Abstract
Flower plantings can increase the abundance of bees and improve pollination services in the surrounding landscape. However, uncertainty remains as to whether flower plantings play a role in wild bee conservation. The aim of this study has been to examine the contribution of the composition and management of flower plantings to the attraction of bees, particularly of endangered species. In a large-scale monitoring project, wild bee data were collected on 60 flower plantings and 120 semi-natural reference plots in 20 study sites over 2 years. In total, we recorded 60,335 bees belonging to 351 species. In flower plantings, bee species richness and abundance were intricately linked to high plant richness and constant blooming throughout the season. In the first year of this study, a complimentary blooming phenology of annual and perennial plants resulted in a more constant bloom on flower plantings. In the second year, partial mowing of flower plantings mid-season enhanced floral resources during the late season. As a result, bee richness and abundance in flower plantings increased from the first to the second year. Nevertheless, the compositional heterogeneity of bees over all 20 sites in Germany did not increase from the first to the second year. We conclude that diverse and constant blooming throughout the season is the most important factor for promoting bees in flower plantings. To ensure sufficient beta diversity over a large spatial scale, we recommend the adjustment of seed mixtures according to the geographical region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild bees provide key pollination services within natural ecosystems and croplands (Kearns et al. 1998; Bommarco et al. 2012; Holzschuh et al. 2012). Therefore, they play a major role for global food security and in the maintenance of ecosystem stability and biodiversity (Klatt et al. 2013; Potts et al. 2016). Evidence is however growing for a worldwide decline of wild bees (Brittain et al. 2010; Bartomeus et al. 2013a; Powney et al. 2019; Zattara and Aizen 2021). This has prompted concerns about not only the safety of the pollination service provided by wild bees, but also the intrinsic value of wild bee biodiversity (Kleijn et al. 2015). As a result, the aim of several ongoing projects is to safeguard and promote wild bees in agricultural landscapes (Knop et al. 2006; Melathopoulos et al. 2015; Jönsson et al. 2015; Chopra et al. 2015; Ouvrard et al. 2018; Neumüller et al. 2020).
Semi-natural habitats such as extensively managed grassland or ruderal areas offer a diverse set of feeding and nesting sources for wild bees (Steffan-Dewenter et al. 2002; Kennedy et al. 2013; Krimmer et al. 2019; Neumüller et al. 2020). Driven by the agricultural intensification in Europe, many of these valuable habitats have been lost during the last few decades (Stoate et al. 2001) and many flowering plants are in decline (Eichenberg et al. 2021). These observations suggest that the shortage of available floral resources is one of the main drivers of the current wild bee decline (Tilman et al. 2001; Biesmeijer et al. 2006; Scheper et al. 2014). Accordingly, the provision of adequate floral resources is considered to be essential for sustainable habitat management targeting wild bees (Scheper et al. 2014; Sutter et al. 2017). Nevertheless, the restoration or establishment of new semi-natural habitats is often expensive or impossible (von Haaren and Reich 2006). Alternatively, the establishment of flower plantings on tilled arable land has become an important approach for providing additional floral resources for bees (Haaland et al. 2011). Flower plantings can efficiently increase wild bee abundance and related pollination services in the surrounding landscape (Carvalheiro et al. 2012; Blaauw and Isaacs 2014), although only a few plantings have been adapted to the specific needs of the local bee community and have been shown to enhance wild bee richness (Scheper et al. 2015).
Studies investigating the value of flower plantings have mostly focused on their beneficial effects for a few common generalist bee species (Sutter et al. 2017; Grab et al. 2019; Ahrenfeldt et al. 2019; Albrecht et al. 2020) that are abundant in agroecosystems (Kleijn et al. 2015; Sutter et al. 2017). Among these species are honeybees or common bumblebee species that choose host plants in respect of quantity or nutritional requirements (Leonhardt and Blüthgen 2012). The abundance of bees is strongly driven by the availability of feeding sources in the same location (Holzschuh et al. 2013). In contrast, bee species richness is predominately moderated by the composition and richness of available plant species (Potts et al. 2003; Scheper et al. 2015; Neumüller et al. 2020). In particular, rare bee species mostly rely on key plant species different from those used by most of the important crop pollinators (Sutter et al. 2017; Westrich 2018; Danforth et al. 2019). In this context, many bee species have been shown to prefer specific taxonomic groups of plants and are only active for a limited time period of the year (Westrich 2018; Danforth et al. 2019).
