Abstract
Maintaining heathlands in early successional stages to sustain heather (Calluna vulgaris) is a common, large-scale management practice in Europe. However, allowing patches of long-term natural vegetation development may increase habitat heterogeneity benefitting insects, but empirical evidence is sparse. We investigated how old-growth heathland (> 30 years abandonment) affect species richness and composition of bees (Anthophila), crane flies (Tipuloidea), ground beetles (Carabidae), hoverflies (Syrphidae) and rove beetles (Staphylinidae) in relation to their hygropreference. Adult insects, vegetation and edaphic explanatory variables were collected in old-growth, managed and wet sites and compared in four lowland heathland locations in Denmark. We found 299 species including 24 nationally red-listed. Species composition differed between managed, old-growth and wet heathland for all taxa. Indicator species and richness analyses showed a predominance of xerophilic bee species in managed heathland. Old-growth heathland showed a predominance of mesophilic indicator species, and higher richness of mesophilic crane flies and of hygrophilic ground and rove beetles compared to managed heathland. Wet heathland was generally dominated by hygrophilic species. Soil moisture, bare soil and vegetation height density were important drivers explaining the contrasting responses in richness and composition between heathland types. Our results demonstrate that heathland management focusing solely on early successional vegetation stages may homogenize insect communities. We suggest that management practices should focus on improving structural vegetation heterogeneity. This can be achieved through management regimes that reset the succession and expose bare soil, but also by allowing patches of old-growth vegetation stages to develop and by conserving existing ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
European heathlands are vulnerable semi-natural ecosystems of high conservation value protected under the European Union Habitats Directive (Evans 2006). Management of heathlands is necessary to sustain the cover and growth of ericoid dwarf shrubs and to prevent nutrient accumulation and succession towards forest. This is achieved through management regimes such as prescribed burning, livestock grazing or machine cutting, which deplete nutrients and favor rejuvenation and regeneration of heather (Calluna vulgaris) (Hobbs 1984; Webb 1998). Heathlands that are kept in their early successional vegetation stages are often associated with a higher cover of Calluna, but often develop into homogenous even-aged sites with uniform canopies and low structural diversity (Gimingham 1970). While most heathland management practices have focused on maintaining early successional stages, a growing body of literature shows negative consequences of a homogenous vegetation structure on heathland arthropods (Usher 1992; Bell et al. 2001; Webb et al. 2010; WallisDeVries et al. 2016; Hansen et al. 2020).
Heathlands contain a diverse and unique arthropod fauna with varied ecological requirements and life cycles (Kirby 1992; Usher 1992). Vegetation composition and structure are important drivers of variation in heathland arthropod assemblages. The cover and growth-phases of C. vulgaris (pioneer, building, mature and degenerate, total 30–40 years) are associated with different species assemblages of flies, ground beetles, butterflies and moths, millipedes, phytophagous insects, spiders and springtails (Miller 1975; Gimingham 1985; Usher 1992; Haysom and Coulson 1998). A high graminoid cover in heathlands promotes species richness of grasshoppers (Schirmel et al. 2011), while bare ground patches affect the species composition and community structure of bees, wasps and ground beetles (Gregory and Wright, 2005; Cameron and Leather 2012). An evaluation of the habitat requirements of UK heathland priority species, of which the majority are arthropods, found that shelter (topography or vegetation), bare ground, grasses, and shrubs/trees are essential habitats (Webb et al. 2010) contrasting the common heathland management aiming at a high and homogeneous Calluna cover.
The effects of heathland management on vegetation structure and composition and thus arthropod assemblages are complex and depend on several factors such as management regime and intensity, land-use history and abiotic factors (Gimingham 1975; Riis-Nielsen et al. 1991; Lake et al. 2001; Newton et al. 2009; Kepfer-Rojas et al. 2014; Schellenberg and Bergmeier 2021). Common management regimes such as prescribed burning aim to restart the life cycle of aging heather and prevent colonization of trees (Gimingham 1975). Prescribed burning removes the canopy and accumulated litter and exposes bare ground, which can be beneficial to a wide range of xerophilic epigeal and burrowing insects (Kirby 1992; Schirmel et al. 2012; Buchholz et al. 2013; Bargmann et al. 2015). Livestock grazing aims at increasing structural diversity, and maintaining open dwarf shrub vegetation with a high diversity of plant species while eliminating woody species (Lake et al. 2001). The effect of grazing on heathland arthropod assemblages is taxa specific and largely determined by grazing intensity (WallisDeVries et al. 2016). Intensive heathland management regimes may further affect soil and plant chemical properties with detrimental effects on the insect fauna (De Bruyn et al. 2001; Vogels et al. 2017).
Besides the effects of management, underlying abiotic factors also structure heathland ecosystems and may in turn affect insect communities. A key factor is soil moisture, and insect communities usually differ markedly between wet and dry heathlands (Usher 1992; De Bruyn et al. 2001; Mantilla-Contreras et al. 2011; WallisDeVries et al. 2016). Wet heathland have often been unmanaged for decades or even centuries, and contain a large number of specialized hygrophilic arthropod species of high conservation value (Spitzer and Danks 2006). In large-scale intensively managed heathland locations, long-term unmanaged sites are not common, but allowing dwarf shrub-dominated heathlands to develop naturally through long periods of time (henceforth referred to as old-growth heathland) may benefit certain insect taxa. Over time, old-growth heathlands develop a complex vegetation structure and composition (Riis-Nielsen et al. 2005; Ransijn et al. 2015), including the different growth-phases of Calluna (Gimingham 1985).
The aim of this study was to investigate how old-growth heathland affect species richness and composition of five different insect taxa. Adult insects, vegetation and edaphic explanatory variables were collected in old-growth, managed and wet sites and compared in four lowland heathland locations in Denmark. Wet heathland was included as an outgroup, enabling us to compare the species assemblages to those of managed and old-growth heathland, as we expected old-growth heathland invertebrate communities to show more resemblance towards wet heathland. We focused on bees (Hymenoptera: Apoidea: Anthophila), crane flies (Diptera: Tipuloidea), ground beetles (Coleoptera: Carabidae), hoverflies (Diptera: Syrphidae) and rove beetles (Coleoptera: Staphylinidae), which are species rich families/clades (> 250 species per group in Denmark) commonly found in heathlands. Insect taxa were further divided into groups based on their hygropreferences. We expected that xerophilic species would prefer managed heathland because of the sparse vegetation and bare soil, mesophilic species would prefer old-growth heathland because of the denser vegetation and more varied conditions, and hygrophilic species would prefer wet heathland and to some extend old-growth heathland because of similarity in vegetation structure among habitats. To our knowledge, multi-taxa responses across heathland management regimes and old-growth unmanaged heathlands have not been previously reported.
