Abstract
Assessing landscape connectivity allows defining the degree to which the landscape facilitates or impedes the movement of a species between resource patches. In this phase of climate change and biodiversity crisis, maintaining landscape connectivity by restoring and protecting connecting areas and corridors is a key strategy to ensure the survival of many species. The Eurasian otter (Lutra lutra) is a freshwater top predator that is slowly recovering after a dramatic decline occurred in central and southern Europe in the last century. To assess the chances of otter recolonization of the western Alps, we analyzed environmental connectivity by applying electrical circuit theory to an expert-based resistance surface using the Circuitscape software. The study area included southeastern France, northwestern Italy, and Switzerland. We produced a cumulative current flow map and a gap analysis was also conducted to highlight the “conservation gaps” for optimal corridors. The results revealed that the orography of the landscape was the main factor influencing the quantity and quality of the pathways in the western Alpine landscapes. As main corridors were concentrated on valley bottoms, human pressure could severely diminish animal movement. Despite this, some heavily populated areas showed high connectivity values. Some important pathways did not fall within protected areas, potentially hindering otter dispersal and highlighting the need to expand the system of protected areas in the Alpine arc. Recolonization of Alpine territories by otters can therefore only occur if connectivity and environmental suitability combine to ensure the animals' survival over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Movements of wildlife among habitat patches promote genetic exchange, reduce fluctuations in abundance, and thus promote the persistence of populations over time (Tischendorf and Fahrig 2000). Assessing landscape connectivity allows defining the degree to which the landscape facilitates or impedes the movement of a species between resource patches (Taylor et al. 1993). In this phase of climate change and biodiversity crisis, maintaining landscape connectivity by restoring and protecting core habitat areas and corridors is a key strategy to ensure the survival of many species (Beier and Noss 1998; Tewksbury et al. 2002; Hoegh-Guldberg et al. 2008; Corlatti et al. 2013).
Habitat connectivity is related to both functional and structural connectivity. Functional connectivity is species-specific and it is related to the species' behavior (Doak et al. 1992; Gustafson and Gardner 1996) and the investigated spatio-temporal scale (Wade et al. 2015). Functional connectivity analyses are usually based on measures of movement probability between habitat patches, time spent searching for new patches, immigration rates, and landscape permeability (Kindlmann and Burel 2008). On the other hand, structural connectivity is only related to the landscape structure (Green 1994; With et al. 1997) and is based on the assumption that naturalness and biodiversity increase in areas with low anthropogenic pressure or with uniform abiotic features (Beier and Brost 2010; Theobald et al. 2012). Structural connectivity analysis generally uses approaches based on the configuration of ecological corridors, spacing between elements, and the amount of suitable habitat in the landscape. Connectivity models are especially useful for the study of large mammals (particularly carnivores), birds, reptiles, and amphibians (Correa Ayram et al. 2016; Wood et al. 2022). Also, they can be useful to explore the connectivity in habitats that are particularly vulnerable to anthropic pressures, such as riparian ones (Capon et al. 2013).
Mammals are among the taxonomic groups most affected by habitat fragmentation (Andren 1994; Cardillo et al. 2005; Rivera-Ortíz et al. 2015). The magnitude of this negative effect is related to body size, vagility, degree of specialization, and generation time. In particular, medium- and large-sized or specialized species are more strongly affected by fragmentation (Crooks 2002). Similarly, species with little vagility and short generation times, if isolated, are at greater risk of suffering genetic erosion and becoming extinct (Rivera-Ortíz et al. 2015). Among mammals, carnivores play a key role in regulating ecological communities and ecosystems, even at low densities (Ripple et al. 2014). Predation can certainly limit the presence of herbivores, but it can also affect other carnivores. Carnivores are especially threatened, since they usually have high energy requirements, disperse over large areas in search of prey, and live at low population densities (Ripple et al. 2014). In Europe, for example, the brown bear (Ursus arctos) and Eurasian lynx (Lynx lynx) have been strongly affected by habitat fragmentation (Schmidt et al. 2011; Newbold et al. 2015; Waller and Servheen 2016). The Eurasian lynx, in particular, has suffered a severe contraction of its distribution range over the centuries, and currently, most populations are small and isolated (Von Arx et al. 2004). This has led to the loss of genetic variability, which is one of the major causes of extinction for wild species with a fragmented distribution range (Frankham 2005).
The Eurasian otter (Lutra lutra) is a carnivore mustelid whose ecology is strictly linked to the riparian ecosystem. Despite otters resting and reproducing on the ground, they use waterbodies (lakes, artificial basins, rivers, swamps, and coastal areas) for moving and hunting (Roos et al. 2015). It is considered a flagship species, and its protection helps to drive conservation issues for freshwater habitats and associated species (Dudgeon et al. 2006; Cianfrani et al. 2011; Fuller et al. 2015). The Eurasian otter is one of the species that suffered a strong decline in Europe during the second half of the twentieth century (Mason and Macdonald 1986; Hung and Law 2016). Together with other major threats such as water pollution and human persecution, the destruction, and fragmentation of freshwater habitats (dams construction and clearing of riparian vegetation) led the otter to go extinct in most of Europe (Mason and Macdonald 1986; Kruuk 2006; Ruiz-Olmo et al. 2008; Duplaix and Savage 2018). Nowadays, thanks to conservation policies, its inclusion in Annex I of CITES and Annexes II and IV of the Habitats Directive (Council Directive 92/43/EEC), and the banning of harmful pollutants, the otter is slowly re-colonizing its previous distribution range, and European populations are progressively increasing (Roos et al. 2015; Loy and Duplaix 2020).
