Abstract
Reintroductions are powerful tools for tackling biodiversity loss, but the resulting populations can be intrinsically small and vulnerable. It is therefore critical to maximise the number of individuals that are available to contribute to recovery efforts. To address this, we investigated how demographic parameters from a reintroduced population can reveal threats to long-term persistence, inform thresholds for management interventions, and create targets for removing an endangered species from the IUCN Red List. We calculated capture-mark-recapture population estimates for eastern quolls (Dasyurus viverrinus) which had been reintroduced to a fenced reserve in the Australian Capital Territory. We then incorporated the resulting demographic parameters into population viability analyses (PVAs) to estimate probabilities of persistence under several scenarios, including supplementations and harvests (removal of individuals for translocation to other locations). After determining sustainable harvest rates, we then ‘back-cast’ the population size and occupancy area required to remove the species from the IUCN Red List within 10 years. Our demographic results indicated high mean apparent survival (90% ± 5), and PVAs revealed the probability of persistence over a 50-year time horizon was 50.5% with no interventions, 0% when the population was harvested of > 6 individuals, and 100% if harvests ≤ 54 juveniles were combined with an annual supplementation of ten maternal females (with ≤ 6 young each). Based on this model, a total harvest area of 413 km2 and an occupancy area of 437 km2 would be needed to recover the species within 10 years (i.e., 90 similar fenced reserves, not accounting for edge effects). Due to the inherent difficulty in securing large areas for species recovery, we see these ambitious targets as a call to create coordinated and collaborative sanctuary networks where species can be managed as a metapopulation across multiple sites. By taking advantage of a rapid life history and harvesting the ‘doomed surplus’, managers can achieve their stretch goals for species recovery in the long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Defaunation in the Anthropocene, driven by human-induced environmental change and destruction, threatens biodiversity, ecosystem function, and human health worldwide (Dirzo et al. 2014). Restoration presents a major challenge for the next century, but to avoid long-term goals being limited by short-term human memory of ecosystems (i.e., ‘shifting baseline syndrome’, Pauly 1995; Miller 2005; Manning et al. 2006), robust monitoring and species listings are paramount. The International Union for Conservation of Nature’s Red List of Threatened Species (IUCN 2021) provides a powerful tool used by conservationists and researchers to list declining species, and galvanise conservation action and policy change (Rodrigues et al. 2006; Betts et al. 2020). It provides a repository for information related to species range, population size, threats, and conservation actions, and compares these to broadly applicable and standardised criteria to categorise species from ‘Critically endangered’ to ‘Least concern’. These criteria enable managers to quantify the targets required to downlist (reclassify from higher risk to lower risk categories) or delist (remove from the IUCN Red List) a species, and encourages small-scale projects to be unified under long-term visions for species recovery.
Reintroductions are a critical tool used to reverse defaunation and restore ecosystem function (Armstrong and Seddon 2008). While reintroductions aim to establish a viable and self-sustaining population (IUCN 2013), management decisions must always be made in the face of imperfect knowledge about species and ecosystems (Armstrong and Seddon 2008). This uncertainty can be addressed using robust monitoring of demographic parameters including abundance, survival, and reproduction; which can indicate population self-sustainability, density-dependence, carrying capacity, and threats to these processes (e.g., Manning et al. 2019; Parlato et al. 2021). This is particularly crucial when a population transitions through the three phases of reintroduction, from ‘establishment’ (where post-release effects drive population dynamics) to ‘growth’ (characterised by high rates of expansion) and, finally, to ‘regulation’ (where density dependence limits survival and recruitment, Sarrazin 2007). However, many reintroduction studies span short timeframes (i.e., < 5 years, Parlato et al. 2021), limiting their ability to estimate temporal variation in vital rates over the long term (e.g., Leech et al. 2007; Cremona et al. 2017). From the outset, reintroduction programmes should be focused on a long-term vision of species recovery, which necessarily requires robust demographic monitoring to ensure the population reaches the regulation phase (Armstrong and Reynolds 2012; McCarthy et al. 2012; Nichols and Armstrong 2012).
As part of this long-term approach, demographic parameters from a reintroduced population can be built into stochastic population models (i.e., for population viability analyses [PVAs], Lindenmayer et al. 1993). These models can be used to compare alternative management interventions including supplementations (reinforcement of individuals) and harvests (removal of individuals for translocation to other locations), and thus provide crucial insights to guide these management actions. For example, PVAs have revealed that expanding the reintroduction of bearded vultures (Gypaetus barbatus) would dangerously deplete the captive source population (Bustamante 1996), and have highlighted the need to establish a second population of Eurasian beavers (Castor fiber) to ensure their viability in the Netherlands (Nolet and Baveco 1996). Since population growth is often affected by population size, this can result in a trend toward a constant breeding density (i.e., density-dependence, Sibly et al. 2002). Above this density, excess individuals can be considered a ‘doomed surplus’ (e.g., northern bobwhite Colinus virginianus, Errington 1945, but see Williams et al. 2004). Harvesting these individuals (especially for species with high fertility) could offer an ideal opportunity to maximise the number of individuals available for translocation to begin or reinforce other populations (e.g., demonstrated in black-footed ferrets Mustela nigripes, Biggins et al. 2011). Reintroduced populations, however, can be intrinsically small and vulnerable to stochastic effects (Lacy 2000), and therefore the impacts of harvesting from these populations should be simulated before any individuals are removed. PVAs can inform thresholds for harvests of the doomed surplus for translocation by indicating the maximum sustainable number of individuals available to contribute to species recovery.
We used a reintroduced population of eastern quolls (Dasyurus viverrinus) to investigate how demographic monitoring and PVAs can reveal viable interventions (i.e., supplementations and harvests) that can contribute to removing an endangered species from the IUCN Red List. Specifically, we (1) modelled survival and recruitment rates throughout the establishment, growth, and regulation phases of the eastern quoll reintroduction, (2) incorporated these demographic parameters into stochastic population models to reveal long-term viability under different management scenarios (i.e., no intervention, supplementation, harvest, and combinations of these), and (3) determined the contribution of our programme toward species recovery. Since long-term vision is essential for effective restoration, we set an ambitious end point (or ‘stretch goal’) of eastern quoll species recovery within 10 years, and then retrospectively calculated the area of habitat (henceforth ‘area of occupancy’) and number of harvests required to achieve this goal (also known as ‘back-casting’, Manning et al. 2006).
Australia has suffered the highest rate of mammal extinctions of any continent (Woinarski et al. 2015), due in large part to predation by introduced species (e.g., red fox Vulpes vulpes, and feral cat Felis catus, Kinnear et al. 2002; Radford et al. 2018). To circumvent these threatening processes, significant efforts have been made to reintroduce species where introduced predators are absent, such as in conservation-fenced areas (Hayward and Kerley 2009; Moseby et al. 2011; Legge et al. 2018). The benefits of conducting long-term, large-scale experiments under such fenced conditions are increasingly being recognised, and allow researchers to build an understanding of ecological processes which may otherwise be impossible (Hester et al. 2000; Manning et al. 2009).