We hypothesized that: (1) insect richness and species composition differ between heathland types (managed, old-growth and wet) and are linked to insect hygropreferences, with xerophilic species preferring managed heathland, mesophilic species preferring old-growth heathland, and hygrophilic species preferring wet heathland and to some extent also old-growth heathland; (2) differences in insect richness and composition between heathland types can be explained by differences in vegetation and edaphic conditions; (3) old-growth heathland sites in combination with managed sites provide heterogeneous habitat conditions that increase overall diversity of insects.
Materials and methods
Study locations and experimental design
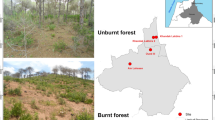
The study was conducted in four heathland locations in Jutland, Denmark (Fig. 1). The locations represent some of the largest inland heaths in Denmark, and were chosen based on historical information regarding past and present management regimes and long continuity. The heathlands are located within 70 km of each other, and are protected within the Natura 2000 network. The mean annual temperature is 8.8 °C and mean annual precipitation is 950 mm. The total annual nitrogen deposition is 11–15 kgN/ha (DCE 2020).
All heathland locations included (1) a dry old-growth heathland site primarily dominated by Calluna vulgaris or other dwarf shrubs (2) sites with different management regimes and (3) a wet heathland site (habitat code 4010) with Erica tetralix, Molinia caerulea, Sphagnum species and different dwarf shrub species (Table 1). The managed sites have a previous history of intensive heathland management through the past decades, and have primarily been managed by prescribed burning, grazing or harvesting, which are among the most common management regimes in Denmark (Buttenschøn and Schmidt, 2015; Riis-Nielsen et al. 1991).
At each site, 8 plots were established randomly with the following criteria: Plots were placed with a minimum of 50 m to the border of the site, and with a minimum of 50 m between plots (Fig. 1) to avoid depletion of pitfall trap catches (Digweed et al. 1995). In addition, four extra plots were established in each site with pitfall traps installed. These traps were active in the same periods as in the other 8 plots, but were only used in the dataset if data from the 8 plots were incomplete, e.g. due to trampling of animals or flooding.
Sampling of insects
Insects were sampled recurrently from early June to early August of 2017 using sweep nets (crane flies and hoverflies), pan traps (bees and hoverflies), and pitfall traps (ground beetles and rove beetles). Sweep net sampling was conducted in a circle (r: 15 m) around the plot center for a total of 10 min shared by 2–3 collectors at three occasions during the summer (Online Appendix A) using a 38 cm diameter sweep net. Sweep net sampling was initiated from the periphery of the circle to ensure least possible disturbance of the fauna. Sweep net catches were emptied in bags containing 70% ethanol. Sampling was conducted from 9 AM to 6 PM in dry weather and wind speed < 8 m/s. Three coloured pan traps (radius: 7.5 cm, volume: 500 ml) in blue, white and yellow, were placed on top of the vegetation or at ground level in each plot and filled with 50% propylene glycol and 50% soap water. Pan traps were active between 8 and 16 days in three periods (Online Appendix A). A pitfall trap was installed in each plot, consisting of a 50 cl plastic cup (diameter: 10 cm) with propylene glycol and a roof to prevent flooding. Pitfall traps were active between 12 and 30 days in three periods (Online Appendix A).
Species identification
Bees were identified to species using identification keys for the different bee genera (Amiet 1996; Amiet et al. 1999, 2001, 2004, 2007; Bogusch and Straka 2012; Schmid-Egger and Scheuchl 1997; Falk and Lewington 2015). Due to the issue of workers having identical appearance within the species complex Bombus terrestris, B. lucorum, B. cryptarum and B. magnus, specimens were pooled together and treated as one species (Bombus terrestris complex Group). Honey bees (Apis mellifera) only occur commercially in Denmark and were excluded from the dataset. Species identification was performed using the key by Stubbs and Kramer (2016) for crane flies; Luff (2007) for ground beetles; Bartsch et al. (2009) for hoverflies, with supplementary information on morphological characters from Van Veen (2010). Females of the genus Sphaerophoria were treated as Sphaerophoria female Group, since morphological identification is uncertain. Similarly, no distinction between species of Melanostoma scalare and Melanostoma mellinum was made and they were treated as Melanostoma Group. Rove beetles were identified using the keys by Hansen (1951; 1952; 1954) as well as Assing and Schülke (2012). Species in the morphological similar subfamily Aleocharinae were separated in two groups, one with the genus Dinarda, and one with the rest of the Aleocharinae, of which most belonged to a single species, Drusilla canaliculata.
Following identification, species were assigned to xerophiles, mesophiles and hygrophiles based on their hygropreference. Bees were assigned according to Cederberg et al. (2022); crane flies according to information contained in the Catalogue of the Craneflies of the World (Oosterbroek 2022); ground beetles according to Lindroth (1985; 1986); hoverflies according to information contained in Syrph the Net Database (Speight et al. 2000); and rove beetles according to Hansen et al. (2018). Red-listed species were assigned according to the Danish Red List (Moeslund et al. 2019), and expert assessments were used to evaluate the status of crane flies and rove beetles, which are not included in the Danish Red List.
Vegetation and edaphic variables
Vegetation analysis was conducted in the plots in a 5 m radius circle. The presence of all vascular plant species and a visual assessment of their cover including bare ground were recorded by estimates of two separate recorders. Vegetation height density was measured using a ruler in the plots at 1 m intervals across the plot in the cardinal directions, as the height where the ruler was no longer visible from a top-down perspective, thus representing a combination of height and density similar to Robel et al. (1970). Plant coverage data were assigned to functional groups (Table 2).
Soil was sampled 5 m from the plot center in the directions N, S, E and W using a soil corer with an inner diameter of 5 cm. The 4 samples were divided into two fractions, one comprising the mor-layer and another the top 10 cm of mineral soil (e-horizon) and pooled respectively. After drying at 55 °C, soil pH was measured on the mineral soil after 2 h extraction in a 0.01 M CaCl2 solution on ratios 1:5 (soil:extractant) using a Radiometer combination-electrode GK2401 (Radiometer, Copenhagen, Denmark). Mineral soil for C:N analysis was further grinded on a Retsch Planetary Ball Mill PM400 (Retsch, Germany) at 370 rpm for 12 min. Carbon and nitrogen contents were measured using dry combustion method on a Thermo Scientific FLASH 2000 soil CN analyzer. Soil moisture content in the top layer was measured using a Theta ML3 Kit (Buch & Holm, Denmark) five meter from the plot center in the directions N, S, E and W at four different occasions during the summer and averaged. Edaphic variables are shown in Table 2.
Data analyses
Before analyses, insect species data from the three collection events were aggregated on plot level, and plots with incomplete data were omitted (Table 1). A total of 82 unspecified specimens were removed from the dataset, crane flies 16 specimens and hoverflies 66 specimens. All analyses were done with the statistical software R (R Core Team 2020), and graphs were created using the packages ‘ggplot2’ (Wickham 2009) and ‘effects’ (Fox et al. 2016). Sample completeness was evaluated on plot level, site level and heathland type level using the package ‘iNEXT’ (Hsieh et al. 2016). Because of low replication among the different management regimes (burned, grazed and harvested), they were treated as one group (managed) in the analyses (Table 1). This grouping was justified as we expected vegetation structure and composition to be relatively uniform among management regimes and different from old-growth and wet heathland.