Notwithstanding an increase in their distribution range and population numbers throughout Europe, otters' re-colonization patterns are slow in the mountainous areas of the Alpine range (Loy and Duplaix 2020). Specifically, Austria and Slovenia are the only countries where the underway recolonization is including the Alpine range. In Switzerland, the presence of otters is limited to a few scattered sites, whereas the French Alps still lack a stable population (Loy and Duplaix 2020). In Italy, the only viable otter populations occur in south-central regions (Balestreri et al. 2016; Giovacchini et al. 2018), whereas only a few scattered records are available for the Alps in the North-East (Alto Adige and Friuli-Venezia-Giulia regions), likely following dispersion from Austria and Slovenia (Angst and Weinberger 2020; Arthur and Barthélemy 2020; Kranz and Poledník 2020; Lapini et al. 2020; Tremolada et al. 2020).
Otter recovery in the Alps is crucial for the survival of European populations, also considering that almost all populations proved to be genetically isolated from each other (Randi et al. 2003; Buglione et al. 2021). Therefore, this work aims to identify pathways for the dispersal of Eurasian otters in the Western Alps through a large-scale connectivity analysis that may i) offer an accurate framework for the number and quality of corridors; and ii) identify gaps in the network of protected areas where to concentrate efforts to promote gene flow and otter dispersal. For this purpose, we applied circuit theory to predict connectivity corridors highlighting connections from peri-Alpine territories, where permanent populations of otters are currently present, to the core of the Western Alps. The results obtained will therefore serve as an indication for future environmental protection and restoration measures.
Materials and methods
Study area
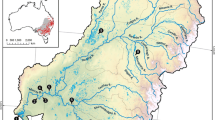
The connectivity analysis was implemented in an area of c. 240,000 km2 including southeastern France, northwestern Italy, and part of Switzerland (Fig. 1). The area is dominated by central European mountain chains: the Alps, French Central Massif, and Jura Massif. Altitude ranges from the sea level to 4810 m above sea level (a.s.l.) of Mont Blanc.
Resistance surface
To describe the relationship between landscape structure and animal movement, we generated a resistance surface as a raster layer where each pixel is assigned a value describing its resistance to the movement of the target species (Adriaensen et al. 2003). To calculate the resistance surface, we selected the following six environmental variables relevant to otter movements and available for the entire study area: the Hydrographic network derived from the HydroSHED dataset (https://www.hydrosheds.org/products/gloric); Digital Elevation Model with a 25 m resolution of the Copernicus program (European Digital Elevation Model, EU-DEM, version 1.1); slope, extracted from the EU-DEM; CORINE Land Cover (scale 1:100.000) with a 100 m resolution; road network (Global Roads Inventory Database, GRIP); dams location (Global Dam Watch dataset, GDW, http://globaldamwatch.org). We decided to exclude datasets describing the width and depth of watercourses of our study area, or distance maps from watercourses o road networks, since the effect of these environmental characteristics on otter dispersal is still unknown, although probably very important for a mammal strongly dependent on water bodies, e.g., for food. Variables were rasterized at 100 m spatial resolution because of the need to have uniform data over the entire study area, but also because the use of high-resolution data for such a large study area would have required an excessively high computational effort, which was not necessary for the result we wanted to achieve, i.e., an overview for the Western Alps. For each variable, we used an expert-based approach to assign a resistance value to each pixel, ranging from 1 (minimum resistance) to 100 (total barrier) (Fig. 2) (Three experts: AL, MdF, CF). We decided to use an expert-based approach because inferential data on otter dispersal are extremely scarce and expensive to obtain and because using inferential data applied to peripheral areas of an expanding species’ range may fail to discriminate between suitable and unsuitable areas since suitable areas not yet be utilized as pathways (Clevenger et al. 2002). The criteria used to determine the resistance values were selected using the Delphi methodology, based on group comparisons between experts (McMillan and Marshall 2006). Specifically, since otters move preferentially along rivers and other water bodies (Tarasoff et al. 1972; Roos et al. 2015), the hydrographic network was expected to provide the least resistance (highest permeability) to otter movements. However, we did not include in our analyses watercourses of Strahler order equal to 1, i.e., those watercourses with a torrential character that have lower flow and are more likely to run dry during certain months of the year (Horton 1945). Also, we considered that otters in Europe are rarely observed above 2000 m a.s.l. (Ruiz-Olmo 1998; Kruuk 2006), have no good climbing skills (Loy et al. 2009; Cianfrani et al. 2013), and tend to avoid intensively cultivated and urbanized areas (Kruuk 2006; Loy et al. 2009). In addition, roads and dams could limit otter movements under specific circumstances, such as the presence of two-lane paved roads (Shepard et al. 2008), or dams located on steep slopes. Details about the permeability scores assigned to each variable are reported in Table S2. Finally, with a swing weights procedure (Malczewski 2000), three experts in agreement ranked the variables in descending order, starting with a score of 100, according to their importance to the otter ecology. Then, the weight of each variable was obtained by dividing the ranking value itself by the sum of all ranking values, results ranged from 0.25 for the hydrographic network to 0.10 for the road network (Table 1), and used as a multiplier when combining the six layers in the final resistance map (Figs. 2, S1). Due to the lack of precise documentation available regarding landscape development plans, land use transformation, or effects of drought on watercourses it was not possible to construct several resistance maps to analyze possible future scenarios.