In central Europe, government-funded seed mixtures that fulfil the criteria of agri-environmental schemes are often limited in the number of plant species that they contain and are designed to implement flower plantings that are re-established every year (Carvell et al. 2007; Batáry et al. 2015; Agrarmarkt Austria 2021; Landwirtschaftskammer Nordrhein-Westfalen 2021; Mastel et al. 2021). Although annual flower plantings have been shown to promote common crop pollinators and the associated pollination service in a landscape (Jönsson et al. 2015; Campbell et al. 2017; Rundlöf et al. 2018; Klatt et al. 2020), evidence has been presented that species-rich and adequately managed flower plantings support a greater spectrum of bee species (Carvell et al. 2007; Pfiffner et al. 2019). On this basis, the mixing of annual and perennial plants and mowing during the season in flower plantings might therefore promote more constant blooming throughout the season and might attract complementary groups of wild bee species (Williams et al. 2015; Kirmer et al. 2018; Martins et al. 2018). The temporal continuity of floral resources is important for high bee species richness as it strongly affects the stability of a bee community (Blüthgen and Klein 2011; Scheper et al. 2015; Williams et al. 2015; Crowther and Gilbert 2020). If the goals of the establishment of a flower planting is not only to promote pollination service, but also to conserve wild bee diversity, then we need to provide flower plantings that serve both target groups: common crop pollinators and rare species (Sutter et al. 2017). Enhancement of wild bee diversity has been shown to increase pollination success in crop cultures (Klein et al. 2003; Hoehn et al. 2008) by stabilizing pollination over space and time (Hector et al. 2010; Blüthgen and Klein 2011; Bartomeus et al. 2013b). Hence, multiple goals can be achieved by promoting general bee diversity.
Bee communities greatly differ between regions because of the high grade of habitat specialization of wild bees. They require a range of floral and nesting resources and are adapted to specific climatic conditions (Westrich 2018). As a result, the composition of wild bee communities shows a high geographical turnover, resulting in a high compositional heterogeneity of bees on a large spatial scale (Rubene et al. 2015; Winfree et al. 2018; Orr et al. 2021). A previous study involving a comparison of bee communities in semi-natural habitats and locally adapted flower plantings has revealed that the grade of compositional heterogeneity (compositional turnover between sample plots) of bees is reduced on flower plantings in the first year after establishment, when compared with surrounding semi-natural habitats (Neumüller et al. 2020). However, uncertainty remains as to whether flower plantings with a longer succession can attain a similar compositional heterogeneity to that of semi-natural habitats.
In this large-scale study over 20 study sites in Germany, we investigated wild bee communities in flower plantings (flower strips) containing annual and perennial plant species over 2 years. We explored changes in plant and bee communities in the flower plantings from the first year of establishment to the second year (Fig. 1). To control for yearly changes in bee occurrence in the flower plantings, we also monitored, at each site, the bee communities on two different semi-natural habitats. Bee communities in flower plantings were explored while paying special attention to bee species classified as threatened or vulnerable (Red list of Germany, Westrich et al. 2011).
Flower planting for wild bee conservation established at the 20 study sites [photos H. R. Schwenninger (A) and H. Burger (B–D)]. The images show various developmental stages of the same flower planting (A) in the first year (dominating flowers: Anthemis tinctoria, followed by Centaurea cyanus), B in the second year after establishment (flowers such as Echium vulgare, Reseda luteola and Carduus nutans) and C late flowers after partial mowing (left: unmown, right: mown part with dominating Daucus carota flowers, followed by Centaurea jacea). D Examples of wild bees visiting flowers of the plantings (from top left to bottom right): Andrena flavipes on Tripleurospermum perforatum, Bombus sylvarum on Trifolium pratense, Bombus pascuorum on Carduus nutans, Halictus tumulorum on Anthemis tinctoria)
As wild bee communities strongly depend on floral resources, we expected that, in flower plantings, the abundance and richness of bees would be determined by the local availability and diversity of the flowering plants. In this context, we furthermore hypothesized that, in the second year of the study, perennial plants would produce a more diverse and continuous flowering aspect throughout the year. Rare bee species are predicted to depend on specific plant species and are therefore hypothesized to benefit particularly from enhanced flowering in the second year. Previous observations on field margins suggest that mowing during the season can promote constant blooming throughout the season (Kirmer et al. 2018). Hence, we hypothesized that, in the second year, partial mowing of the flower plantings mid-season would enhance flower cover during the late summer. Finally, by providing enhanced floral resources in the second year of this study, flower plantings might attract a broader spectrum of bees from the local community. Hence, we hypothesized that compositional heterogeneity between flower plantings over whole Germany would increase from the first to the second year.
