. non-natural (CC-OC transects), were compared, differences in diversity were not were not statistically significant between sampling stations for the foliage assemblages. In the case of the ground assemblages, Shannon’s entropy was only significantly higher in one transect type (CC-G), where the G30 station was significantly higher than that of the CC15 and CC60 stations (Table 3; Fig. 2).
Violin plots showing changes in overall Shannon’s entropy for each biotope, as well changes in Shannon’s entropy along each sampled transect type and for each sampled assemblage. Plots in the first column represent the ground assemblage, and the second column represents the foliage assemblage. Each row represents the same transect type. Abbreviations used: CC – Closed canopy plantations (red colour fill), F – Forest (dark green colour fill), G – Grasslands (light green colour fill) and OC – Open canopy plantations (orange fill colour fill). See Table 2 for detailed pairwise comparisons
By comparing the species richness of specialist fidelity groups (cultural and stenotopic species) at individual sampling stations along each transect, the degree of spillover between biotopes can be determined. In this case, in both ground and foliage assemblages, little or no spillover occurred among the specialist fidelity groups. Where species showed fidelity to a natural biotope (grassland or forest stenotopic species), species richness was significantly higher at transect sampling stations in the natural biotopes than in the two plantation types (Fig. 3). This was the case for grassland species in both assemblage types along both the CC-G and OC-G transects, as well as for the forest species along the F-CC transects for both assemblage types (Table 4).
Violin plots showing how species richness changes across transect types appropriate for each fidelity group. The first column is the ground assemblage, and the second column the foliage assemblage. Each row is a transect fidelity group combination. Abbreviations used: CC – Closed canopy plantations (red colour fill), F – Forest (dark green colour fill), G – Grasslands (light green colour fill) and OC – Open canopy plantations (orange fill colour fill). See Table 3 for detailed pairwise comparisons
Cultural species diversity of both assemblage types was much lower than that of the grassland or forest associated species (Table 2) and as a direct result, species richness of cultural species along the different transects was very low, with a maximum of four species in the foliage assemblage at the OC60 sampling station along the OC-G transect. Among the cultural species in both assemblage types, there were no significant differences between sampling stations at any of the biotope combination. Furthermore, for the ground active species, models run on the cultural species richness along the F-CC and CC-G transects failed due to too few species present (Fig. 3).
The ubiquitous species spilled over among the four different biotopes. In the case of the ground assemblage, sampling station did not significantly influence species richness (Table 4). However, for the foliage assemblage, sampling station was significant for ubiquitous species richness along the CC-G and F-CC transects, yet no significant pairwise differences were evident (Table 4). For both assemblage types, species richness of ubiquitous species was similar along the sampling stations of the five different transect types (Fig. 4).
Violin plots showing how ubiquitous species richness changes across the five different transect types. Ground assemblage is shown in the first column and foliage assemblage in the second column. Each row represents a transect type. CC – Closed canopy plantations (red colour fill), F – Forest (dark green colour fill), G – Grasslands (light green colour fill) and OC – Open canopy plantations (orange fill colour fill). See Table 3 for detailed pairwise comparisons
Discussion
There was little spillover among the grassland and forest stenotopic species, as well as the cultural species, across the boundaries of natural and transformed biotopes. In contrast, as the ubiquitous species group comprised species with associations to a mix of biotopes, there was much spillover across all biotope edge combinations. Furthermore, there was no stenotopic species spillover between natural grassland and forest. These results are not surprising, as spillover is limited by species specialisation, with species adapted to one biotope not expected to persist in biotopes to which they are not adapted. Interestingly, spillover was present between the open and closed canopy plantation stands. This pattern was maintained in the cultural species where there was spillover of cultural species between plantation types and observed among both the ground and foliage assemblages. Nonetheless, the number of cultural species overall was very low.