Quantifying landscape connectivity
To estimate the connectivity in the study area, we used the Circuitscape software version 4.0 (https://circuitscape.org), which adapts concepts from circuit theory to animal movement. The metric used to estimate connectivity is the ''resistance distance'', defined as the effective resistance between a pair of nodes, also considering multiple paths separating them (McRae et al. 2008), in contrast to minimum cost analysis, which instead only identifies the path with the lowest cost and therefore shortest distance (Adriaensen et al. 2003). Even if Circuitscape is mostly used for modeling the dispersal of terrestrial species, it was recently used also for works focusing on species that disperse along linear routes and are strongly related to fluvial habitats, such as the manatee (Haase et al. 2017) and the water vole (Foltête et al. 2016) and also many other aquatic vertebrate and invertebrate species (Dickson et al. 2018). The software reads the resistance surface map as an electric circuit, where habitat patches and dispersal connections are replaced by nodes and resistors (McRae et al. 2008). The current flows from one patch to another and the current values at each pixel are interpreted in terms of the probability that a random “walker” (i.e., an otter in this case) passes through the cell (McRae et al. 2008). A high current flow value of a pixel represents a high probability of passage, and the degree of connectivity between patches increases with the number of connections between pixels. This approach, therefore, highlights the best connection corridors in the study area (McRae et al. 2008). As focal nodes (animal dispersion sources), we used the centroids of peri-Alpine basins in which the otter presence is known (Loy and Duplaix 2020) (Table S3). We produced a cumulative current flow map, showing the sum of all current flows between all possible patches, useful for highlighting essential areas for the maintenance of connectivity in the entire study area.
Gap analysis
To represent only the pathways with a high probability of being undertaken by a random walker, from the cumulative current flow map we extracted a layer containing all pixels with a current flow value ≥ the mean value + 1(SD) of all current flow values (Elliot et al. 2014). The map of protected areas (https://opendata.swiss, combined with https://www.eea.europa.eu) was overlapped with the resulting map to identify high-probability pathways needing protection (Ducci et al. 2019). In addition, for protected areas crossed by optimal corridors, we calculated the area-weighted centrality score, defined as the sum of current flow values passing through all pixels in each protected area divided by the surface of the protected area. This score shows the relative importance of each protected area in providing connectivity to the network (Dickson et al. 2013).
Results
Cumulative current flow map
Values in the final resistance map ranged from 1 to 100 (mean = 15.65, SD = 14.10) (Fig. 2). On the French territory, the basin of River Rhone showed high connectivity downstream to its mouth in the Camargue region. In particular, in the southeastern area (nodes 6, 9, 10) the River Durance, a direct tributary of the Rhone, and its tributaries showed good connectivity values up to their springs, located close to the Italian border (Fig. 3). Further North, in the Savoy region, the River Isère and its tributaries are excellent corridors through the core area of the Western Alps (Fig. 3). Compared to the French-Italian border, the northern part of the study area showed lower current intensities and over smaller portions of territory, generally corresponding to the valley bottom of major watercourses. The lowest connectivity values were detected along the border between Italy and Switzerland, the central area of the Pennine Alps (Fig. 3). In the North of the Rhone, in the Swiss Pre-Alps, connectivity increased due to the presence of several water bodies, such as the Lake of Geneva. In Italy, the Po Valley showed overall low connectivity values due to the absence of nearby focal nodes, although the network including the rivers Adda, Ticino, Dora Baltea, Dora Riparia, Po, and Tanaro offered several low-resistance connections. Their tributaries originate in the Alpine territories of Liguria, Piedmont, Valle d'Aosta, and Lombardy regions forming along the eastern perimeter of the Alpine chain a low resistance belt, which starts from the Maritime Alps and reaches the Swiss border (Fig. 3).
Cumulative current flow map displayed using histogram equalization to increase contrast; numbers refer to focal nodes. The cumulative current flow map represents the sum of the intensity of the currents when all pairs of nodes are connected simultaneously. The map highlights which areas are most important for the connectivity of the whole study area
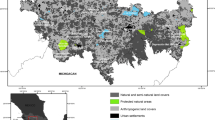
Gap analysis and protected area’s centrality
The area occupied by high probability pathways (optimal surface for dispersal) covered 21,575 km2, of which 77% fell in France (16,580 km2), 12% in Italy (2556 km2), and 11% in Switzerland (2414 km2). About 42% (9095 km2) of high probability pathways were included in protected areas and their buffer zones, while the remaining 52% (12,480 km2) do not (Fig. 4; Table 2). France hosts 48% (7903 km2) of the best-protected corridors and 69% (8677 km2) of the unprotected ones, Italy hosts 29% (742 km2) of the best-protected corridors and 14% (1814 km2) of the unprotected ones, and Switzerland hosts 19% (450 km2) of the protected and 16% (1964 km2) of the unprotected ones. French corridors were concentrated in the Provence-Alps-Côte Azur and Auvergne Rhone Alpes regions (Fig. 4). Several regional nature parks and their buffer zones are crossed by them and may guarantee protection to otters, such as the Préalpes D’Azur Regional Natural Park, Geological Nature Reserve of Digne les Bains (buffer zone included), Provençal Baronies Regional Natural Park, the Vercors Regional Nature Park, Chartreuse and Les Bauges Regional Nature Parks (Fig. 4; Table 3, S4). In Switzerland, the best corridors fell in Bern, Luzern, Uri, Ticino, and Graubünden’s cantons (Fig. 4; Table 3, S4). Protected areas showing high values of centrality were Les Grangettes Nature Reserve, the Rive Sud du lac de Neuchatel, and the Fanel et Chablais de Cudrefin RAMSAR sites and, Piano di Magadino Park, (Fig. 4; Table 3, S4). In Italy, optimal corridors were mainly located in western Liguria and Piedmont, where large lakes Como and Maggiore are found (Fig. 4). A large part of the protected corridors of the Liguria region fell in Natura 2000 protected sites, e.g., Lecceta di Langan, Monte Galero and Lago di Osiglia Sites of Community Importance (SCI) (Fig. 4, Table 3, S4). In the Piedmont region, the Gran Bosco di Salbertrand Natural Special Protection Area (SPA) in the western part of Torino municipality, along the River Dora Riparia, showed higher centrality values than other protected areas bordering French regions. In the Po Valley, the most important protected areas were the Valle del Ticino Park and the System of Protected Areas of the Po River Banks (Fig. 4; Table 3, S4).