Our eastern quoll reintroduction took place in this fenced context, and presents a unique model for testing the effect of conservation actions on species recovery because (1) the species is categorised as ‘Endangered’ under the IUCN Red List (Burbidge and Woinarski 2016), (2) the founding population was small but within the normal range for mammal reintroductions (n = 44), (3) reintroductions to fenced areas provide an ideal opportunity to undertake ecological experiments, (4) the population has been robustly monitored for over five years, and (5) the programme emulates small conservation projects across the globe, creating broad relevance of our outcomes to long-term reintroduction planning.
Materials and methods
Ethics statement
Translocations were carried out under licenses from the Tasmanian Department of Primary Industries, Parks, Water and Environment (permits TFA 16025 and 17091, export licences 12818/16 and 13528/17), Victorian Department of Environment, Land, Water and Planning (permit 14505167), and Australian Capital Territory Government (scientific licence LT2017959, import licence L120161261). Reintroduction (protocol A2016/02) and monitoring procedures (protocol A2020/40) were approved by The Australian National University Animal Experimentation Ethics Committee.
Study area
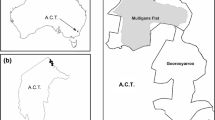
This study took place at Mulligans Flat Woodland Sanctuary (henceforth Mulligans Flat), a 485 ha public nature reserve containing critically endangered box-gum grassy woodland (McIntyre et al. 2010) situated on Ngunnawal and Ngambri Country in the Australian Capital Territory (−35.167, 149.158). Mulligans Flat is part of the Mulligans Flat-Goorooyarroo Woodland Experiment (https://www.mfgowoodlandexperiment.org.au/) and functions as an ‘outdoor laboratory’ where restoration techniques are trialed (Manning et al. 2011; Shorthouse et al. 2012). This includes the reintroduction of locally extinct species, such as the eastern bettong (Bettongia gaimardi, Manning et al. 2019), bush stone-curlew (Burhinus grallarius, Rapley 2020), and New Holland mouse (Pseudomys novaehollandiae, Abicair et al. 2020, Smith et al. 2022). To enable these reintroductions, the sanctuary is enclosed by a 11.5 km fence which excludes introduced species including the red fox, feral cat, European rabbit (Oryctolagus cuniculus), and European hare (Lepus europaeus, Shorthouse et al. 2012). While these threatening and destructive species have been eradicated from within the sanctuary, conditions are otherwise like other unfenced woodlands in the region. The fence design includes an overhang on the outside which prevents entry by introduced species (Shorthouse et al. 2012), but does not prevent agile species from climbing out of the sanctuary into the surrounding landscape.
Study species
The eastern quoll (‘murunguny’ in the Indigenous Ngunnawal language) is a solitary, small-to-medium (0.7–1.9 kg, Jones et al. 2001) marsupial carnivore (family Dasyuridae, Stannard and Old 2013). It is an opportunistic hunter with a diet dominated by invertebrates, but will also depredate birds, reptiles, and mammals, and scavenge on carcasses (Blackhall 1980; Godsell 1983).
The species is promiscuous and facultatively monoestrous, breeding synchronously in early Austral winter (Fletcher 1985), giving birth after 20 days gestation, and weaning ≤ 6 young in spring when food availability is high (Godsell 1983). Eastern quolls are sexually dimorphic, with males being larger (0.9–2 kg) and having larger home ranges (mean 44 ha) than females (0.7–1.1 kg, mean 35 ha, Godsell 1983). Populations reach their highest densities in early summer when the annual cohort of juveniles disperse from their natal dens, and lowest densities during winter largely due to juvenile mortality (Godsell 1983). The combination of a short lifespan (3–4 years) and these seasonal population fluctuations result in high population turnover (Jones et al. 2001).
Historically, eastern quolls were distributed throughout south-eastern Australia until the 1960s. The species disappeared from all but the southern island state of Tasmania due to a combination of habitat destruction, disease, human persecution, and predation by introduced species (particularly red foxes, Jones et al. 2001; Peacock and Abbott 2014). While there is no robust assessment of population size, state-wide spotlighting surveys revealed a 52% decline in sightings at 150 sites across Tasmania between 1999 and 2009 (i.e., 10,400 decline in population size from a 20,000 estimate, Fancourt et al. 2013). In addition, trapping surveys revealed a 61–100% decline at historical ‘hotspots’ (with disproportionately high eastern quoll densities compared with other parts of Tasmania, i.e., Cradoc, Cradle Mountain, and Buckland) compared with trapping conducted 18–31 years earlier. In response, the species was listed as ‘Endangered’ by the IUCN (Burbidge and Woinarski 2016) and the Australian Threatened Species Scientific Committee (2015).
Reintroduction
To reintroduce eastern quolls to Mulligans Flat, we adopted an adaptive translocation tactics approach (sensu Batson et al. 2015); involving a series of iterative trials where learnings were used to refine tactics for the following trial. When the first trial in 2016 revealed high male mortality associated with increased conspecific aggression and overdispersal (87.5% mortality, Wilson et al. 2020; Wilson et al. 2021), we selected only female founders for subsequent trials to maximise survival (12.5–23.1% mortality). By translocating maternal females (henceforth mothers) in winter, several were either pregnant or carrying pouch young, allowing us to reintroduce juvenile males and females ‘via the pouch’. This also potentially increased genetic diversity because multiple sires can be represented in a litter (B. Brockett unpublished data).
Forty-four (female = 36, male = 8) founding individuals (henceforth founders) were translocated to Mulligans Flat between 2016 and 2019, with a presumed total of 33 founders surviving the establishment period (42 days post-release, Wilson et al. 2020). Founders were either captive-bred (sourced from Mt Rothwell Biodiversity Interpretation Centre, henceforth Mt Rothwell, https://www.mtrothwell.com.au/) or wild-caught (sourced from free-ranging populations across four regions in Tasmania, as per Wilson et al. 2020).
Monitoring
We used a Robust Design capture-mark-recapture (CMR) framework to conduct demographic monitoring of the reintroduced eastern quoll population (Pollock 1982; Kendall and Nichols 2002). We conducted eight CMR primary sessions, each consisting of two trap nights 2–3 days apart (secondary sessions), in Austral summer and autumn each year between 2017 and 2022. Trapping during summer was intended to detect the greatest population density following juvenile dispersal in spring, and trapping during autumn was intended to detect the size of the breeding population. We integrated one night of free-feeding before each trap night to encourage the capture of more ‘trap-shy’ individuals (Biro 2013).
We standardised an array of 92 trap sites across Mulligans Flat, each placed 25 m from vehicle tracks and approximately 200 m apart (Fig. 1). We used wire cage traps (31 cm × 31 cm × 70 cm) baited with sardines, and for each trapped animal we inserted a microchip for identification, sampled fur, scat, and skin (biopsy for genetic material), and recorded sex, weight, and pouch occupancy (as per Portas et al. 2020).