Species richness
To assess the influence of heathland type (managed, old-growth and wet) on species richness of the different taxa according to their hygropreferences (xerophilic, mesophilic and hygrophilic), we performed generalized linear mixed effects models (GLMMs) using the ‘lme4’ package (Bates et al. 2007) or ‘glmmTMB’ package (Magnusson et al. 2017). Heathland type (managed, old-growth and wet) was included as a fixed effect and heathland location as a random effect. Significance was assessed using the ‘lmerTest’ package (Kuznetsova et al. 2017), and in case of significance followed by a Tukey’s post hoc test using the ‘Multcomp’ package (Hothorn et al. 2016). Assumptions of independence, homoscedasticity and normality of the residuals were assessed using the ‘DHARMa’ package (Hartig 2022). In most cases, a Poisson distribution for the count richness data was appropriate, but some models showed signs of underdispersion and a proper distribution was chosen. Model validation and full results are included in Online Appendix B.
Vegetation and edaphic variables
We compared if vegetation and edaphic variables differed between managed, old-growth and wet heathland. For vegetation cover, we performed GLMMs (Bates et al. 2007) with a negative binomial distribution. For edaphic variables we performed Linear mixed models (LMMs) (Bates et al. 2007) with a Gaussian distribution on log-transformed data. In case of significance, we used Tukey’s post hoc test for pairwise comparisons (Table 2).
To assess the effect of vegetation and edaphic variables on species richness of the different insect taxa according to their hygropreference, we performed GLMMs using the ‘lme4’ package (Bates et al. 2007) or ‘glmmTMB’ package (Magnusson et al. 2017). Before analyses, collinearity of all measured predictor variables were assessed, and a generalized variance inflation factor (GVIF) with a threshold of 3 was used to remove collinear variables (Zuur et al. 2010). After exclusion of collinear variables, the remaining predictor variables were scaled to unit variance and included in GLMMs as fixed effects. Heathland location was used as a random effect. Model validation was done using the ‘DHARMa’ package (Hartig 2022). In most cases, a Poisson distribution for the count richness data was appropriate, but some models showed signs of underdispersion and a proper distribution was chosen. In addition, some models showed signs of non-linearity between dependent and independent variables. This was accounted for by visually inspecting relationships and adding a quadratic term to the predictor variable. Model validation and full results are included in Online Appendix C.
Species composition
Non-Metric Multidimensional Scaling (NMDS) was used to visualize the species composition of the insect taxa between heathland types using the function metaMDS in the ‘vegan’ package (Oksanen et al. 2016). Plots with fewer than three specimens were omitted to achieve a clear interpretation of data. We used a Bray-Curtis dissimilarity matrix calculated for Hellinger transformed abundance data. Ordinations were set to 999 iterations and stress values below 0.20 were accepted. Stress values above 0.20 were evaluated against the distribution of stress values from independent permutations of the dataset to assess its difference from a random ordination structure (Dexter et al. 2018). To assess the influence of vegetation and edaphic variables on species composition we used the envfit function in the ‘vegan’ package (Oksanen et al. 2016). This function was fitted onto NMDS axes 1 and 2 for insect community data, and statistical significance was assessed using 9999 permutations.
Permutational multivariate analysis of variance (PERMANOVA; Anderson 2001) was used to test for differences in species composition between heathland types and locations using the Adonis function in the ‘vegan’ package (Oksanen et al. 2016). We independently conducted a PERMANOVA for heathland type (with permutations restricted within heathland locations using the argument strata) and heathland location. The pseudo p-values were generated using 9999 permutations. In the case of a significant PERMANOVA result, pairwise comparisons were performed for heathland types with Bonferroni-corrected p-values using the ‘pairwiseAdonis’ package (Arbizu 2017). As PERMANOVA can be sensitive to heterogeneity in dispersions (Anderson 2013), we tested for differences in dispersions among heathland types using the betadisper function in the ‘Vegan’ package (Oksanen et al. 2016).
To evaluate differences in species occurrence among heathland types we further performed indicator species analyses using the multipatt function in the ‘indicspecies’ package (De Cáceres et al. 2010). Indicator association values (IndVal) were calculated for each species related to either managed, old-growth and wet heathland or a combination, as the square root of the product of the specificity and the fidelity. We restricted permutations within heathland locations, and assessed statistical significance using 9999 permutations.
Results
In total, 14 702 identified specimens belonging to 299 species including 5 complexes of insects were found. Species diversity comprised between 6 and 26% of the total Danish species within each taxon. Xerophiles constituted 76 species, mesophiles 139 species and hygrophiles 84 species. 18 species were DK red-listed species, and 6 species were considered red-listed/rare by expert opinion (Table 3). Sample completeness was generally high for bees, ground beetles, hoverflies and rove beetles (plot level x̄: 0.82; site level x̄: 0.95; type level x̄: 0.99) (Online Appendix D), while crane flies had too few specimens in managed and old-growth sites to make any robust estimation. Roughly 1/3 of all species were singletons and doubletons. A full species list is found in Online Appendix E.
Richness of insect taxa
Xerophilic species richness varied among insect taxa and heathland types with plot median values ranging from 2 to 6 for bees, 0 for crane flies, 0–1 for ground beetles, 2–3 for hoverflies, and 0–2 for rove beetles (Fig. 2). Bees were the only taxon showing a significantly higher richness of xerophilic species in managed plots compared to old-growth plots, while xerophilic bees, ground beetles and rove beetles showed a significantly higher species richness in old-growth plots compared to wet plots (Fig. 2).
Species richness of xerophilic (Xero), mesophilic (Meso) and hygrophilic (Hygro) insect taxa in managed, old-growth and wet plots. Black dots indicate outliers. The letters indicate statistically significant difference at p < 0.05 between managed, old-growth and wet heathland for the different hygropreferences. NS not significant, NA not available
Mesophilic species richness was generally higher than that of xerophilic species richness with median values ranging from 3 to 4 for bees, 0–1 for crane flies, 2–7 for ground beetles, 5 for hoverflies, and 1–5 for rove beetles (Fig. 2). Crane flies were the only taxon showing a significantly higher richness of mesophilic species in old-growth plots compared to managed plots, while both mesophilic ground beetles and rove beetles showed a significantly lower species richness in wet plots compared to managed and old-growth plots (Fig. 2).
Hygrophilic species richness showed median values ranging from 0 for bees, 0–3 for crane flies, 0–2 for ground beetles, 2–3 for hoverflies, and 1–3 for rove beetles (Fig. 2). Hygrophilic ground beetle and rove beetle richness were significantly higher in old-growth plots compared to managed plots. Hygrophilic crane fly and ground beetle richness were significantly higher in wet plots compared to managed and old-growth plots, while no difference in hygrophilic rove beetle richness was found between old-growth and wet plots (Fig. 2).