Gap analysis and centrality of protected areas results. The protected areas are colored by a gradient following their area-weighted centrality score. The centrality score was obtained as the sum of all current values into each protected area’s perimeter divided by its area. The numbers refer to the ranking position of PAs by centrality score (see also Table 3): SCI Lecceta di Langan (1); The Grangettes Nature Reserve (14); SCI Lago di Osiglia (19); The Préalpes d'Azur Regional Natural Park (44); The Vercors Regional Nature Park (49); Chartreuse Natural Regional Parks (53); The Natural Regional Park Massif des Bauges (55); Provençal Baronies Regional Natural Park (62); Protection perimeter of the Geological Nature Reserve of Digne les Bains (68); Fanel et Chablais de Cudrefin Ramsar site (82); Gran Bosco di Salbertrand SPA (146); Val Calanca Park (167); Mercantour National Parc (177); Haut-Jura Natural Regional Park (211); UNESCO Biosphere Entlebuch (218); Gantrisch Natural Park (255); Ecrins National Park (276); Gran Paradiso National Park (303)
The conservation gap affected most of the corridors located close to French, Italian, and Switzerland borders (Fig. 4). Among the tributaries of the French Rhone, the rivers Isere, Arc, Doron de Bozel, and Durance were highlighted as threatened. In Switzerland, only a small portion of the corridors along the rivers Rhone, Ticino, Adda, and Anterior Rhine are protected. In Italy, the watercourses flowing from the Alps and pre-Alps towards the Po Valley, such as the rivers Tanaro, Doria Riparia, and Dora Baltea, and their tributaries (i.e., Stura di Demonte, Maira, Variata) are far from the available network of protected areas (Fig. 4).
Discussion
In this work, we showed that landscape characteristics affect both the quantity and quality of connectivity corridors for Eurasian otters across the Western Alps. Multiple potential corridors were identified by the connectivity analysis, especially in the French peri-Alpine territories at the Alpine foothills, where existing populations are well connected and could act as a source of dispersers in the next future.
Valley bottoms were identified as the areas most conducive to otter dispersal, despite a high rate of human pressure. In these areas, the increasing urban development along river banks may negatively affect the likelihood of otter dispersal. In Europe, the main cause of death of Eurasian otters is roadkill, which occurs mainly within a 100 m radius buffer from watercourses and on roads with relatively low traffic density (Philcox et al. 1999; Poledník et al. 2011; Červinka et al. 2015). Therefore, urbanized areas may act as barriers, but they are not the only element that negatively affects dispersal movements. This is evident, for example, from the high connectivity values obtained along the entire basin of the River Rhone, where human-made development is strong to its mouth in the Camargue region. Moreover, otters seem to be more able of colonizing sub-optimal environments than previously believed (Baltrulnaite et al. 2009; Pita et al. 2009; Romanowski et al. 2013; Weinberger et al. 2016). In Portugal, for example, a positive correlation between otter abundance and irrigation canals in a cultivated landscape has been demonstrated (Pita et al. 2009). This is encouraging, as a large part of northern Italian regions are characterized by similar agricultural landscapes that could therefore guarantee otters’ dispersal and stabilization.
Dams did not seem to have a significant effect on dispersal paths. In the Alpine regions, most dams are located near the springs of watercourses, where the environmental conditions are already unfavorable for the passage of otters (i.e., high elevation and steep slope). At lower elevations, the presence of multiple passages, both in water and on land, seems to compensate for the interruptions of the current flow. However, weirs along watercourses should be analyzed at a more detailed scale to more accurately interpret how otters perceive them.
Although the presence of steep terrain can compromise otter dispersal (Janssens et al. 2008), altitude is certainly a more limiting factor (Kruuk 2006). In this regard, noteworthy is the high connectivity values obtained along the southern limits of the Alpine range, where the availability of low-altitude passes is higher. In Maritimes and Ligurian Alps watercourses are fed almost exclusively by rainfall and are therefore subject to periods of low water in summer. These catchments of water are usually not able to sustain breeding populations, although otters have been shown to visit small watercourses to hunt or to use them as migration routes (Sulkava et al. 2007; O’Néill et al. 2009; Romanowski et al. 2013). In this study, we did not incorporate data on the availability of trophic resources and the seasonality of watercourses, since they were not available for the entire study area. However, previous observations in France (Malthieux 2020) and southern Italy (Giovacchini et al. 2018) suggest that otter populations persist and may even expand where water availability is limited, thus supporting our speculation on the Maritimes and Ligurian Alps.