A clear general picture emerges from these results despite the distribution of species in agro-forestry ecosystems often being highly variable (Brockerhoff et al. 2008). As there is little evidence of spillover of insect diversity from natural to transformed regions in this landscape, retaining and conserving patches of remnant natural vegetation is essential for maintaining specialist insect diversity across the landscape. This was unexpected because even in high contrast landscape such the apple orchard and fynbos landscape mosaics of the Western Cape South Africa and using similar methods as we did here, van Schalkwyk et al., (2020) showed that there was limited spillover of cultural species. In addition, the forest patches in this landscape are naturally fragmented, having been naturally isolated before human transformation and are thus unlikely to show recent extinction debt (Pryke et al. 2013; Yekwayo et al. 2016). Furthermore, species richness and diversity of both assemblages were much higher in natural vegetation than in open or closed canopy plantation stands, which further underscores this point.
In sum, while we hypothesized that both ground and foliage assemblages in grassland would show higher spillover into open-canopy young plantation stands compared to mature closed-canopy plantation stands due to additional pioneer plant species and less habitat structure contrast, this was not the case. Furthermore, we also expected natural forest species to show higher spillover than grassland species into mature plantation blocks as these patches have lower contrast, which again did not happen. Lastly, we expected stenotopic species to show the highest habitat fidelity and show little spillover from natural vegetation into plantation stands, which was the case.
Overall diversity and spillover between biotopes
Often driven by resource availability and population density, spillover requires that species can forage or persist to a degree in neighbouring biotopes (Loreau et al. 2013). For this to occur, essential resources, such as food or host species, must be present close to the home biotope (Rand et al. 2006). Lower contrast between biotopes in terms of similarities in vegetation structure, allows for biotope edges to be more permeable to species, and therefore enables spillover of species able to access resources beyond their natal biotope (Didham and Lawton 1999; Collinge and Palmer 2002; Inclán and Marini 2015; Evans et al. 2016). Within the context of our results, spillover was limited to cultural species between the open and closed canopy plantation stands, suggesting that both the open and closed canopy plantation stands provide equally suitable environments to both the ground and foliage insect assemblages that favour these transformed local environments. Given that these two plantation types are comprised of a monoculture of a single tree species, similarities in vegetation structure, and resource availability can be inferred, even if the canopies and tree ages differed.
Contrast between biotopes is not always dichotomous, but rather can vary along a gradient (Peyras et al. 2013), which here resulted in boundaries between the two plantation stand types, young and mature, being permeable to species of both ground and foliage assemblages, allowing for bi-directional spillover. Greatly differing micro-habitats and thermal environments are present where there is sharp contrast between biotopes, as between grassland and indigenous forest here, and between grassland and the two plantation stand types (Leege and Murphy 2001). Such sharp boundaries greatly reduce permeability, preventing spillover, a well-known phenomenon (Reis and Sisk 2004; Boetzl 2016; Evans et al. 2016). This is evident here, as the cultural species we sampled did not spillover into the natural biotopes, whether grassland or forest.
Biotope fidelity and spillover
Species displaying biotope fidelity were present here among both ground and foliage assemblages. The grasslands and forests supported the highest proportion of stenotopic species among both assemblages. This was not unexpected as semi-natural and natural patches in agroecosystems are essential for the maintenance of landscape-wide species diversity (Duelli and Obrist 2003). In contrast, cultural species were rare among both assemblages, not surprisingly as exotic plantation forestry in this area has been present for < 50 years, a relatively short time for native assemblages to adapt to local plantation conditions.
Our approach here of assessing edge patterns of different fidelity groupings allowed us to test the directionality of spillover of species richness and diversity. Directionality of spillover is important as the specialisation of insect species drives the direction of spillover. Frost et al. (2015) found in a New Zealand system, spillover of generalist hymenopteran predators from pine plantations to native forest was greater than that of specialist parasitoid species. Since generalist species do not require specific prey to be present in the recipient biotope, the spillover of these species depends solely on the abundance of potential prey species, and dual-directional movement of these species between plantation and forest occurs as food resources are available in both biotopes (Frost et al. 2015). This is emphasized by predacious coccinellid beetles following their prey irrespective of the nature of the vegetation, natural or crop, in southern Africa (Magagula and Samways 2001).