In addition, the test was conducted on the number of red-listed species across all taxa, and no difference was found between heathland types (GLMM: Chi = 0.01, P = 0.99).
Insect richness in relation to vegetation and edaphic variables
Overall, the measured explanatory variables differed between heathland types (Table 2). In total, three variables were removed by the GVIF and excluded from the models (Table 2). A Pearson correlation matrix is included in Online Appendix F.
Xerophilic bee richness was negatively associated to veg. height density (GLMM: est = − 0.28 ± 0.11, Z = − 2.6, p = 0.01) and showed a nonlinear negative association to soil moisture (GLMM: est = − 0.28 ± 0.12, Z = − 2.3, p = 0.02) (Fig. 3), while mesophilic bee richness showed no significant association to any variable.
Partial residual plots for the significant effects of explanatory variables on species richness of xerophilic, mesophilic and hygrophilic insect taxa derived from GLMMs (Online Appendix C). Partial residuals and regression lines have been back transformed to the original scale. Plots do not include random effects. Extreme outliers have been excluded
Mesophilic crane fly richness showed a negative association to Calluna cover (GLMM: est = − 0.58 ± 0.23, Z = − 2.6, p = 0.01) and a positive association to other dwarf shrub cover (GLMM: est = 0.29 ± 0.13, Z = 2.2, p = 0.03), while hygrophilic crane fly richness showed a positive association to percentage bare soil (GLMM: est = 0.62 ± 0.18, Z = 3.4, p < 0.001), soil moisture (GLMM: est = 1.25 ± 0.23, Z = 5.4, p < 0.001), other dwarf shrub cover (GLMM: est = 0.28 ± 0.13, Z = 2.1, p = 0.03) and vegetation height density (GLMM: est = 0.31 ± 0.14, Z = 2.2, p = 0.03) (Fig. 3).
Xerophilic ground beetle richness was positively associated to forb cover (GLMM: est = 0.23 ± 0.09, Z = 2.4, p = 0.01) and negatively associated to soil moisture (GLMM: est = − 0.69 ± 0.20, Z = − 3.3, p < 0.001). Mesophilic ground beetle richness were negatively associated to soil moisture (GLMM: est = − 0.54 ± 0.08, Z = − 6.9, p < 0.001) and percentage bare soil (GLMM: est = − 0.14 ± 0.06, Z = − 2.4, p = 0.02), while hygrophilic ground beetle richness was negatively associated to bare soil (GLMM: est = − 1.1 ± 0.41, Z = − 2.7, p = 0.01) (Fig. 3).
Xerophilic rove beetle richness was negatively associated to soil moisture (GLMM: est = − 0.95 ± 0.18, Z = − 5.3, p < 0.001), while mesophilic rove beetles richness was negatively associated to percentage bare soil (GLMM: est = − 0.20 ± 0.08, Z = − 2.6, p = 0.01), Calluna cover (GLMM: est = − 0.13 ± 0.06, Z = − 2.1, p = 0.04) and soil moisture (GLMM: est = − 0.59 ± 0.1, Z = − 6.1, p < 0.001) (Fig. 3). Hygrophilic rove beetle richness was negatively associated to Calluna cover (GLMM: est = − 0.25 ± 0.1, Z = − 2.7, p = 0.01) and positively associated to veg. height density (GLMM: est = 0.25 ± 0.1, Z = 2.5, p = 0.01) (Fig. 3).
Richness of xerophilic, mesophilic and hygrophilic hoverflies showed no significant association to any variable.
Species composition
Species compositions of all insect taxa were significantly different between heathland types, explaining 12–28% of the variation (Table 4), whereas heathland location was significant for all taxa and explained 20%, 18%, 23% and 26% for bees, ground beetles, hoverflies and rove beetles, respectively. Crane flies were omitted from species composition analyses because of a limited number of specimens in managed and old-growth plots. Bees showed a stress value of 0.25, but the ordination was considered different from a random ordination structure (Online Appendix G).
Pairwise tests showed a significant difference in the species composition of all insect taxa in all possible comparisons, except for hoverflies which were not different between managed and old-growth heathland (Table 4; Fig. 4). However, bees, ground beetles and hoverflies violated the assumptions of similar multivariate dispersions (betadisper test) indicating that differences in β-diversity between heathland types might be causing the differences in composition between heathland types. The ordinations show that this is the case only for bees, where there is an overlap between old-growth and wet heathlands.
Non-metric Multi-Dimensional Scaling (NMDS) ordination (based on Hellinger transformed Bray-Curtis dissimilarity) showing the species composition of the different insect taxa in Managed (red), Old-growth (green) and Wet (blue) heathland plots. The ellipses represent the standard deviation of plot scores for each heathland type. Arrows indicate the direction and strength of correlation between significant variables and NMDS axes. Stress: (Bees: 0.25. Ground beetles: 0.19. Hoverflies 0.18. Rove beetles: 0.19)
Several vegetation and edaphic variables were significantly associated to the NMDS axes 1 and 2 (Fig. 4). Insect assemblages in managed plots were generally associated with exposed bare soil and high pH, whereas old-growth plots were associated with other dwarf shrub and forb cover. Wet plots were associated with high soil moisture. In addition, both old-growth and managed plots were associated with the cover of Calluna, while wet and old-growth plots were associated with vegetation height density (Fig. 4).
Indicator species analysis identified 23, 18 and 19 species significantly associated to managed, old-growth and wet heathland, respectively (Table 5). The species associated to managed heathland primarily comprised xerophilic species, while old-growth heathland primarily comprised mesophilic species, and wet heathland was dominated by hygrophilic species (Table 5). Full indicator species analysis is included in Online Appendix H.
Discussion
Despite the strong dependence of lowland European heathlands on management, few studies have contrasted the impact of managed heathland to old-growth succession heathland on insect communities. Our analyses across multiple insect taxa showed that species richness and composition generally differed between heathland types (managed, old-growth and wet heathland), and that differences were linked to shifts in insect hygropreferences. The measured vegetation and edaphic variables to some extent explained the differences in species richness and composition between heathland types. However, the results were taxa-specific and contrasted among taxa.
We hypothesized that xerophilic species would show a preference for managed heathland because of the sparse vegetation and bare soil. This was, however, only the case for xerophilic bees, which showed a much higher richness and number of xerophilic indicator species compared to old-growth and wet heathland. Xerophilic bee assemblages consisted predominantly of ground-nesting species, which are highly affected by soil conditions, where soil moisture (Julier and Roulston 2009; Pane and Harmon-Threatt 2017) and bare soil (Gregory and Wright 2005; Potts et al. 2005) are important factors. This corresponded with the analysis, where xerophilic bee species richness in managed heathland was explained by low soil moisture and low vegetation height density.