Some relevant conservation gaps for important corridors were revealed in the study area, especially in the regions Auvergne-Rhône-Alpes, in the territories between the Haute Jura Natural Regional Park and the Massif des Bauge and Chartreuse Natural Regional Parks, and further south, in the territories between the Vercors, Baronnies Provençales and Ecrins National Parks. Then between the Alpes-Cote d'Azur province and the Liguria region, the coastal territories especially those south of the Mercantour National Park. In Aosta Valley, north of the Gran Paradiso National Park along the Dora Baltea river, and in the cantons Valais (along the Rhone river), Bern (in the territories between the Gantrisch Natural Park, Diemtigtal Natural Park, and the UNESCO Biosphere Entlebuch), and Ticino (along Ticino river, in the proximity of Val Calanca Park). Protected areas are the main tool used to cope with the loss of biodiversity (Spalding et al. 2008; Pacifici et al. 2020; Chen et al. 2022). Although many species of mammals, amphibians, and birds have become extinct in recent decades, the rate of extinction could have been 20% higher in the absence of protected areas (Hoffmann et al. 2010). Moreover, today, compared to the past, the distribution range of many wild species falls mainly in protected areas, this is due in minor part to the increase in the global protected area, but, mainly, to the disappearance of these species from unprotected territories (Pacifici et al. 2020). The success of dispersal movement depends on the dispersal capabilities of the species and the permeability of the matrix between protected areas, which is why a high number of protected areas favors the dispersal of wild species (Santini et al. 2016). Due to an inadequate state of protection and therefore a significant human presence, the connectivity in cross-border territories between nations is usually lower than within a national protection network (Santini et al. 2016). For these reasons the absence of protected areas comprising the corridors along the Isére, Dora Baltea, Dora Riparia, Tanaro, and Anterior Rhine rivers could significantly slow the dispersal and recolonization processes for otters. To account for these problems, many conservation projects have been implemented. Among these, the Target 11 of the Strategic Plan for Biodiversity 2011–2020 of the Convention on Biological Diversity, aims to expand the current protected area network to cover 17% of the terrestrial environment while maintaining and improving network connectivity. In the Alps, the Continuum Project (Kohler et al. 2008) is another example of a conservation initiative designed to enhance transboundary connectivity. An effective action for creating connections would be to increase structures that mitigate wildlife mortality and interactions with humans (green infrastructures), which would greatly benefit several animal species, including the Eurasian otter (Villalva et al. 2013; Niemi et al. 2014). In our study, areas with a high area-weighted centrality score represent in a straightforward manner the territories that could be a good conservation investment given their role in the stability of the entire network. This result also shows that even the smaller protected areas, which are generally also the most vulnerable, can play a key role in maintaining connectivity and thus in the survival of wild animals. Ensuring connections even between small areas could be equally important.
Conclusion
For wild animal species, the ability to move freely is a necessary condition for dispersal, reproduction, and persistence (Turner et al. 2001; Nathan et al. 2008). However, nowadays animal movements are often hindered by the destruction and fragmentation of their habitats (Lindenmayer and Fischer 2006). Despite the agricultural land abandonment in mountain areas over the past years due to socio-economics changes (Dax et al. 2021), the Alpine environment has suffered important transformations by humans in the last century, such as the mechanization and intensification of agriculture (Britschgi et al. 2006; Scolozzi and Geneletti 2011), urbanization for tourism, and the increase of road networks (Caprio et al. 2011). Nevertheless, the Alpine region is still rich in protected areas that can help maintain and increase wildlife populations (Geldmann et al. 2013; Walston et al. 2016). Otters are also capable of adapting their ecological requirements to available conditions during recolonization processes (Baltrulnaite et al. 2009; Clavero et al. 2010; Romanowski et al. 2013; Weinberger et al. 2016). Therefore, our results stand as a necessary reference for environmental restoration actions aiming to promote the recolonization of the Western Alps by otters that can therefore only occur if connectivity and environmental suitability combine to ensure the animals' survival over time and reduce the mortality of dispersing animals.
References
Adriaensen F, Chardon JP, De Blust G et al (2003) The application of “least-cost” modelling as a functional landscape model. Landsc Urban Plan 64:233–247. https://doi.org/10.1016/S0169-2046(02)00242-6
Andren H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 23:355–366. https://doi.org/10.2307/3545823
Angst C, Weinberger I (2020) Status of the Eurasian otter (Lutra lutra) in Switzerland. J Mt Ecol 13:23–30
Arthur C, Barthélemy V (2020) The state of conservation of the Otter, Lutra lutra, in the French alps. What does the future hold? J Mt Ecol 13:9–22
Balestreri A, Remonti L, Prigioni C (2016) Towards extinction and back: decline and recovery of otter populations in Italy. Problematic wildlife: a cross-disciplinary approach. Springer, Cham, pp 91–105