Lack of spillover among stenotopic and cultural species here from natural to transformed biotopes, further emphasises the role that a species biotope fidelity plays in preventing cross boundary spillover in this production landscape. For example, the only biotope where ants showed fidelity was grassland, due to specialist ants not spilling over into neighbouring biotopes. Ant assemblages are sensitive to changes in contrast, and their assemblages are known to change significantly across grassland-forest ecotones due to fundamental differences in both microclimates and micro-habitats among the two highly contrasting biotope types (Dröse et al. 2019).
The contrast in this production landscape is due to structural differences among grassland, forest, and plantation types. Each biotope has distinct set of environmental factors driving the insect assemblages, and consequently species assemblages are comprised of species that are best suited to each biotope (Rand et al. 2006). Grasslands stenotopic species here did not spillover into either plantation stand type or into the forests. Structural changes between grassland and forest and between open and closed plantations is associated with changes in thermal environments through shading and woody bush encroachment, microhabitats, and resource availability, effectively excluding open grassland specialists from these woodier environments (Dröse et al. 2019). The implication here is that afforestation of grasslands by exotic timber stands significantly alters the physical environment, and so excludes grassland specialist species through loss of suitable environmental conditions for them (Reino et al. 2009; Dröse et al. 2019). Spillover within this landscape is largely limited to the ubiquitous species that occur in a variety of biotope combinations and move across boundaries and utilise resources in neighbouring biotopes.
The landscape sampled here is designed predominantly in accordance with land sparing principles - plantation timber stands are present within an interconnected web of grasslands and small patches of forests, forming a larger ecological network (Samways and Pryke 2016). Should further expansion of the plantations occur, leading to the grassland and forest patches being replaced with plantation timber, highly specialist species would likely be locally lost from the system unless an insightful land sparing approach is taken. However, land sparing approaches can lead to the isolation of insect populations (Kremen 2015). Although here in this physically well-connected landscape this will likely not be the case as grassland or forest species are able to persist in the large and extensive network of corridors, which provide structural, compositional, and functional connectivity, independent of the impoverishing effects of the plantation stands (Samways and Pryke 2016), and which support only a homogenized fauna of cultural and some ubiquitous species.
Conclusion
As with many production landscapes, large-scale networks of conservation corridors of high-quality natural habitat, contribute significantly to the conservation and maintenance of biodiversity. Here, both grassland and forest insects were significantly richer in species than plantation stands. These ecological networks are diverse, providing essential habitat heterogeneity. Eucalyptus plantation stands, although not as diverse as grassland and forests, support some ground-active and foliage-dwelling species, with some species even showing preference for timber stands. Furthermore, there is some spillover among the plantation stands of different ages, suggesting that the species diversity in the plantation stands is self-sustaining and does not rely on spillover of individuals from the indigenous grassland and forest.
The high fidelity of species to specific vegetation types does raise concern over reduced functional connectivity between remnant patches, which potentially could promote ecological relaxation. However, this is averted by the ecological networks of conservation corridors providing many interconnected habitats, refuges, and topographic opportunities which feed into resilient ecosystems. Overall, the results here distinctly suggest that there are two essentially parallel ecosystems, natural habitat and plantation stands. Neither of these two ecosystems share much insect diversity with the other in terms of both ground active and foliage insects. Thus, we advocate that understanding species fidelity is essential to ensure correct future conservation decisions are made.
Data Availability
Upon publication, data underpinning this study will be made available.