Data analyses
Demography
We fitted Robust Design Pradel Recruitment Closed Population Estimation models (Kendall et al. 1995, 1997; Pradel 1996) which incorporate closed sampling periods (secondary sessions) within open sampling periods (primary sessions, Pollock 1982; Kendall and Nichols 2002). We assumed emigration and mortality only occurred between primary sessions and that population growth reflected young eastern quolls recruited to adulthood (and not immigration, since Mulligans Flat is fenced). The Pradel Robust Design allowed us to derive estimates of population size (N) at each primary session from initial capture probability (p), recapture probability (c), apparent survival (φ), and recruitment (f) using a logit link function. Since the length of time between primary and secondary sessions varied (either by design or logistical constraints), we ensured the sampling regime was reflected in the models.
We tested 12 candidate models, which included whether p was equal to c or varied from each other (e.g., due to ‘trap shyness’), and whether p and c were constant over time (null), or varied by session, season, trap night, minimum temperature (°C), maximum temperature (°C), and rainfall on the day of trapping (Bureau of Meteorology 2021), and whether there was an additive effect of trap availability (fraction of traps available to eastern quolls after removing traps made unavailable by other species and defective traps), across all individuals and between sexes (Table 1). We assessed models based on Akaike’s Information Criterion corrected for small sample sizes (AICc) to predict the final parameter estimates (Burnham and Anderson 2002).
We also investigated whether body weight varied by primary session or estimated population size (N) by fitting linear models. Since the eastern quoll is sexually dimorphic, females and males were modelled separately. Finally, we estimated eastern quoll density by dividing the trapped area (485 ha) by the mean female and male estimates (N) across all sessions excluding autumn 2017 (where the population was still establishing). Demographic analyses were conducted within the R environment (version 4.1.2, R Core Team 2022) using the packages AICcmodavg (Mazerolle 2017), ggplot2 (Wickham 2011), lme4 (Bates et al. 2015), lsmeans (Lenth 2016), MuMIn (Barton 2016), and RMark (Laake 2013) to interface with the program MARK (version 9.0, White 2016).
Viability
We explored the long-term viability of the reintroduced eastern quoll population over a 50-year time horizon using individual-based models simulating a hypothetical, non-spatially explicit population incorporating demographic parameters from our top-ranking model (determined above), and ecological data available for the species (Table 2). Due to their rapid life history (3–4 year lifespan), we considered only two life stages where individuals > 1 year old were classed as adults, otherwise they were classed as juveniles.
Since eastern quolls, and especially males, have naturally high rates of mortality during the juvenile dispersal (summer) and breeding periods (early winter, Godsell 1983; Wilson et al. 2020), we aimed to determine a threshold for sustainably harvesting (i.e., removing individuals for translocation to other locations) this ‘doomed surplus’ (animals that would never survive the seasonal bottleneck, Errington 1945). In Mulligans Flat, this process likely manifests as an exodus over the conservation fence due to limited territory. In addition, supplementing mothers (carrying ≤ 6 pouch young) allows managers to translocate ‘seven for the price of one’ (Wilson et al. 2020). As such, we ordered the simulated events in a year as: (1) setting of annual rates (EV), (2) aging, (3) carrying capacity (K) truncation, (4) breeding (early winter) with a census, (5) supplementation (mid-winter) with a census, (6) growth rate (r) calculation, (7) harvest (late spring) with a census, and (8) mortality (summer) with a census (for definitions see Lacy and Pollak 2021).
We modelled the following annual scenarios based on a stable stage distribution: (a) no intervention, (b) harvest (i.e., removed) of six (1:1 sex ratio) juveniles, (c) supplementation (i.e., reinforcement) with one mother (carrying six young, effectively n = 7), (d) supplementation with one mother (effectively n = 7) and harvest of four juveniles, and (e) supplementation with 10 mothers (effectively n = 70) and harvest of 54 juveniles. We simulated models with 1000 iterations to account for stochasticity in parameter estimates and increase model precision, and did not include catastrophes. Inbreeding depression was included for the ‘no interventions’ and harvest-only scenarios, but was not included for scenarios involving supplementations because inbreeding effects would likely be negated (Mills and Allendorf 1996). Parameters derived from the PVAs included population growth rate (λ) and probability of persistence (percent). PVAs were conducted using Vortex 10.5.5 (Lacy and Pollak 2021) and post-simulation visualisations were generated using the vortexR package (Pacioni and Mayer 2017) in R version 4.1.2 (R Core Team 2022).
Recovery
To reveal a roadmap towards eastern quoll species recovery, we summarised the IUCN Red List criteria for the status of ‘Critically endangered’, ‘Endangered’, and ‘Vulnerable’ (IUCN 2021), and recommended actions that would result in delisting the species based on the eastern quoll assessment (i.e., Burbidge and Woinarski 2016). We then incorporated these targets with our PVA results (i.e., sustainable harvests) to calculate the annual contribution of Mulligans Flat towards species recovery (i.e., offsetting the population reductions and increasing geographic range that placed the species in the IUCN Red List ‘Vulnerable’ category). Finally, we back-casted the number of harvests and the area of occupancy that would be required achieve our stretch goal of species recovery within 10 years (Manning et al. 2006).
Results
Demography
During 1,472 trapping nights, we made 421 eastern quoll captures (155 unique individuals, 101 females, 54 males) at Mulligans Flat over eight trapping sessions between 2017 and 2022. 56.44% of females and 70.37% of males were recaptured at least once. The top-ranking CMR model included capture probability (p) and recapture probability (c) varying from each other, and trap night (Table 1).
In the first monitoring session in autumn 2017, we caught one founder and seven sanctuary-born eastern quolls (n and N = 8). Between 2018 and 2020 population estimates oscillated, with summer estimates being expectedly greater (coinciding with juvenile dispersal, mean = 37 ± 6.24), and autumn estimates being lower and more variable (between the dispersal and breeding periods, mean = 34.56 ± 14.14, Fig. 2a). Sex ratios were relatively balanced until summer 2018 (1.12 females: 1 male), but by the following summer 2019 session they skewed heavily toward females (2.24 females: 1 male). The autumn 2021 population estimate deviated from this oscillating trend and reached a new peak (N = 51), with a sex ratio that was also skewed toward females (2.13 females: 1 male). The final autumn 2022 session achieved a similar population estimate (N = 47) and sex ratio (2.35 females: 1 male).
Estimated population size (a N, ± 95% CI) and mean body weight (b kg, ± 95% CI) based on eight capture-mark-recapture sessions for female, male, and all (‘both’) reintroduced eastern quolls (Dasyurus viverrinus) at Mulligans Flat Woodland Sanctuary, Australian Capital Territory. Mean body weights were calculated in R version 4.1.2 (R Core Team 2022), and population estimates were calculated using the RMark package (Laake 2013) to interface with the program MARK version 9.0 (White 2016)
Excluding 2017 where the population was still establishing, mean apparent survival (φ) across all individuals was 90% (± 5) between 2018 and 2022, with females having similar survival rates (91% ± 6) to males (89% ± 6). Estimated recruitment (f) was similar between sexes (females 7% ± 5, and males 7% ± 7).