Surprisingly, xerophilic ground and rove beetles showed no difference in richness between managed and old-growth heathland, and only a few xerophilic ground and rove beetle indicator species were found. Xerophilic ground beetles have previously been associated with high species prevalence and many indicator species in young successional vegetation stages of dry habitats (Schirmel and Buchholz 2011; Buchholz et al. 2013). This indicates that xerophilic epigeic heathland insect communities may be able to persist after 30–120 years of abandonment, or this could simply be a spillover effect from nearby managed sites. Some rarely encountered xerophilic species were even unique to old-growth areas such as the rove beetle Platydracus latebricola found in Nørholm. It should, however, be noted that the investigated old-growth sites were primarily dominated by dwarf-shrubs with low to intermediate levels of grass and tree encroachment. Heathland dominated by grasses and trees would likely have a negative effect on xerophilic beetle communities (Desender and Turin 1989; Schirmel and Buchholz 2011; Buchholz et al. 2013).
In accordance with our hypothesis, old-growth heathland showed a predominance of mesophilic indicator species, and higher richness of mesophilic crane flies and hygrophilic ground and rove beetles compared to managed heathland. Mesophilic crane fly richness showed a weak negative relationship to Calluna cover and a positive relationship to other dwarf shrub cover, which provides little biological explanation. It is thus more likely, that mesophilic crane flies simply benefit from the accumulation of organic matter and mosses in old-growth areas, as many species are considered detritivores and bryophagous (Pritchard 1983). In addition, rove beetles have a high affinity to microhabitats, especially congregated piles of various types of organic matter, e.g. litter, dung, fungi and animal nests (Betz et al. 2018; Hansen et al. 2018), and early successional heathland stages with bare soil may lack such substrates. This is in accordance with mesophilic and hygrophilic rove beetle richness showing a negative relationship to bare soil and a positive relationship to vegetation height density, respectively.
While species richness was generally low in wet heath compared to managed and old-growth heathland, most taxa except bees had distinct assemblages of hygrophilic species, with a comparable number of indicator species as managed and old-growth heathland. Crane flies were highly abundant and species rich in wet areas compared to dry areas, which can be attributed to the hydrophilic nature of the larvae (Pritchard 1983; Byriel et al. 2020). Some rare crane fly species findings included the peat bog-specialists Phylidorea heterogyna and Erioptera nielseni, which were only known from a few old records in Denmark and were considered threatened or even extinct (Salmela 2012), but occurred in high abundances in three of the four wet heath sites. Bee communities in wet heathland resembled communities in old-growth heathland as indicated by the NMDS plot. Contribution to similarity could include an overlap in floral ericoid resources, e.g. Vaccinium spp., which are attractive to Bombus spp. (Moquet et al. 2017) and the oligolectic Andrena lapponica, which was identified as an indicator species of old-growth and wet heathland.
As opposed to the other taxa investigated, hoverflies showed remarkably similar richness median values and no response to vegetation and edaphic variables. Species composition indicated low variation among managed and old-growth heathland, while wet heathland showed distinct assemblages. In contrast to bees, hoverflies do not have parental care and are not restricted to a certain home range after oviposition (Rotheray and Gilbert 2011), which together with their high mobility makes them more suitable for environmental evaluation at larger scales (Sommaggio 1999). Common heathland hoverfly assemblages include long-foraging aphidophagous species associated with e.g. coniferous and deciduous forests (Moquet et al. 2018), thus explaining the low affinity to local vegetation characteristics. Wet heathland may show distinct hoverfly communities as they provide breeding habitats for saprophagous and aquatic larvae (Moquet et al. 2018), which corresponded with the three hygrophilic indicator species identified from wet heathland.
Several studies have shown that heathland management regimes such as grazing, harvesting and burning influence the structure of arthropod communities (Bell et al. 2001; Bargmann et al. 2015; WallisDeVries et al. 2016; Hansen et al. 2020). Although our study did not allow for a robust comparison between different management regimes, we observed taxa-specific responses to management as inferred from the NMDS plots. Especially insect assemblages in harvested/burned plots varied from other management regimes. These plots were characterized by a high cover of bare soil and forbs, which are known to influence pollinating and soil-dwelling insects through changes in food availability, temperature and nesting conditions (Gregory and Wright 2005; Meyer et al. 2009; Cameron and Leather 2012; Schirmel et al. 2012; Moquet et al. 2018). Grazed heathland was similarly characterized by distinguished hoverfly and rove beetle communities, and none of the measured variables seemed to explain this relationship. Large grazing herbivores are known to affect insect communities directly by creating microhabitats such as dung or trampled soil (Dennis et al. 1997; van Klink et al. 2020), which are important substrates for many species of hoverflies and rove beetles e.g. the dung-associated hoverfly Eristalinus sepulchralis found as an indicator of managed heathland.
Study limitations
Because of low replication among the different management regimes (burned, grazed and harvested), we were unable to elucidate the effect of individual management regimes on insect taxa diversity. Therefore, the results presented here should be viewed as an initial exploration of the mechanisms separating insect communities in managed vs. old-growth succession heathland. A balanced dataset including the different management regimes would likely provide a more nuanced depiction of insect diversity and associated drivers across managed and old-growth heathland. Despite this limitation, our study demonstrated a clear effect of the different heathland types on the investigated insect taxa, highlighting the importance of old-growth successional stages in structuring insect communities. Future studies should disentangle the effects of individual management regimes on the investigated insect taxa, as it would be of great conservation value.
The sampling conducted in this study was done at three different occasions from early June to early August. This interval was chosen as we expected it would represent the main peak in heathland insect diversity across all taxa. However, the interval may have been too short to cover the full phenology of all the insect taxa investigated. Sampling in the spring and autumn would unquestionably have yielded additional species, but we were limited by the number of samples that we would be able to sort and identify.
Implications for conservation management
The high number of insect species recorded as well as the large proportion of nationally red-listed species (~ 8% of all identified species) highlight the significance of heathlands in insect conservation and the value of preserving this semi-natural habitat. With our multi-taxa approach, we found supporting evidence for the hypothesis that old-growth heathland sites in combination with managed heathland sites provide heterogeneous habitat conditions that increase overall diversity of insects. Heathland management focusing solely on maintaining early successional stages with a high cover of even-aged Calluna may be beneficial to certain xerophilic insect species, but may limit a large proportion of the mesophilic and hygrophilic heathland insect fauna, which require more buffered conditions provided by the dense vegetation and humid microclimate.
Our study challenges the general conception that heathland succession deteriorate xerophilic insect communities and endangered species as previously reported for ground beetles (Buchholz et al. 2013) and butterflies (Schirmel and Buchholz 2011). Hence, we found no difference in red-listed species between managed, old-growth and wet heathland, and xerophilic species seemed to persist to some extent in old-growth heathland sites. In addition, no association between red-list status and hygropreference was found in this study with 10 xerophilic, 9 mesophilic and 5 hygrophilic species being red-listed. In contrast to Schirmel and Buchholz (2011) and Buchholz et al. (2013) this study did not investigate succession from dry heathland to grass-invaded and tree-dominated sites, which likely explain the discrepancies. Additional studies are needed to understand to what extent xerophilic and endangered heathland species may persist following heathland succession.