References
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Boetzl FA (2016) Asymmetric carabid beetle spillover between calcareous grasslands and coniferous forests. J Insect Conserv 20:49–57. https://doi.org/10.1007/s10841-015-9838-6
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv 17:925–951. https://doi.org/10.1007/s10531-088-9308-x
Brudvig LA, Damschen EI, Tewksbury JJ, Haddad NM, Levey DJ (2009) Landscape connectivity promotes plant biodiversity spillover into non-target habitats. PNAS 106:9328–9332. https://doi.org/10.1073/pnas.0809658106
Chao A, Gotelli NJ, Hsieh TC, Sander EL, MA KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Collinge SK, Palmer TM (2002) The influences of patch shape and boundary contrast on insect response to fragmentation in California grasslands. Landsc Ecol 17:647–656. https://doi.org/10.1023/A:1021536302195
De Cáceres M, Legendre M (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574. 10.189/08-1823.1
Didham RK, Lawton JH (1999) Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments. Biotropica 31:17–30. https://doi.org/10.1111/j.1744-7429.1999.tb00113.x
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20. https://doi.org/10.18637/jss.v022.i04
Dröse W, Podgasiski LR, Dias CF, de Souza Mendonca M (2019) Local and regional drivers of ant communities in forest-grassland ecotones in South Brazil: a taxonomic and phylogenetic approach. PLoS ONE 14:1–20. https://doi.org/10.1371/journal.pone.0215310
Duelli P, Obrist MK (2003) Regional biodiversity in an agricultural landscape: the contribution of seminatural habitat islands. Basic Appl Ecol 4:129–138. https://doi.org/10.1078/1439-1791-00140
Evans MJ, Banks SC, Davies KF, Mccleanahan J, Melbourne B, Driscoll DA (2016) The use of traits to interpret responses to large scale-edge effects: a study of epigaeic beetle assemblages across a Eucalyptus forest and pine plantation edge. Landsc Ecol 31:1815–1831. https://doi.org/10.01007/s10980-016-0364-z
Fischer J, Brosi B, Daily GC, Ehrlich PR, Goldman R, Goldstein J, Lindenmayer DB, Manning AD, Mooney HA, Pejchar L, Ranganathan J, Tallis H (2008) Should agricultural policies encourage land sparing or wild-life friendly farming? Front Ecol Environ 6:380–385. https://doi.org/10.1890/070019
Fox J, Weisberg S (2019) An R companion to applied regression, third edition. Sage, Thousand Oaks, CA
Frost CM, Didham RK, Rand TA, Peralta G, Tylianakis JM (2015) Community-level net spillover of natural enemies from managed to natural forest. Ecology 96:193–202. https://doi.org/10.1890/14-0696.1
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850. https://doi.org/10.1007/s10841-013-9565-9
Grau R, Kuemmerle T, Macchi L (2013) Beyond ‘land sparing versus land sharing’: environmental heterogeneity, globalization and the balance between agricultural production and nature conservation. Curr Opin Environ Sustain 5:477–483. https://doi.org/10.1016/j.cosust.2013.06.001
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for interpolation and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
Li D (2018) hillR: taxonomic, functional, and phylogenetic diversity and similarity through Hill numbers. J Open Source Softw 3:1041. https://doi.org/10.21105/joss.01041
Inclán DJ, Marini L (2015) Landscape composition affects parasitoid spillover. Agric Ecosyst Environ 208:48–54. https://doi.org/10.1016/j.agee.2015.04.027
Kremen C (2015) Reframing the land sparing/land sharing debate for biodiversity conservation. Ann N Y Acad Sci 1355:52–76. https://doi.org/10.1111/nyas.12845
Leege LM, Murphy PG (2001) Ecological effects of the non-native Pinus nigra on sand dune communities. Can J Bot 79:429–437. https://doi.org/10.1139/b01-015
Loreau M, Daufresne T, Gonzalez A, Gravel D, Leroux SJ, Loeuille N, Mouquet N (2013) Unifying sources and sinks in ecology and Earth sciences. Biol Rev 88:365–379. 10.1111.brv/12003
Magagula CN, Samways MJ (2001) Maintenance of ladybeetle diversity across a heterogeneous african agricultural/savanna land mosaic. Biodivers Conserv 10:209–222. https://doi.org/10.1023/A:1008900302267
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria, South Africa
Peyras M, Vespa NI, Isabel M, Zurita B GA (2013) Quantifying edge effects: the role of habitat contrast and species specialization. J Insect Conserv 17:807–820. https://doi.org/10.1007/s10841-013-9563-y