Baltrulnaite L, Balčiauskas L, Matulaitis R, Stirke V (2009) Otter distribution in Lithuania in 2008 and changes in the last decade. Est J Ecol 58:94–102. https://doi.org/10.3176/eco.2009.2.03
Beier P, Brost B (2010) Use of land facets to plan for climate change: conserving the arenas, not the actors. Conserv Biol 24:701–710. https://doi.org/10.1111/j.1523-1739.2009.01422.x
Beier P, Noss RF (1998) Do habitat corridors provide connectivity? Conserv Biol 12:1241–1252. https://doi.org/10.1111/j.1523-1739.1998.98036.x
Britschgi A, Spaar R, Arlettaz R (2006) Impact of grassland farming intensification on the breeding ecology of an indicator insectivorous passerine, the Whinchat Saxicola rubetra: lessons for overall Alpine meadowland management. Biol Conserv 130:193–205. https://doi.org/10.1016/j.biocon.2005.12.013
Buglione M, Petrelli S, Troiano C et al (2021) Spatial genetic structure in the Eurasian otter (Lutra lutra) meta-population from its core range in Italy. Contrib to Zool 90:70–92. https://doi.org/10.1163/18759866-BJA10012
Capon SJ, Chambers LE, Mac Nally R et al (2013) Riparian ecosystems in the 21st century: hotspots for climate change adaptation? Ecosystems 16:359–381. https://doi.org/10.1007/s10021-013-9656-1
Caprio E, Chamberlain DE, Isaia M, Rolando A (2011) Landscape changes caused by high altitude ski-pistes affect bird species richness and distribution in the Alps. Biol Conserv 144:2958–2967. https://doi.org/10.1016/j.biocon.2011.08.021
Cardillo M, Mace GM, Jones KE, et al (2005) Evolution: multiple causes of high extinction risk in large mammal species. Science (80) 309:1239–1241. https://doi.org/10.1126/science.1116030
Červinka J, Riegert J, Grill S, Šálek M (2015) Large-scale evaluation of carnivore road mortality: the effect of landscape and local scale characteristics. Mammal Res 60:233–243. https://doi.org/10.1007/s13364-015-0226-0
Chen C, Liu R, Brodie JF et al (2022) Global camera trap synthesis highlights the importance of protected areas in maintaining mammal diversity. Conserv Lett. https://doi.org/10.1111/conl.12865
Cianfrani C, Le LG, Maiorano L et al (2011) Adapting global conservation strategies to climate change at the European scale: the otter as a flagship species. Biol Conserv 144:2068–2080. https://doi.org/10.1016/j.biocon.2011.03.027
Cianfrani C, Maiorano L, Loy A et al (2013) There and back again? Combining habitat suitability modelling and connectivity analyses to assess a potential return of the otter to Switzerland. Anim Conserv 16:584–594. https://doi.org/10.1111/acv.12033
Clavero M, Hermoso V, Brotons L, Delibes M (2010) Natural, human and spatial constraints to expanding populations of otters in the Iberian Peninsula. J Biogeogr 37:2345–2357. https://doi.org/10.1111/j.1365-2699.2010.02377.x
Clevenger A, Wierzchowski J, Chruszcz B, Gunson K (2002) GIS-generated, expert-based models for identifying wildlife habitat linkages and planning mitigation passages. Conserv Biol 16:503–514. https://doi.org/10.1046/j.1523-1739.2002.00328.x
Corlatti L, Bassano B, Valencak TG, Lovari S (2013) Foraging strategies associated with alternative reproductive tactics in a large mammal. J Zool 291:111–118. https://doi.org/10.1111/jzo.12049
Correa Ayram CA, Mendoza ME, Etter A, Salicrup DRP (2016) Habitat connectivity in biodiversity conservation: a review of recent studies and applications. Prog Phys Geogr 40:7–37. https://doi.org/10.1177/0309133315598713
Crooks KR (2002) Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv Biol 16:488–502
Dax T, Schroll K, Machold I et al (2021) Land abandonment in mountain areas of the EU: an inevitable side effect of farming modernization and neglected threat to sustainable land use. Land. https://doi.org/10.3390/land10060591
Dickson BG, Albano CM, Anantharaman R et al (2018) Circuit-theory applications to connectivity science and conservation. Conserv Biol 33:239–249. https://doi.org/10.1111/cobi.13230
Dickson BG, Roemer GW, McRae BH, Rundall JM (2013) Models of regional habitat quality and connectivity for pumas (Puma concolor) in the Southwestern United States. PLoS ONE 8:e81898. https://doi.org/10.1371/journal.pone.0081898
Doak DF, Marino PC, Kareiva PM (1992) Spatial scale mediates the influence of habitat fragmentation on dispersal success: implications for conservation. Theor Popul Biol 41:315–336. https://doi.org/10.1016/0006-3207(93)90456-b
Ducci L, Roscioni F, Carranza ML et al (2019) The role of protected areas in preserving habitat and functional connectivity for mobile flying vertebrates: the common noctule bat (Nyctalus noctula) in Tuscany (Italy) as a case study. Biodivers Conserv 28:1569–1592. https://doi.org/10.1007/s10531-019-01744-5
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc 81:163–182. https://doi.org/10.1017/S1464793105006950
Duplaix N, Savage M (2018) The global otter conservation strategy
Elliot NB, Cushman SA, Macdonald DW, Loveridge AJ (2014) The devil is in the dispersers: predictions of landscape connectivity change with demography. J Appl Ecol 51:1169–1178. https://doi.org/10.1111/1365-2664.12282
Foltête JC, Couval G, Fontanier M et al (2016) A graph-based approach to defend agro-ecological systems against water vole outbreaks. Ecol Indic 71:87–98. https://doi.org/10.1016/j.ecolind.2016.06.033
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140. https://doi.org/10.1016/j.biocon.2005.05.002
Fuller MR, Doyle MW, Strayer DL (2015) Causes and consequences of habitat fragmentation in river networks. Ann N Y Acad Sci 1355:31–51. https://doi.org/10.1111/nyas.12853
Giovacchini S, Marrese M, Loy A (2018) Good news from the south: filling the gap between two otter populations in Italy. IUCN Otter Spec Gr Bull 35:212–221
Green G (1994) Connectivity and complexity in landscapes and ecosystems. Pacific Conserv Biol 1:194. https://doi.org/10.1071/pc940194