Overall, our results demonstrate that heathland management focusing on early successional vegetation stages may homogenize insect communities, emphasizing the need for rethinking current management practices. We suggest that management practices should focus on improving structural vegetation heterogeneity. This can be achieved through management regimes that reset the succession and expose bare soil, but also by allowing patches of old-growth vegetation stages to develop and by conserving existing ones.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Amiet F (1996) Hymenoptera, Apidae, 1. Teil. Allgemeiner Teil, Gattungsschlüssel, die Gattungen Apis, Bombus und Psithyrus, vol 1. Mitt Schweiz Entomol Ges, Neuchâtel
Amiet F, Müller A, Neumeyer R (1999) Apidae 2. Colletes, Dufourea, Hylaeus, Nomia, Nomioides, Rhophitoides, Rophites, Sphecodes, Systropha, vol 4. Mitt Schweiz Entomol Ges, Neuchâtel
Amiet F, Herrmann M, Müller A, Neumeyer R (2001) Apidae 3. Halictus, Lasioglossum, vol 6. Mitt Schweiz Entomol Ges, Neuchâtel
Amiet F, Herrmann M, Müller A, Neumeyer R (2004) Apidae 4. Anthidium, Chelostoma, Coelioxys, Dioxys, Heriades, Lithurgus, Megachile, Osmia, Stelis, vol 9. Mitt Schweiz Entomol Ges, Neuchâtel
Amiet F, Herrmann M, Müller A, Neumeyer R (2007) Apidae 5. Ammobates, Ammobatoides, Anthophora, Biastes, Ceratina, Dasypoda, Epeoloides, Epeolus, Eucera, Macropis, Melecta, Melitta, Nomada, Pasites, Tetralonia, Thyreus, Xylocopa, vol 20. Mitt Schweiz Entomol Ges, Neuchâtel
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26(1):32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ, Walsh DC (2013) PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr 83(4):557–574. https://doi.org/10.1890/12-2010.1
Arbizu PM (2017) pairwiseAdonis: pairwise multilevel comparison using adonis. R package version 0.0.1
Assing V, Schülke M (2012) Die Käfer Mitteleuropas. Band 4. Staphylinidae I. Zweite neubearbeitete Auflage. [The beetles of Central Europe. Volume 4. Staphylinidae I. Second revised edition]. Spektrum Akademischer Verlag, I-XII, Heidelberg und Berlin, p 560
Bargmann T, Hatteland BA, Grytnes JA (2015) Effects of prescribed burning on carabid beetle diversity in coastal anthropogenic heathlands. Biodivers Conserv 24:2565–2581. https://doi.org/10.1007/s10531-015-0945-1
Bartsch H, Binkiewicz E, Klintbjer A, Rådén A, Nasibov E (2009) Nationalnyckeln till Sveriges flora och Fauna. Tvåvingar: Blomflugor: Syrphidae. Diptera: Syrphidae: Syrphinae. & Syrphidae: Eristalinae & Microdontinae. Artdatabanken, Uppsala
Bates D, Sarkar D, Bates MD, Matrix L (2007) The lme4 package. R package version 1.1–21
Bell JR, Wheater CP, Cullen WR (2001) The implications of grassland and heathland management for the conservation of spider communities: a review. J Zool 255(3):377–387. https://doi.org/10.1017/S0952836901001479
Betz O, Irmler U, Klimaszewski J (2018) Biology of rove beetles (Staphylinidae): life history, evolution, ecology and distribution. Springer, Switzerland
Bogusch P, Straka J (2012) Review and identification of the cuckoo bees of central Europe (Hymenoptera: Halictidae: Sphecodes). Zootaxa 3311:1–41. https://doi.org/10.11646/zootaxa.3311.1.1
Buchholz S, Hannig K, Schirmel J (2013) Losing uniqueness–shifts in carabid species composition during dry grassland and heathland succession. Anim Conserv 16(6):661–670. https://doi.org/10.1111/acv.12046
Buttenschøn RM, Schmidt IK (2015) De danske hedetyper, deres udbredelse og tilstand. Flora og Fauna 121(3–4):75–78
Byriel DB, Schmidt IK, Justesen MJ, Pape T, Hansen AK, Riis-Nielsen T, Kepfer-Rojas S (2020) Forest management affects crane fly (Tipuloidea) community structure through changes in edaphic conditions. For Ecol Manag 457:117756. https://doi.org/10.1016/j.foreco.2019.117756
Cameron KH, Leather SR (2012) Heathland management effects on carabid beetle communities: the relationship between bare ground patch size and carabid biodiversity. J Insect Conserv 16:523–535. https://doi.org/10.1007/s10841-011-9438-z
Cederberg B, Holmström G, Hall K, Berg A (2022) Svenska bin Artfakta. SLU Artdatabanken. https://artfakta.se/. Accessed Mar 2022
DCE (2020) Danish Centre For Environment And Energy, University of Aarhus. Department of Environmental Science. https://envs.au.dk/. Accessed Sep 2020
De Bruyn L, Thys S, Scheirs J, Verhagen R (2001) Effects of vegetation and soil on species diversity of soil dwelling Diptera in a heathland ecosystem. J Insect Conserv 5:87–97. https://doi.org/10.1023/A:1011319417994
De Cáceres M, Legendre P, Moretti M (2010) Improving indicator species analysis by combining groups of sites. Oikos 119(10):1674–1684. https://doi.org/10.1111/j.1600-0706.2010.18334.x
Dennis P, Young MR, Howard CL, Gordon IJ (1997) The response of epigeal beetles (Col.: Carabidae, Staphylinidae) to varied grazing regimes on upland Nardus stricta grasslands. J Appl Ecol 34(2):433–443. https://doi.org/10.2307/2404888
Desender K, Turin H (1989) Loss of habitats and changes in the composition of the ground and tiger beetle fauna in four west European countries since 1950 (Coleoptera: Carabidae, Cicindelidae). Biol Conserv 48(4):277–294. https://doi.org/10.1016/0006-3207(89)90103-1
Dexter E, Rollwagen-Bollens G, Bollens SM (2018) The trouble with stress: a flexible method for the evaluation of nonmetric multidimensional scaling. Limnol Oceanogr: Methods 16(7):434–443
Digweed SC, Currie CR, Carcamo HA, Spence JR (1995) Digging out the “digging-in effect” of pitfall traps: influences of depletion and disturbance on catches of ground beetles (Coleoptera: Carabidae). Pedobiologia 39:561–576
Evans D (2006) The habitats of the European Union habitats directive. Biol Environ: Proc R Ir Acad 106B(3):167–173. https://doi.org/10.3318/BIOE.2006.106.3.167
Falk S, Lewington R (2015) Field guide to the bees of Great Britain and Ireland. Bloomsbury Publishing, London
Fox J, Weisberg S, Friendly M, Hong J, Andersen R, Firth D, Taylor S (2016) Effect displays for linear, generalized linear, and other models. R package version, 4.2-1