Mean body weights (females 0.84 kg ± 0.13, males 1.13 kg ± 0.15) oscillated between a maximum in autumn and minimum in summer; the opposite to the trend observed for population estimates (Fig. 2b). However, body weights were unexpectedly lower in autumn 2021 compared to previous autumns (females 0.74 kg ± 0.04, males 1.02 kg ± 0.08), however this was followed by the greatest body weights observed across the study period in autumn 2022 (females 1.02 kg ± 0.04, males 1.35 kg ± 0.08). When fitting a linear model with body weights (kg) against estimated population size (N), we found a significantly negative association (p = 0.034), suggesting the population had become density-dependent six years after the first trial reintroduction.
The mean density of eastern quolls across sessions (excluding autumn 2017 where the population was still establishing) was one female per 19.57 ha (± 2.84), one male per 37.51 ha (± 1.06), and for both sexes, one individual per 12.97 ha (± 3.76). The maximum density of eastern quolls in Mulligans Flat was one individual per 9.53 ha in autumn 2021, indicating a maximum carrying capacity of 51 adults (34 females: 17 males).
Viability
PVAs revealed that with no interventions, the Mulligans Flat population would have a 50.5% probability of persistence over the next 50 years (Fig. 3). This probability fell to 0% when > 6 juveniles were harvested from the population annually, but rose to 100% if the population was supplemented with at least one mother annually (carrying ≤ 6 young, effectively n = 7). Further, four juveniles could be sustainably (100% probability of persistence) harvested from the population annually as long as one mother (effectively n = 7) was also supplemented into the population annually. Finally, 54 juveniles could be sustainably harvested if ten mothers (effectively n = 70) were supplemented annually. The deterministic annual population growth rate (r) was 0.5063 across all scenarios, and probability of persistence was sensitive to carrying capacity (K) and the number of individuals supplemented and/or harvested.
Simulated population size (N) for reintroduced eastern quolls (Dasyurus viverrinus) at Mulligans Flat Woodland Sanctuary, Australian Capital Territory. ‘F’ refers to the number of females, ‘M’ to males, and ‘J’ to juveniles. Scenarios were simulated using 1000 iterations over a 50-year time horizon with parameters provided in Table 2. Population viability analyses were conducted using Vortex 10.5.5 (Lacy and Pollak 2021) and post-simulation visualisation was generated using the package vortexR (Pacioni and Mayer 2017) in R version 4.1.2 (R Core Team 2022)
Recovery
The estimated eastern quoll population size in Tasmania was ~ 20,000 prior to its 52% decline (Fancourt et al. 2013). To avoid meeting the criteria for IUCN Red List ‘Vulnerable’ category, the estimated current population of ~ 9600 would need to be increased to 14,200 (i.e., a < 30% decline from the original population size, Table 3). This provides us with a stretch goal of producing 4600 eastern quolls, equivalent to 460 individuals per year for 10 years, to achieve species recovery. Since we can sustainably harvest the Mulligans Flat population of 54 individuals per year across its 4.85 km2 area, this implies that to harvest 460 juveniles, an area of 41.31 km2 would be needed (this area would need to have conditions comparable to Mulligans Flat, i.e., without introduced predators). Finally, a total of 437.45 km2 (4600 individuals / Mulligans Flat density [10.52 individuals per km2]) would be required to sustain these individuals beyond 10 years (i.e., 90 sanctuaries similar to Mulligans Flat, not accounting for edge effects).
In addition, eastern quolls need to occur in > 10 ‘locations’ (“geographically or ecologically distinct areas in which a single threatening event can rapidly affect all individuals of the taxon present”, IUCN 2001) to avoid meeting the criteria for the IUCN Red List ‘Vulnerable’ category (Table 3). We interpreted a ‘single threatening event’ as being spatially and temporally explicit (e.g., flood) as opposed to a threatening process which is spatially and temporally dynamic (e.g., disease, climate change). Burbidge and Woinarski (2016) indicated there were two locations: Tasmania and Bruny Island. We suggest that Mulligans Flat and Mt Rothwell should be also considered locations because they support self-sustaining, density-dependent, and geographically distinct populations since their reintroductions in 2016 and 2003, respectively. Thus, an additional seven locations with similar levels of introduced predator mitigation will be required. Finally, while not quantified, Burbidge and Woinarski (2016) reported a decline in the eastern quoll’s extent of occurrence (EOO) and area of occupancy (AOO). These criteria could be addressed by increasing the number of eastern quoll locations on mainland Australia.
Discussion
We have demonstrated how incorporating a reintroduced population’s demographic parameters into PVAs to determine the viability of harvesting a ‘doomed surplus’ can contribute measurably to species recovery. We also highlighted how back-casting can reveal the pathway to removing an endangered species from the IUCN Red List within 10 years (i.e., by reversing population declines and increasing the number of locations). While our targets may appear daunting, a stretch goal, by definition, must be ambitious enough to inspire the creativity and innovation to achieve long-term outcomes that currently seem impossible (Manning et al. 2006). Progress toward large-scale conservation and restoration requires such innovation to prevent the ‘locking-in’ of the current shifting baseline (Evans et al. 2022).
Demography
The reintroduced eastern quoll population at Mulligans Flat grew rapidly in the absence of introduced predators, despite a limited number of founders (n = 44). After autumn 2017, the population oscillated between maximums in summer and minimums in autumn for the next 3 years (Fig. 2a), depicting a transition from the establishment phase (2017) to the growth phase (2018–2019), and finally, to the regulation phase (2020–2022, sensu Sarrazin 2007). The combination of a rapid life history (2–3 year lifespan), high reproductive success (100% female breeding, Godsell 1983), and high fertility (≤ 6 progeny per year) created an ideal scenario for producing an insurance population on mainland Australia.
Mean body weights oscillated between maximums in autumn and minimums in winter (Fig. 2b) and had a significantly negative association with population estimates (p = 0.034). This indicates density-dependence, a fundamental objective of any reintroduction, and mechanistically could have resulted from conspecific competition for prey or territory (e.g., dens). Interestingly, the autumn 2021 and 2022 sessions deviated from the oscillating trends in both population estimates and mean body weights. Australia suffered long-term rainfall deficiencies between 2017 and 2020, with conditions easing in March 2021 (Bureau of Meteorology 2021). The severe climatic conditions likely affected available prey for eastern quolls (similarly observed in Tasmania by Fancourt et al. 2018), thereby limiting the carrying capacity of Mulligans Flat in these years. In the 2021 session the population estimates reached a new maximum (N = 51) but mean body weights were lower (0.82 kg ± 0.04) than in previous autumn sessions (mean 1.033 kg ± 0.11). This suggests that as population size increased, resources (e.g., prey items) may have become limiting, resulting in lower body weights.
Interestingly, mean apparent survival was similar for females (91% ± 6) and males (89% ± 6) in Mulligans Flat, whereas historical survival rates in Tasmania differed between the sexes (females 63%, males 25%, Godsell 1983). Sex ratios were relatively balanced until summer 2018 (1.12 females: 1 male), after which it became heavily skewed toward females (2.24 females: 1 male in summer 2019). Our population estimates indicate a maximum carrying capacity of 34 females, each with longer lifespans (2–3 years) than males (1–2 years). While females have smaller and overlapping home ranges (Wilson et al. 2020), there may not be enough territory to support all the juveniles they produced (34 females produce ≤ 204 young per year, Godsell 1983); explaining high adult survival but low recruitment. There is no evidence to suggest eastern quolls reduce their fecundity with density-dependence (unlike reintroduced eastern bettongs, Manning et al. 2019). Rather, if favourable environmental conditions increased the carrying capacity of Mulligans Flat, territory could become limiting by lowering the fecundity of females that cannot secure natal territory (as observed in bobcats Lynx rufus, Knick 1990).