Plath E, Rischen T, Mohr T, Fischer K (2021) Biodiversity in agricultural landscapes: grassy field margins and simi-natural fragments both foster spider diversity and body size. Agric Ecosys Environ 316. https://doi.org/10.1016/j.agee.2021.107457
Pryke JS, Roets F, Samways MJ (2013) Importance of habitat heterogeneity in remnant patches for conserving dung beetles. Biodivers Conserv 22:2857–2873. https://doi.org/10.1007/s10531-013-0559-4
Pryke JS, Samways MJ (2012) Conservation management of complex natural forest and plantation edge effects. Landsc Ecol 27:73–85. https://doi.org/10.1007/s10980-011-9668-1
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Rand TA, Tylianakis JM, Tscharntke T (2006) Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol Lett 9:603–614. https://doi.org/10.1111/j.1461-0248.2006.00911.x
Reino L, Beja P, Osborne PE, Morgado R, Fabiao A, Rotenberry JT (2009) Distance to edges, edge contrast and landscape fragmentation: interactions affecting farmland birds around forest plantations. Biol Conserv 2:824–838. https://doi.org/10.1016/j.biocon.2008.12.011
Reis L, Sisk TD (2004) A predictive model of edge effects. Ecology 85:2917–2926. https://doi.org/10.1890/03-8021
Samways MJ, Pryke JS (2016) Large-scale ecological networks do work in an ecologically complex biodiversity hotspot. Ambio 45:161–172. https://doi.org/10.1007/s13280-015-0697-x
Scholtz C, Holm E (eds) (1985) Insects of Southern Africa. Butterworth Publishers, Durban, South Africa
Tscharntke T, Rand TA, Bianchi FJJA (2005) The landscape context of trophic interactions: insect spillover across the crop-noncrop interface. Ann Zool Fenn 42:421–432. https://www.jstor.org/stable/23735887
van Schalkwyk J, Pryke JS, Samways MJ, Gaigher R (2020) Environmental filering and spillover explain multi-species edge responses across agricultural boundaries in a biosphere reserve. Sci Rep 10:14800. https://doi.org/10.1038/s41598-020-71724-1
Venables WN, Ripley BD (2002) Modern applied statistics with S, fourth edition. Springer, New York
Wimp GM, Murphy S (2021) Habitat edges alter arthropod community composition. Landsc Ecol 36:2849–2861. https://doi.org/10.1007/s10980-021-01288-6
Yekwayo I, Pryke JS, Roets F, Samways MJ (2016) Conserving a variety of ancient forest patches maintains historic arthropod diversity. Biodivers Conserv 25:887–903. https://doi.org/10.1007/s10531-016-1096-8
Acknowledgements
This project was supported by Mondi Group. Collection permit (OP 399/2018) was issued by Ezemvelo KZN Wildlife. Access to field sites was kindly provided by Mondi Group, NCT Forestry Agricultural Co-Oprative Limited, as well as by the private landowners of Minerva Heights Private Nature Reserve. Furthermore, both Mondi Group and NCT Forestry Agricultural Co-Operative Limited kindly provided GIS layers regarding the timber compartments on their properties, enabling site selection and the creation of the locality map. Assistance in field work was provided by Kondwani Shawula, Nhlanhla Shandu and Tima Sánchez.
Funding
Funding for this study was provided by Mondi Group.
Open access funding provided by Stellenbosch University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the planning of this study. Aileen van der Mescht conducted the fieldwork, sorted the samples and identified species, as well as statistical analyses with input from James Pryke. The initial draft of the manuscript was written by Aileen van der Mescht, with all authors contributing substantially to its revision.
Corresponding author
Ethics declarations
Ethical approval
Collection permit for invertebrate sampling was issued by Ezemvelo KZN Wildlife (OP 399/2018).
Competing Interests
The authors have no relevant financial or non-financial interests to disclose. Mondi Group had no input into the design or sampling of this study, all conclusions reached are the authors own.
Additional information
Communicated by Nigel Stork.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Mescht, A.C., Pryke, J.S., Gaigher, R. et al. Remnant habitat patches provide high value for a wide range of insect species in a timber plantation mosaic. Biodivers Conserv 32, 1755–1775 (2023). https://doi.org/10.1007/s10531-023-02574-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-023-02574-2