Geldmann J, Barnes M, Coad L et al (2013) Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol Conserv 161:230–238. https://doi.org/10.1016/j.biocon.2013.02.018
Gustafson EJ, Gardner RH (1996) The effect of landscape heterogeneity on the probability of patch colonization. Ecology 77:94–107. https://doi.org/10.2307/2265659
Haase CG, Fletcher RJ, Slone DH et al (2017) Landscape complementation revealed through bipartite networks: an example with the Florida manatee. Landsc Ecol 32:1999–2014. https://doi.org/10.1007/s10980-017-0560-5
Hoegh-Guldberg O, Hughes L, Mcintyre S, et al (2008) Assisted colonization and rapid climate change. Science (80–) 321:345–346
Hoffmann M, Hilton-Taylor C, Angulo A, et al (2010) The impact of conservation on the status of the world’s vertebrates. Science (80–) 330:1503–1509. https://doi.org/10.1126/science.1194442
Horton RE (1945) Erosional development of streams and their drainage basins, hydrophysical approach to quantitative morphology
Hung N, Law CJ (2016) Lutra Lutra (Carnivora: Mustelidae). Mamm Species 48:109–122. https://doi.org/10.1093/mspecies/sew011
Janssens X, Fontaine MC, Michaux JR et al (2008) Genetic pattern of the recent recovery of European otters in southern France. Ecography (cop) 31:176–186. https://doi.org/10.1111/j.2007.0906-7590.04936.x
Kindlmann P, Burel F (2008) Connectivity measures: a review. Landsc Ecol 23:879–890. https://doi.org/10.1007/s10980-008-9245-4
Kohler Y, Plassmann G, Ullrich A et al (2008) The continuum project. Mt Res Dev 28:168–172. https://doi.org/10.1659/mrd.1010
Kranz A, Poledník L (2020) Recolonization of the Austrian Alps by otters: conflicts and management. J Mt Ecol 13:31–40
Kruuk H (2006) Otters ecology, behaviour and conservation, 2nd edn. Oxford University Press
Lapini L, Pontarini R, Molinari P et al (2020) The return of the Eurasian otter in north-eastern Italy. New challenges for biological conservation from Friuli Venezia Giulia Region. J Mt Ecol 13:41–50
Lindenmayer DB, Fischer J (2006) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280. https://doi.org/10.1111/j.1466-8238.2007.00287.x
Loy A, Carranza ML, Cianfrani C et al (2009) Otter Lutra lutra population expansion: assessing habitat suitability and connectivity in southern Italy. Folia Zool 58:309–326
Loy A, Duplaix N (2020) Decline and recovery of the otter in Europe. Lessons learned and future challenges. J Mt Ecol 13:1–8
McMillan D, Marshall K (2006) The Delphi process—an expert-based approach to ecological modelling in data-poor environments. Anim Conserv 9:11–19. https://doi.org/10.1111/j.1469-1795.2005.00001.x
Malczewski J (2000) On the use of weighted linear combination method in GIS: common and best practice approaches. Trans GIS 4:5–22. https://doi.org/10.1111/1467-9671.00035
Malthieux L (2020) La Loutre d’ Europe Lutra lutra (Linnaeus, 1758 ) en Roya-Bévéra: relique ou retour? Prospections, état des lieux et implications
Mason CF, Macdonald SM (1986) Otters: ecology and conservation. Cambridge University Press
McRae BH, Dickson BG, Keitt TH, Shah VB (2008) Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 89:2712–2724. https://doi.org/10.1890/07-1861.1
Nathan R, Getz WM, Revilla E et al (2008) A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA 105:19052–19059. https://doi.org/10.1073/pnas.0800375105
Newbold T, Hudson LN, Hill SLL et al (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50. https://doi.org/10.1038/nature14324
Niemi M, Jääskeläinen NC, Nummi P et al (2014) Dry paths effectively reduce road mortality of small andmedium-sized terrestrial vertebrates. J Environ Manag 144:51–57. https://doi.org/10.1016/j.jenvman.2014.05.012
O’ Néill L, Veldhuizen T, de Jongh A, Rochford J (2009) Ranging behaviour and socio-biology of Eurasian otters (Lutra lutra) on lowland mesotrophic river systems. Eur J Wildl Res 55:363–370. https://doi.org/10.1007/s10344-009-0252-9
Pacifici M, Di Marco M, Watson JEM (2020) Protected areas are now the last strongholds for many imperiled mammal species. Conserv Lett 13:1–7. https://doi.org/10.1111/conl.12748
Philcox CK, Grogan AL, Macdonald DW (1999) Patterns of otter Lutra lutra road mortality in Britain. J Appl Ecol 36:748–761. https://doi.org/10.1046/j.1365-2664.1999.00441.x
Pita R, Mira A, Moreira F et al (2009) Influence of landscape characteristics on carnivore diversity and abundance in Mediterranean farmland. Agric Ecosyst Environ 132:57–65. https://doi.org/10.1016/j.agee.2009.02.008
Poledník L, Poledníková K, Větrovcová J et al (2011) Causes of deaths of Lutra lutra in the Czech Republic (Carnivora: Mustelidae). Lynx Nová Ser 42:145–157
Randi E, Davoli F, Pierpaoli M et al (2003) Genetic structure in otter (Lutra lutra) populations in Europe: implications for conservation. Anim Conserv 6:93–100. https://doi.org/10.1017/S1367943003003123
Ripple WJ, Estes JA, Beschta RL, et al (2014) Status and ecological effects of the world’s largest carnivores. Science (80–). https://doi.org/10.1126/science.1241484
Rivera-Ortíz FA, Aguilar R, Arizmendi MDC et al (2015) Habitat fragmentation and genetic variability of tetrapod populations. Anim Conserv 18:249–258. https://doi.org/10.1111/acv.12165
Romanowski J, Brzeziński M, Żmihorski M (2013) Habitat correlates of the Eurasian otter Lutra lutra recolonizing Central Poland. Acta Theriol (warsz) 58:149–155. https://doi.org/10.1007/s13364-012-0107-8
Roos A, Loy A, de Silva P, et al (2015) Lutra lutra. The IUCN red list of threatened species 2015: e.T12419A21935287
Ruiz-Olmo J (1998) Influence of altitude on the distribution, abundance and ecology of the otter (Lutra lutra). Behav Ecol Riparian Mamm. https://doi.org/10.1017/cbo9780511721830.011
Ruiz-Olmo J, Loy A, Cianfrani C, et al (2008) Lutra lutra. In: IUCN 2009. IUCN Red List of threatened species.