Estimated recruitment was low but consistent (females 7 ± 5%, males 7 ± 7%), suggesting that outcompeted individuals either died in Mulligans Flat or emigrated over the conservation fence into the surrounding landscape (note that we estimated apparent, rather than true, survival because we could not distinguish mortality from emigration, Williams et al. 2002). Such overdispersal is a problem in reintroductions, where individuals disperse away from the recipient site and do not contribute to population establishment (Richards and Short 2003). Serendipitously, some of these migrants have colonised the adjoining Goorooyarroo Nature Reserve which now also has an conservation fence (S. Stratford pers comms), thereby founding a new population (the Mulligans Flat fence only allows one-way passage to Goorooyarroo). Additionally, this spill-over or halo effect (Tanentzap and Lloyd 2017) could be used to colonise the landscape ‘beyond-the-fence’ if introduced predators can be maintained below the tolerance levels of eastern quolls (sensu Evans et al. 2021).
It has been suggested that a population must contain at least 1000 individuals to maintain “adequate adaptive potential … in the face of environmental change” (Willi et al. 2006; Weeks et al. 2011). Due to its limited size this target is not feasible for Mulligans Flat, but reinforcement translocations using the one-migrant-per-generation method (Mills and Allendorf 1996) could negate the effects of small population size (i.e., inbreeding depression, Weeks et al. 2011). It is encouraging that 100% probability of persistence was achieved by supplementing the population with one new mother annually, highlighting the need to increase gene flow between isolated populations. As such, we recommend that Mulligans Flat and other reintroduction locations be treated as a metapopulation; translocating individuals between them to promote in situ genetic diversity (Weeks et al. 2011; Frankham 2015).
Viability
Despite reaching the regulation phase, the Mulligans Flat eastern quoll population is inherently small and vulnerable to demographic stochasticity (Caughley 1994), and self-sustainability does not necessarily translate to long-term persistence (Seddon 1999). There is a 50.5% likelihood of persistence over the next 50 years (Fig. 3), emphasising the importance of ongoing management interventions. Annual supplementation of one mother could stabilise the population over 50 years, though we note two assumptions: ecological conditions will remain similar to the most recent monitoring sessions (autumn 2021 and 2022), and the population will maintain similar vital rates. Though we assumed high juvenile mortality (females 64.17% ± 19.92, males 64.93% ± 19.87, based on Godsell 1983), similar studies have demonstrated how increases in juvenile mortality can trigger comprehensive mortality and recruitment failure (e.g., in northern quolls Dasyurus hallucatus, Cremona et al. 2017, and African lions Panthera leo, Barthold et al. 2016). For example, after persisting for two decades, reintroduced Arabian oryx (Oryx leucoryx) suffered a poaching epidemic which rendered the population non-viable (Stanley Price 1989). While we present more than the minimum 5 years of vital rates required to identify temporal parameters driving variation (Gelman and Hill 2006; Parlato et al. 2021), each additional year of monitoring will improve our inferences; highlighting the value of long-term datasets that inform long-term goals.
Recovery
We explored how our programme could contribute measurably to eastern quoll species recovery. For the species to be delisted, we need to produce ≥ 4,600 eastern quolls to raise the total number from 9,600 (after 52% decline from 20,000 estimate in Tasmania, Fancourt et al. 2013) to the 14,200 (≤ 71% of 20,000 estimate) required to avoid meeting the IUCN Red List criteria for the ‘Vulnerable’ category (i.e., ≥ 30% decline). In addition, the eastern quoll must occur in > 10 locations (“geographically or ecologically distinct areas in which a single threatening event can rapidly affect all individuals of the taxon present”, IUCN 2001). While there are currently two recognised locations (Tasmania and Bruny Island, Burbidge and Woinarski 2016), based on the definition for ‘location’ we suggest that Mulligans Flat and Mt Rothwell should also be recognised. Further, we posit that Tasmania (2,320 km2 occupancy area) may represent more than one location, since significant genetic structure with consistent regional differentiation related to geographic distance has been found between populations (Cardoso et al. 2014). This geographical and/or behavioural separation suggests a single threatening event is unlikely to endanger all eastern quolls in Tasmania.
To achieve a stretch goal of species recovery within 10 years, a 41 km2 harvest area with conditions comparable to Mulligans Flat (i.e., without introduced predators) will need to be harvested of 460 individuals annually, and a 437 km2 occupancy area would be required to sustain these individuals beyond 10 years (i.e., 90 sanctuaries similar to Mulligans Flat, not accounting for edge effects). Due to the inherent difficulty in securing large areas for species recovery, we see these ambitious targets as a call to create a coordinated and collaborative sanctuary network where the eastern quoll, and other species, can be managed as a metapopulation across multiple sites (e.g., South Eastern Australia Sanctuary Operations Network or ‘SEASON’, Sharp 2021). In such a network, decisions regarding management interventions should be based on robust monitoring of the populations’ demographics and genetic composition. This network would buffer against the demographic and genetic perils facing isolated populations, and limit edge effects associated with small occupancy areas (McGregor et al. 2020). To identify appropriate areas for future reintroduction sites, we recommend incorporating eastern quoll occurrence data from established mainland populations, such as Mulligans Flat, into broad-scale habitat modelling across the species’ former range (e.g., maximum entropy species distribution modelling).
While fenced sanctuaries have produced insurance populations of at least 38 species that are susceptible to introduced predators, they are limited in area and capacity to expand, and maintaining them comes at a cost (Ringma et al. 2017; Legge et al. 2018). Reintroducing species ‘beyond-the-fence’ where introduced predators are actively managed (and adaptively calibrated) to remain below species’ tolerance levels (sensu Evans et al. 2021) is the next frontier to establish viable, self-sustaining populations and return ecological functions to our increasingly defaunated landscape (James and Eldridge 2007). Maintaining such a ‘Goldilocks zone’ of tolerance (the ‘just right’ predation level needed to drive selection for predator-resistant traits, Evans et al. 2021) in the area surrounding a conservation fence could deliver a great return on investment by protecting migrants and aiding in their establishment ‘beyond-the-fence’ (i.e., spill-over or halo effect, Tanentzap and Lloyd 2017). Finally, to prevent the “locking-in” of the degraded shifting baseline (where native species vulnerable to introduced predators are accepted as permanently absent from the wild), we must explore innovative solutions to drive or enable adaptive evolution of threatened species and introduced predators alike (i.e., ‘coexistence conservation’, Evans et al. 2022).