Gimingham CH (1970) British heathland ecosystems: the outcome of many years of management by fire. In: Proceedings of the annual tall timbers fire ecology conference 10: 293–321
Gimingham CH (1985) Age-related interactions between Calluna vulgaris and phytophagous insects. Oikos 44(1):12–16. https://doi.org/10.2307/3544036
Gimingham CH (1975) An introduction to heathland ecology. Oliver & Boyd, Edinburgh
Gregory S, Wright I (2005) Creation of patches of bare ground to enhance the habitat of ground-nesting bees and wasps at Shotover Hill, Oxfordshire, England. Conserv Evid 2:139–141
Hansen V (1951) Danmarks Fauna 57. Biller XV. Rovbiller 1. [The danish Fauna 57. Beetles XV. Rove beetles 1.]. G. E. C. Gads Forlag, Copenhagen, p 274
Hansen V (1952) Danmarks Fauna 58. Biller XVI. Rovbiller 2. [The danish Fauna 58. Beetles XVI. Rove beetles 2.]. G. E. C. Gads Forlag, Copenhagen, p 251
Hansen V (1954) Danmarks Fauna 59. Biller XVII. Rovbiller 3. [The danish Fauna 59. Beetles XVII. Rove beetles 3.]. G. E. C. Gads Forlag, Copenhagen, p 499
Hansen AK, Justesen MJ, Kepfer-Rojas S, Byriel DB, Pedersen J, Solodovnikov A (2018) Ecogeographic patterns in a mainland-island system in Northern Europe as inferred from the rove beetles (Coleoptera: Staphylinidae) on Læsø island. Eur J Entomol 115:256–263. https://doi.org/10.14411/eje.2018.025
Hansen RR, Nielsen KE, Offenberg J, Damgaard C, Byriel DB, Schmidt IK, Sørensen PB, Kjær C, Strandberg MT (2020) Implications of heathland management for ant species composition and diversity—is heathland management causing biotic homogenization? Biol Conserv 242:108422. https://doi.org/10.1016/j.biocon.2020.108422
Hartig F (2022) DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.5. http://florianhartig.github.io/DHARMa/. Accessed Apr 2022
Haysom KA, Coulson JC (1998) The Lepidoptera fauna associated with Calluna vulgaris: effects of plant architecture on abundance and diversity. Ecol Entomol 23(4):377–385. https://doi.org/10.1046/j.1365-2311.1998.00152.x
Hobbs RJ, Gimingham CH (1984) Studies on fire in scottish heathland communities II. Post-fire vegetation development. J Ecol 72(2):585–610. https://doi.org/10.2307/2260069
Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A, Scheibe S (2016) Package ‘multcomp’. Simultaneous inference in general parametric models. R package version 1.4-10
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7(12):1451–1456
Julier HE, Roulston TH (2009) Wild bee abundance and pollination service in cultivated pumpkins: farm management, nesting behavior and landscape effects. J Econ Entomol 102:563–573. https://doi.org/10.1603/029.102.0214
Kepfer-Rojas S, Schmidt IK, Ransijn J, Riis‐Nielsen T, Verheyen K (2014) Distance to seed sources and land‐use history affect forest development over a long‐term heathland to forest succession. J Veg Sci 25(6):1493–1503. https://doi.org/10.1111/jvs.12203
Kirby P (1992) Habitat management for invertebrates: a practical handbook. Royal Society for the Protection of Birds, Sandy
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82(13):1–2. https://doi.org/10.18637/jss.v082.i13
Lake S, Bullock JM, Hartley SE (2001) Impacts of livestock grazing on lowland heathland in the UK. English Nature Research Reports 422, Peterborough
Lindroth CH (1985) The Carabidae (Coleoptera) of Fennoscandia and Denmark. Fauna Entomol Scand 15:1–225
Lindroth CH (1986) The Carabidae (Coleoptera) of Fennoscandia and Denmark. Fauna Entomol Scand 15:226–497
Luff ML (2007) The Carabidae (ground beetles) of Britain and Ireland. Handbooks for the identification of British insects. Royal Entomological Society, London
Magnusson A, Skaug H, Nielsen A, Berg C, Kristensen K, Maechler M, van Bentham K, Bolker B, Sadat N, Lüdecke D, Lenth R, O’Brien J, Geyer CJ, McGillycuddy M, Brooks MM (2017) Package ‘glmmTMB’. R package version 1.1.2.3
Mantilla-Contreras J, Schirmel J, Zerbe S (2011) Influence of soil and microclimate on species composition and grass encroachment in heath succession. J Plant Ecol 5(3):249–259. https://doi.org/10.1093/jpe/rtr031
Meyer B, Jauker F, Steffan-Dewenter I (2009) Contrasting resource-dependent responses of hoverfly richness and density to landscape structure. Basic Appl Ecol 10(2):178–186. https://doi.org/10.1016/j.baae.2008.01.001
Miller BJF (1975) Studies of changes in the populations of invertebrates associated with cyclical processes in heathland. Ph. D. Thesis, University of Aberdeen
Moeslund JE, Nygaard B, Ejrnæs R, Bell N, Bruun LD, Bygebjerg R, Carl H, Damgaard J, Dylmer E, Elmeros M, Flensted K, Fog K, Goldberg I, Gønget H, Helsing F, Holmen M, Jørum P, Lissner J, Læssøe T, Madsen HB, Misser J, Møller PR, Nielsen OF, Olsen K, Sterup J, Søchting U, Wiberg-Larsen P, Wind P(2019) The Danish Red List. Aarhus Universitet, DCE—Nationalt Center for Miljø og Energi. www.redlist.au.dk . Accessed Jan 2020
Moquet L, Bacchetta R, Laurent E, Jacquemart AL (2017) Spatial and temporal variations in floral resource availability affect bumblebee communities in heathlands. Biodivers Conserv 26:687–702. https://doi.org/10.1007/s10531-016-1266-8
Moquet L, Laurent E, Bacchetta R, Jacquemart AL (2018) Conservation of hoverflies (Diptera, Syrphidae) requires complementary resources at the landscape and local scales. Insect Conserv Divers 11(1):72–87. https://doi.org/10.1111/icad.12245
Newton AC, Stewart GB, Myers G, Diaz A, Lake S, Bullock JM, Pullin AS (2009) Impacts of grazing on lowland heathland in north-west Europe. Biol Conserv 142(5):935–947. https://doi.org/10.1016/j.biocon.2008.10.018
Oksanen J et al (2016) Vegan: community ecology package. R package version 2.5-6
Oosterbroek P (2022) Catalogue of the craneflies of the world (Diptera, Tipuloidea: Pediciidae, Limoniidae, Cylindrotomidae, Tipulidae). Available from: http://ccw.naturalis.nl/ . Accessed Mar 2022
Pane AM, Harmon-Threatt AN (2017) An assessment of the efficacy and peak catch rates of emergence tents for measuring bee nesting. Appl Plant Sci 5(6):1700007. https://doi.org/10.3732/apps.1700007
Potts SG, Vulliamy B, Roberts S, O’Toole C, Dafni A, Ne’eman G, Willmer P (2005) Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol Entomol 30:78–85. https://doi.org/10.1111/j.0307-6946.2005.00662.x