Santini L, Saura S, Rondinini C (2016) Connectivity of the global network of protected areas. Divers Distrib 22:199–211. https://doi.org/10.1111/ddi.12390
Schmidt K, Ratkiewicz M, Konopiński MK (2011) The importance of genetic variability and population differentiation in the Eurasian lynx Lynx lynx for conservation, in the context of habitat and climate change. Mamm Rev 41:112–124. https://doi.org/10.1111/j.1365-2907.2010.00180.x
Scolozzi R, Geneletti D (2011) Spatial rule-based assessment of habitat potential to predict impact of land use changes on biodiversity at municipal scale. Environ Manag 47:368–383. https://doi.org/10.1007/s00267-011-9613-8
Shepard DB, Kuhns AR, Dreslik MJ, Phillips CA (2008) Roads as barriers to animal movement in fragmented landscapes. Anim Conserv 11:288–296. https://doi.org/10.1111/j.1469-1795.2008.00183.x
Spalding MD, Fish L, Wood LJ (2008) Toward representative protection of the world’s coasts and oceans-progress, gaps, and opportunities. Conserv Lett 1:217–226. https://doi.org/10.1111/j.1755-263x.2008.00030.x
Sulkava RT, Sulkava PO, Sulkava PE (2007) Source and sink dynamics of density-dependent otter (Lutra lutra) populations in rivers of central Finland. Oecologia 153:579–588. https://doi.org/10.1007/s00442-007-0774-3
Tarasoff FJ, Bisaillon A, Piérard J, Whitt AP (1972) Locomotory patterns and external morphology of the river otter, sea otter, and harp seal (Mammalia). Can J Zool 50:915–929. https://doi.org/10.1139/z72-124
Taylor PD, Fahrig L, Henein K, Merriam G (1993) Connectivity is a vital element of landscape structure. Oikos 68:571–573
Tewksbury JJ, Levey DJ, Haddad NM et al (2002) Corridors affect plants, animals, and their interactions in fragmented landscapes. Proc Natl Acad Sci USA 99:12923–12926. https://doi.org/10.1073/pnas.202242699
Theobald DM, Reed SE, Fields K, Soulé M (2012) Connecting natural landscapes using a landscape permeability model to prioritize conservation activities in the United States. Conserv Lett 5:123–133. https://doi.org/10.1111/j.1755-263X.2011.00218.x
Tischendorf L, Fahrig L (2000) On the usage and measurement of landscape connectivity. Oikos 90:7–19. https://doi.org/10.1034/j.1600-0706.2000.900102.x
Tremolada P, Smiroldo G, Verduci F, et al (2020) The otter population of the River Ticino (N Italy ) 20 years after its reintroduction. 13:51–62
Turner MG, Gradner RH, O’Neill RV (2001) Landscape ecology in theory and practice. Springer New York
Villalva P, Reto D, Santos-Reis M et al (2013) Do dry ledges reduce the barrier effect of roads? Ecol Eng 57:143–148. https://doi.org/10.1016/j.ecoleng.2013.04.005
Von Arx M, Breitenmoser C, Würsten FZ, Breitenmoser U (2004) Status and conservation of the Eurasian lynx (Lynx)
Wade AA, McKelvey KS, Schwartz MK (2015) Resistance-surface-based wildlife conservation connectivity modeling: summary of efforts in the united states and guide for practitioners
Waller J, Servheen C (2016) Effects of transportation infrastructure on grizzly bears in northwestern montana. J Wildl Manag 69:985–1000
Walston J, Stokes EJ, Hedges S (2016) The importance of Asia’s protected areas for safeguarding commercially high value species. In: Protected areas: are they safeguarding biodiversity? pp 190–207
Weinberger IC, Muff S, de Jongh A et al (2016) Flexible habitat selection paves the way for a recovery of otter populations in the European Alps. Biol Conserv 199:88–95. https://doi.org/10.1016/j.biocon.2016.04.017
With KA, Gardner RH, Turner MG (1997) Landscape connectivity and population distributions in heterogeneous environments. Oikos 78:151. https://doi.org/10.2307/3545811
Wood SLR, Martins KT, Dumais-Lalonde V et al (2022) Missing interactions: the current state of multispecies connectivity analysis. Front Ecol Evol. https://doi.org/10.3389/fevo.2022.830822
Acknowledgements
We would like to thank the Swiss Species Information Centre (Info Species) and the French Society for the Study and Protection of Mammals (SFEPM) for sharing their data.
Funding
Open access funding was provided by Università degli Studi di Torino within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AL: conceptualization, supervision; CF: conceptualization, writing—review and editing, supervision; FL: conceptualization, formal analysis, investigation, resources, data curation, writing—original draft, visualization; MDF: conceptualization, methodology; PS: data curation, writing—review and editing. All Authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Communicated by Sandro Lovari.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leoncini, F., Semenzato, P., Di Febbraro, M. et al. Come back to stay: landscape connectivity analysis for the Eurasian otter (Lutra lutra) in the western Alps. Biodivers Conserv 32, 653–669 (2023). https://doi.org/10.1007/s10531-022-02517-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02517-3