Conclusion
Here we demonstrated how demographic parameters from a reintroduced population can inform management interventions and create targets for delisting an endangered species. We also highlighted the value of conducting ecological experiments within conservation-fenced sanctuaries. If treated as ‘outdoor laboratories’, these areas provide unique opportunities to measure vital rates in free-ranging, endangered species when it would otherwise be difficult or impossible (Hester et al. 2000; Manning et al. 2009). Ironically, these populations may be better understood than the extant populations (e.g., there are no equivalent robust eastern quoll population estimates for Tasmania, Burbidge and Woinarski 2016) for which knowledge of their population dynamics would greatly assist conservation efforts (Ashbrook et al. 2016). The current extinction crisis highlights the need for managers to use evidence and collaboration to orient decisions and contribute lasting progress towards species recovery.
Data availability
Data presented in this manuscript will be accessible at ANU Data Commons upon acceptance.
References
Abicair K, Manning AD, Ford F, Newport J, Banks SC (2020) Habitat selection and genetic diversity of a reintroduced ‘refugee species.’ Anim Conserv 23:330–341. https://doi.org/10.1111/acv.12550
Armstrong DP, Reynolds MH (2012) Modelling reintroduced populations: the state of the art and future directions. Reintroduction Biol 12:165
Armstrong DP, Seddon PJ (2008) Directions in reintroduction biology. Trends Ecol Evol 23:20–25. https://doi.org/10.1016/j.tree.2007.10.003
Ashbrook K, Taylor A, Jane L, Carter I, Székely T (2016) Impacts of survival and reproductive success on the long-term population viability of reintroduced great bustards Otis tarda in the UK. Oryx 50:583–592. https://doi.org/10.1017/S0030605315000368
Barthold JA, Loveridge AJ, Macdonald DW, Packer C, Colchero F (2016) Bayesian estimates of male and female African lion mortality for future use in population management. J Appl Ecol 53:295–304. https://doi.org/10.1111/1365-2664.12594
Bartoń, K (2016) R package ‘MuMIn’: multi-model inference (version 1.46). Vienna, Austria. https://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Batson WG, Gordon IJ, Fletcher DB, Manning AD (2015) Translocation tactics: a framework to support the IUCN guidelines for wildlife translocations and improve the quality of applied methods. J Appl Ecol 52:1598–1607. https://doi.org/10.1111/1365-2664.12498
Betts J, Young RP, Hilton-Taylor C, Hoffmann M, Rodríguez JP, Stuart SN, Milner-Gulland EJ (2020) A framework for evaluating the impact of the IUCN Red List of threatened species. Conserv Biol 34:632–643. https://doi.org/10.1111/cobi.13454
Biggins DE, Godbey JL, Horton BM, Livieri TM (2011) Movements and survival of black-footed ferrets associated with an experimental translocation in South Dakota. J Mammal 92:742–750. https://doi.org/10.1644/10-MAMM-S-152.1
Biro PA (2013) Are most samples of animals systematically biased? Consistent individual trait differences bias samples despite random sampling. Oecologia 171:339–345. https://doi.org/10.1007/s00442-012-2426-5
Blackhall S (1980) Diet of the eastern native-cat, Dasyurus viverrinus (Shaw), in Southern Tasmania. Aust Wildl Res 7:191–197. https://doi.org/10.1071/WR9800191
Burbidge AA, Woinarski J. 2016. Dasyurus viverrinus. The IUCN Red List of Threatened Species 2016: e.T6296A21947190DOI: https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T6296A21947190.en. https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T6296A21947190.en.
Bureau of Meteorology (2021) Annual climate summary for Canberra
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Bustamante J (1996) Population viability analysis of captive and released bearded vulture populations. Conserv Biol 10:822–831. https://doi.org/10.1046/j.1523-1739.1996.10030822.x
Cardoso MJ, Mooney N, Eldridge MDB, Firestone KB, Sherwin WB (2014) Genetic monitoring reveals significant population structure in eastern quolls: implications for the conservation of a threatened carnivorous marsupial. Aust Mammal 36:169–177
Caughley G (1994) Directions in conservation biology. J Anim Ecol 63:215–244. https://doi.org/10.2307/5542
Cremona T, Crowther MS, Webb JK (2017) High mortality and small population size prevent population recovery of a reintroduced mesopredator. Anim Conserv 20:555–563. https://doi.org/10.1111/acv.12358
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B (2014) Defaunation in the Anthropocene. Science 345:401–406. https://doi.org/10.1126/science.1251817
Errington PL (1945) Some contributions of a fifteen-year local study of the northern bobwhite to a knowledge of population phenomena. Ecol Monogr 15:2–34. https://doi.org/10.2307/1943293
Evans MJ et al (2021) The “Goldilocks Zone” of predation: the level of fox control needed to select predator resistance in a reintroduced mammal in Australia. Biodivers Conserv. https://doi.org/10.1007/s10531-021-02166-y
Evans MJ et al (2022) Coexistence conservation: Reconciling threatened species and invasive predators through adaptive ecological and evolutionary approaches. Conserv Sci Pract 4:e12742. https://doi.org/10.1111/CSP2.12742
Fancourt BA, Hawkins CE, Nicol SC (2013) Evidence of rapid population decline of the eastern quoll (Dasyurus viverrinus) in Tasmania. Aust Mammal 35:195–205
Fancourt BA, Hawkins CE, Nicol SC (2018) Mechanisms of climate-change-induced species decline: spatial, temporal and long-term variation in the diet of an endangered marsupial carnivore, the eastern quoll. Wildl Res 45:737–750
Fletcher TP (1985) Aspects of reproduction in the male eastern quoll, Dasyurus viverrinus (Shaw) (Marsupialia: Dasyuridae), with notes on polyoestry. Aust J Zool 33:101–110
Frankham R (2015) Genetic rescue of small inbred populations: meta-analysis reveals large and consistent benefits of gene flow. Mol Ecol 24:2610–2618. https://doi.org/10.1111/mec.13139
Gelman A, Hill J (2006) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, Cambridge
Godsell J (1983) Ecology of the eastern quoll Dasyurus viverrinus (Dasyuridae: Marsupialia). The Australian National University, Canberra
Hayward MW, Kerley GIH (2009) Fencing for conservation: Restriction of evolutionary potential or a riposte to threatening processes? Biol Conserv 142:1–13. https://doi.org/10.1016/j.biocon.2008.09.022
Hester AJ, Edenius L, Buttenschøn RM, Kuiters AT (2000) Interactions between forests and herbivores: the role of controlled grazing experiments. Forestry 73:381–391. https://doi.org/10.1093/forestry/73.4.381
International Union for Conservation of Nature and Natural Resources (2001) IUCN Red List categories and criteria version 3.1. IUCN Species Survival Commission, Gland, Switzerland and Cambridge, UK.
International Union for Conservation of Nature and Natural Resources (2013) Guidelines for reintroductions and other conservation translocations. IUCN Species Survival Commission, Glandd. https://doi.org/10.1016/j.biocon.2015.07.030
International Union for Conservation of Nature and Natural Resources (2021) The IUCN Red List of Threatened Species. https://www.iucnredlist.org.