Pritchard G (1983) Biology of Tipulidae. Annu Rev Entomol 28(1):1–22. https://doi.org/10.1146/annurev.en.28.010183.000245
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.R-project.org/. Accessed Mar 2022
Ransijn J, Kepfer-Rojas S, Verheyen K, Riis-Nielsen T, Schmidt IK (2015) Hints for alternative stable states from long-term vegetation dynamics in an unmanaged heathland. J Veg Sci 26(2):254–266. https://doi.org/10.1111/jvs.12230
Riis-Nielsen T, Søchting U, Johansson M, Nielsen P (1991) Hedeplejebogen—de danske heders historie, pleje og udforskning. Miljøministeriet, Copenhagen
Riis-Nielsen T, Schmidt IK, Frandsen B, Binding T (2005) Nørholm Hede—En langtidsundersøgelse af hedens vegetationsudvikling og tilgroning. The research series. Forest & Landscape 35, KVL
Robel RJ, Briggs JN, Dayton AD, Hulbert LC (1970) Relationships between visual obstruction measurements and weight of grassland vegetation. J Range Manag 23:295–298
Rotheray GE, Gilbert F (2011) The natural history of hoverflies. Forrest Text, Carmarthen, UK
Salmela J (2012) Updates to the danish crane fly fauna (Diptera, Tipuloidea) and notes on Tipula crassicornis Zett. Entom Meddel 80(2):119–125
Schellenberg J, Bergmeier E (2021) The Calluna life cycle concept revisited: implications for heathland management. Biodivers Conserv. https://doi.org/10.1007/s10531-021-02325-1
Schirmel J, Buchholz S (2011) Response of carabid beetles (Coleoptera: Carabidae) and spiders (Araneae) to coastal heathland succession. Biodivers Conserv 20(7):1469–1482. https://doi.org/10.1007/s10531-011-0038-8
Schirmel J, Mantilla-Contreras J, Blindow I, Fartmann T (2011) Impacts of succession and grass encroachment on heathland Orthoptera. J Insect Conserv 15:633–642. https://doi.org/10.1007/s10841-010-9362-7
Schirmel J, Blindow I, Buchholz S (2012) Life-history trait and functional diversity patterns of ground beetles and spiders along a coastal heathland successional gradient. Basic Appl Ecol 13(7):606–614. https://doi.org/10.1016/j.baae.2012.08.015
Schmid-Egger C, Scheuchl E (1997) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs und Berücksichtigung der Arten der Schweiz. Band III: Schlüssel der Arten der Familie Andrenidae. Eigensverlag, Velden
Sommaggio D (1999) Syrphidae: can they be used as environmental bioindicators? Agric Ecosyst Environ 74(1–3):343–356. https://doi.org/10.1016/S0167-8809(99)00042-0
Speight MCD, Castella E, Obrdlik P (2000) Use of the Syrph the Net database 2000. Syrph the Net, the database of European Syrphidae, Vol. 25, p 99
Spitzer K, Danks HV (2006) Insect biodiversity of boreal peat bogs. Annu Rev Entomol 51:137–161. https://doi.org/10.1146/annurev.ento.51.110104.151036
Stubbs AE, Kramer J (2016) Key to the Tipulomorpha of Great Britain. A–J, Total p 141. Available from http://ccw.naturalis.nl/. Accessed Aug 2018
Usher MB (1992) Management and diversity of arthropods in Calluna heathland. Biodivers Conserv 1:63–79. https://doi.org/10.1007/BF00731035
van Klink R, van Laar-Wiersma J, Vorst O, Smit C (2020) Rewilding with large herbivores: positive direct and delayed effects of carrion on plant and arthropod communities. PLoS ONE 15(1):e0226946. https://doi.org/10.1371/journal.pone.0226946
van Veen MP (2010) Hoverflies of Northwest Europe: identification keys to the Syrphidae. KNNV Publishing, Zeist
Vogels JJ, Verberk WCEP, Lamers LPM, Siepel H (2017) Can changes in soil biochemistry and plant stoichiometry explain loss of animal diversity of heathlands? Biol Conserv 212(B):432–447. https://doi.org/10.1016/j.biocon.2016.08.039
WallisDeVries MF, Noordijk J, Colijn EO, Smit JT, Veling K (2016) Contrasting responses of insect communities to grazing intensity in lowland heathlands. Agric Ecosyst Environ 234:72–80. https://doi.org/10.1016/j.agee.2016.04.012
Webb NR (1998) The traditional management of european heathlands. J Appl Ecol 35(6):987–990. https://doi.org/10.1111/j.1365-2664.1998.tb00020.x
Webb JR, Drewitt AL, Measures GH (2010) Managing for species: Integrating the needs of England’s priority species into habitat management. Part 1 Report. Natural England Research Reports, Sheffield. Number 024
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York. https://doi.org/10.1007/978-3-319-24277-4 (R package version 3.2.1)
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
We are grateful to the Aage V. Jensen Nature Foundation for funding this work. We wish to thank the Danish Nature Agency for access to Harrild and Randbøl, Karl and Martha Nielsen for access to Nørholm and AVJNF for access to Ovstrup. A special thanks to students Marianne Debue, Chloé Malaisé, Jeanne Cazaillon, Faustine Pommarel, Estelle Guérin and Perrine Etheimer for help with fieldwork. The authors are thankful to Henning Bang Madsen and Jan Pedersen (University of Copenhagen), Palle Jørum (Entomologisk Forening) and Rune Bygebjerg (Lund University) for help with species identification. Finally, we thank the anonymous reviewers for their useful feedback.
Funding
Funding was provided by Aage V. Jensens Fonde (Grant No. Naturlig Dynamik i Hedeplejen).
Author information
Authors and Affiliations
Contributions
DBB and IKS conceived the ideas and designed methodology; DBB, HRP, AKH, RRH, MJJ, EK, CBM and IKS collected, sorted and processed the data; DBB and SKR analyzed the data; DBB led manuscript writing with editing from IKS, SKR, RRH and contributions from all authors. All authors gave final approval of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
All persons who collected insects for this study received the necessary authorization from the competent authority.
Additional information
Communicated by Andreas Schuldt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Byriel, D.B., Ro-Poulsen, H., Kepfer-Rojas, S. et al. Contrasting responses of multiple insect taxa to common heathland management regimes and old-growth successional stages. Biodivers Conserv 32, 545–565 (2023). https://doi.org/10.1007/s10531-022-02511-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02511-9