James AI, Eldridge DJ (2007) Reintroduction of fossorial native mammals and potential impacts on ecosystem processes in an Australian desert landscape. Biol Conserv 138:351–359. https://doi.org/10.1016/j.biocon.2007.04.029
Jones ME, Rose RK, Shaw D (2001) Dasyurus Viverrinus. Mammalian Species 1410:1–9. https://doi.org/10.1644/1545-1410(2001)677%3c0001:DV%3e2.0.CO;2
Kendall WL, Nichols JD (2002) Estimating state-transition probabilities for unobservable states using capture-recapture/resighting data. Ecology 83:3276–3284. https://doi.org/10.1890/0012-9658(2002)083[3276:ESTPFU]2.0.CO;2
Kendall WL, Nichols JD, Hines JE (1997) Estimating temporary emigration using capture-recapture data with Pollock’s robust design. Ecology 78:563–578. https://doi.org/10.1890/0012-9658(1997)078[0563:ETEUCR]2.0.CO;2
Kendall WL, Pollock KH, Brownie C (1995) A likelihood-based approach to capture-recapture estimation of demographic parameters under the robust design. Biometrics 51:293–308. https://doi.org/10.2307/2533335
Kinnear JE, Sumner NR, Onus ML (2002) The red fox in Australia-an exotic predator turned biocontrol agent. Biol Conserv 108:335–359. https://doi.org/10.1016/S0006-3207(02)00116-7
Knick ST (1990) Ecology of bobcats relative to exploitation and a prey decline in southeastern Idaho. Wildl Monogr 108:3–42
Laake JL (2013) RMark: an R interface for analysis of capture-recapture data with MARK. AFSC processed report 2013-01 Alaska Fisheries Science Center, National Marine Fisheries Service, NOAA, Seattle, Washington.
Lacy RC (2000) Considering threats to the viability of small populations using individual-based models. Ecol Bull 48:39–51
Lacy RC, Pollak JP (2021) Vortex: a stochastic simulation of the extinction process. Chicago Zoological Society, Brookfield
Leech TJ, Craig E, Beaven B, Mitchell DK, Seddon PJ (2007) Reintroduction of rifleman Acanthisitta chloris to Ulva Island, New Zealand: evaluation of techniques and population persistence. Oryx 41:369–375. https://doi.org/10.1017/S0030605307000517
Legge S et al (2018) Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators. Wildl Res 45:627–644
Lenth R V. 2016. Least-Squares Means: The R Package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01.
Lindenmayer DB, Clark TW, Lacy RC, Thomas VC (1993) Population viability analysis as a tool in wildlife conservation policy: With reference to Australia. Environ Manag 17:745–758. https://doi.org/10.1007/BF02393895
Manning AD et al (2019) Transition to density dependence in a reintroduced ecosystem engineer. Biodivers Conserv 28:3803–3830. https://doi.org/10.1007/s10531-019-01852-2
Manning AD, Gordon IJ, Ripple WJ (2009) Restoring landscapes of fear with wolves in the Scottish Highlands. Biol Conserv 142:2314–2321. https://doi.org/10.1016/j.biocon.2009.05.007
Manning AD, Lindenmayer DB, Fischer J (2006) Stretch goals and backcasting: approaches for overcoming barriers to large-scale ecological restoration. Restor Ecol 14:487–492. https://doi.org/10.1111/j.1526-100X.2006.00159.x
Manning AD, Wood JT, Cunningham RB, McIntyre S, Shorthouse DJ, Gordon IJ, Lindenmayer DB (2011) Integrating research and restoration: the establishment of a long-term woodland experiment in south-eastern Australia. Aust Zool 35:633–648. https://doi.org/10.7882/AZ.2011.016
Mazerolle MJ (2017) Package ‘AICcmodavg.’ CRAN
McCarthy MA, Armstrong DP, Runge MC (2012) Adaptive management of reintroduction. Reintroduction Biol 12:256
McGregor H, Read J, Johnson CN, Legge S, Hill B, Moseby K (2020) Edge effects created by fenced conservation reserves benefit an invasive mesopredator. Wildl Res 47:677–685
McIntyre S, Stol J, Harvey J, Nicholls AO, Campbell M, Reid A, Manning AD, Lindenmayer D (2010) Biomass and floristic patterns in the ground layer vegetation of box-gum grassy eucalypt woodland in Goorooyarroo and Mulligans Flat Nature Reserves, Australian Capital Territory. Cunninghamia 11:319–357
Miller JR (2005) Biodiversity conservation and the extinction of experience. Trends Ecol Evol 20:430–434. https://doi.org/10.1016/j.tree.2005.05.013
Mills LS, Allendorf FW (1996) The one-migrant-per-generation rule in conservation and management. Conserv Biol 10:1509–1518. https://doi.org/10.1046/j.1523-1739.1996.10061509.x
Moseby KE, Read JL, Paton DC, Copley P, Hill BM, Crisp HA (2011) Predation determines the outcome of 10 reintroduction attempts in arid South Australia. Biol Conserv 144:2863–2872. https://doi.org/10.1016/j.biocon.2011.08.003
Nichols JD, Armstrong DP (2012) Monitoring for reintroductions. Reintroduction biology: integrating science and management. Wiley-Blackwell, Oxford
Nolet BA, Baveco JM (1996) Development and viability of a translocated beaver Castor fiber population in the Netherlands. Biol Conserv 75:125–137. https://doi.org/10.1016/0006-3207(95)00063-1
Pacioni C, Mayer F (2017) vortexR: an R package for post Vortex simulation analysis. Wiley, New York. https://doi.org/10.1111/2041-210X.12786
Parlato EH, Ewen JG, McCready M, Gordon F, Parker KA, Armstrong DP (2021) Incorporating data-based estimates of temporal variation into projections for newly monitored populations. Anim Conserv. https://doi.org/10.1111/acv.12702
Pauly D (1995) Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol 10:430
Peacock D, Abbott I (2014) When the “native cat” would “plague”: historical hyperabundance in the quoll (Marsupialia: Dasyuridae) and an assessment of the role of disease, cats and foxes in its curtailment. Aust J Zool 62:294–344. https://doi.org/10.1071/ZO14029
Pollock KH (1982) A capture recapture design robust to unequal probability of capture. J Wildl Manag 46:752–757. https://doi.org/10.2307/3808568
Portas TJ et al (2020) Baseline health and disease assessment of founder eastern quolls (Dasyurus viverrinus) during a conservation translocation to mainland Australia. J Wildl Dis 56:1–14. https://doi.org/10.7589/2019-05-120
Pradel R (1996) Utilization of capture-mark-recapture for the study of recruitment and population growth rate. Biometrics 52:703–709. https://doi.org/10.2307/2532908
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://doi.org/10.1007/978-3-540-74686-7.
Radford JQ et al (2018) Degrees of population-level susceptibility of Australian terrestrial non-volant mammal species to predation by the introduced red fox (Vulpes vulpes) and feral cat (Felis catus). Wildl Res 45:645–657
Rapley S (2020) Spatial ecology informs reintroduction tactics for warabin (Burhinus grallarius; bush stone-curlew). The Australian National University, Canberra
Richards JD, Short J (2003) Reintroduction and establishment of the western barred bandicoot Perameles bougainville (Marsupialia: Peramelidae) at Shark Bay, Western Australia. Biol Conserv 109:181–195. https://doi.org/10.1016/S0006-3207(02)00140-4
Ringma JL, Wintle B, Fuller RA, Fisher D, Bode M (2017) Minimizing species extinctions through strategic planning for conservation fencing. Conserv Biol 31:1029–1038. https://doi.org/10.1111/cobi.12922
Rodrigues ASL, Pilgrim JD, Lamoreux JF, Hoffmann M, Brooks TM (2006) The value of the IUCN Red List for conservation. Trends Ecol Evol 21:71–76. https://doi.org/10.1016/j.tree.2005.10.010
Sarrazin F (2007) Introductory remarks: a demographic frame for reintroductions. Ecoscience 17:iii–v
Seddon PJ (1999) Persistence without intervention: assessing success in wildlife reintroductions. Trends Ecol Evol 14:503. https://doi.org/10.1016/S0169-5347(99)01720-6
Sharp N (2021) The business of biodiversity: the role of Odonata. Proc R Soc Victoria 133:32–35
Shorthouse DJ, Iglesias D, Jeffress S, Lane S, Mills P, Woodbridge G, Mcintyre S, Manning AD (2012) The “making of” the Mulligans Flat—Goorooyarroo experimental restoration project. Ecol Manag Restor 13:112–125. https://doi.org/10.1111/j.1442-8903.2012.00654.x
Sibly RM, Hone J, Clutton-Brock TH, Sibly RM, Hone J (2002) Population growth rate and its determinants: an overview. Philos Trans R Soc Lond Ser b 357:1153–1170
Smith KJ, Evans MJ, Gordon IJ, Pierson JC, Stratford S, Manning AD (2022) Mini Safe Havens for population recovery and reintroductions ‘beyond-the-fence’. Biodiver Conserv 12:1–23. https://doi.org/10.1007/s10531-022-02495-6
Stanley Price MR (1989) Animal reintroductions: the Arabian oryx in Oman. Cambridge University Press, Cambridge
Stannard HJ, Old JM (2013) Digestibility of two diet items by captive eastern quolls (Dasyurus viverrinus). Zool Biol 32:417–422. https://doi.org/10.1002/zoo.21073
Tanentzap AJ, Lloyd KM (2017) Fencing in nature? Predator exclusion restores habitat for native fauna and leads biodiversity to spill over into the wider landscape. Biol Conserv 214:119–126. https://doi.org/10.1016/j.biocon.2017.08.001
Threatened Species Scientific Committee (2015) Dasyurus viverrinus (eastern quoll) conservation advice. Department of the Environment, Canberra, pp 1–14
Weeks AR et al (2011) Assessing the benefits and risks of translocations in changing environments: a genetic perspective. Evol Appl 4:709–725. https://doi.org/10.1111/j.1752-4571.2011.00192.x
White G (2016) Program MARK. Department of Fish, Wildlife, and Conservation Biology, Colorado State University, Fort Collins
Wickham H (2011) Package “ggplot2.” https://doi.org/10.1002/wics.147.
Willi Y, Van Buskirk J, Hoffmann AA (2006) Limits to the adaptive potential of small populations. Annu Rev Ecol Evol Syst 37:433–458. https://doi.org/10.1146/annurev.ecolsys.37.091305.110145
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations. Academic Press, San Diego
Williams CK, Guthery FS, Applegate RD, Peterson MJ (2004) The northern bobwhite decline: scaling our management for the twenty-first century. Wildl Soc Bull 1973–2006(32):861–869
Wilson BA et al (2020) Adapting reintroduction tactics in successive trials increases the likelihood of establishment for an endangered carnivore in a fenced sanctuary. PLoS ONE 15:1–17. https://doi.org/10.1371/journal.pone.0234455
Wilson BA et al (2021) Reintroduction of the eastern quoll to Mulligans Flat Woodland Sanctuary, Australia using trials, tactics and adaptive management. In: Soorae PS (ed) Global conservation translocation perspectives: 2021. Case studies from around the globe, 7th edn, pp 194–199. Gland, Switzerland: IUCN SSC Conservation Translocation Specialist Group, Environment Agency - Abu Dhabi and Calgary Zoo, Canada.
Woinarski JCZ, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proc Natl Acad Sci USA 112:4531–4540. https://doi.org/10.1073/pnas.1417301112
Acknowledgements
We acknowledge and respect the Ngunnawal and Ngambri (ANU, Mulligans Flat), Wathaurong (Mt Rothwell), and Palawa (Tasmania) peoples, the Traditional Custodians of the lands on which this work was undertaken. This study was conducted as part of the Mulligans Flat-Goorooyarroo Woodland Experiment (https://www.mfgowoodlandexperiment.org.au/) and is part of the Australian Research Council-funded ‘Bringing Back Biodiversity’ project (LP140100209). We thank our collaborative partners, the ACT Government (Parks and Conservation Service and Conservation Research Unit), Tasmanian Department of Primary Industries, Parks, Water and Environment (DPIPWE), Mt Rothwell, Odonata, and the Woodlands and Wetlands Trust for their support. We thank Andrew Crane, Annika Everaardt, Claire Hawkins, and Robbie Gaffney of DPIPWE for their assistance, Emily Belton, Dean Heinze, Mark Holdsworth, and Nick Mooney for their support in sourcing Tasmanian eastern quolls, and Annette Rypalski and Nigel Sharp and the Mt Rothwell and Odonata teams for their advice and supply of captive-bred eastern quolls. We also thank Catherine Ross, Christine Mauger, Daniel Iglesias, Dave Whitfield, Greg Hocking, Helen Crisp, Jason Cummings, Joel Patterson, John Lawler, Kate Grarock, Katherine Jenkins, Katherine Moseby, Kristi Lee, Lyall Marshall, Margaret Kitchin, Mark Smith, Mark Sweeney, Melissa Snape, Michelle White, Millie Sutherland-Saines, Sam Banks, Sam Reid, Shoshana Rapley, Simon Stratford, Suzie Fowler, Tim Andrewartha, Tim Portas, and many more for their assistance during this project. We thank Jason Cummings and Simon Stratford for their comments on an earlier draft. Finally, we thank the anonymous reviewers for their valuable feedback.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Conceptualisation: BAW, MJE, IJG, WGB, ADM; methodology: BAW, MJE, IJG, JCP, WGB, ADM; software: BAW, MJE, JCP; validation: BAW, MJE, JCP; formal analysis: BAW, MJE; investigation: BAW, JN; resources: CW, AR, JN, ADM; data curation: BAW, MJE; writing: BAW; review and editing: BAW, MJE, IJG, JCP, BMB, CW, AR, JN, ADM; visualisation: BAW, MJE; supervision: MJE, IJG, WGB, ADM; project administration: CW, AR, JN, ADM; and funding acquisition: ADM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: Adeline Loyau.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
About this article
Cite this article
Wilson, B.A., Evans, M.J., Gordon, I.J. et al. Roadmap to recovery revealed through the reintroduction of an IUCN Red List species. Biodivers Conserv 32, 227–248 (2023). https://doi.org/10.1007/s10531-022-02496-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02496-5