Methods
This study is part of the BienABest project that aims to safeguard the ecosystem service of pollination and to enhance wild bee diversity in agricultural landscapes (www.bienabest.de). The project incorporates extensive wild bee monitoring, which has provided wild bee data for this study. The methodology of the data acquisition in this study is congruent with that of Neumüller et al. (2020).
Study sites
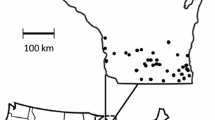
The study was conducted in 2018 and 2019 in 180 sampling plots in 20 different study sites distributed over seven federal states of Germany (Neumüller et al. 2020). The total study area extended over 708 km longitudinally and 507 km latitudinally, with an altitude between 20 and 580 m above sea level (Fig. 2). The mean distance between all study sites was 323.35 kms with a minimum distance of 6.5 kms and a maximum distance of 779.79 kms (also see Fig. 2). The selection of the sites was based mainly on the presence of semi-natural grassland embedded in an agricultural landscape matrix. To evaluate whether the established flower plantings were successful in promoting the local bee communities, we used two semi-natural habitats for comparison. In each study site, the sampling plots were located within a radius of 500 m around a centroid that was, in turn, calculated from the centroids of the nine sampling plots, except for 23 plots that lay close (less than 350 m) to the outer boundary of the 500 m radius.
Map of Germany showing the 20 study sites in which the study was performed. Each dot represents a site. Green areas indicate a low topographical elevation; red areas indicate a high topographical elevation (BKG 2013)
Flower plantings
Within each of the study sites, we established three 0.3-ha flower plantings (flower strips) on tilled arable land, either during the autumn or the spring prior to the first field surveys in 2018. The shape of the flower plantings was quadratic to rectangular. Each flower planting acted as a 0.3-ha sampling plot for variable transect walks (Westphal et al. 2008), resulting in a total of 60 sampling plots of wildflower planting. The planting areas were sown with seed mixtures consisting of between 41 and 53 species encompassing regionally native, naturalized and cultured (non-invasive) plant species with a complementary flower phenology to provide floral resources throughout the season (see Supplementary Material Table S1 for detailed information about our seed mixtures). For the compilation of seed mixtures, previously existing bee-plant interaction data recorded in the ‘Wildbienen Kataster Baden-Württemberg’ (http://www.wildbienen-kataster.de) were evaluated and the most attractive plant species for bees in a respective region were included in the seed mixtures. Although the seed mixtures were similar for all sites, plant composition was adapted to match the distribution of each plant species in a geographic region. In addition to the plants included in the seed mixtures, the spontaneous growth of non-sown wild flowering plants was registered on all flower plantings. In 2019, half of the area of each sampling plot of the flower plantings was mown between the middle of June and the middle of July. Mowing was performed at a height of about 15 cm to encourage further blooming. In the horticultural literature, this practice is referred to as “deadheading” and is known to prolong the bloom period (DeGomez 2002; García-Sogo et al. 2010). In 2018, flower plantings were not mown until the last field survey was performed, because partial mowing was assumed to have no beneficial effect on the annual plant community present in the first year.
Semi-natural habitats
To evaluate the success of the established flower plantings, we used two semi-natural habitats as a reference: grassland and residual habitats. Grassland plots were characterized by extensive management and a high diversity of native flowering plants. The grasslands were managed either by mowing once or twice a year or by grazing by sheep, goats or donkeys. Residual habitats were sampled to monitor bee communities commonly present in agricultural areas. Hence, they were composed of rarely used field paths, field margins and small ruderal areas. Within each of the 20 study sites, we established three 0.3-ha sampling plots for each of the semi-natural habitat types, resulting in a total of 120 reference sampling plots. Additionally, nesting hills were established close to the study plots in all research areas in order to provide nesting resources for bees.
Wild bee surveys
Bee data were collected on the three described habitat types: flower plantings, grassland and residual habitat elements. Bee data from 2018 were taken from Neumüller et al. (2020). Following the methodology of Neumüller et al. (2020), the sampling of each plot in 2019 was performed during five sampling events. Individual events were evenly distributed between April to September, starting with the flowering of dandelion and continuing every 3 to 4 weeks depending on the weather conditions. Sampling was only conducted during sunny weather (cloud cover less than 30%), at temperatures higher than 10 °C and under low wind conditions.
While sampling, collectors could freely move within the plots, following the distribution of relevant nesting and feeding resources for the bees (Westphal et al. 2008; Schindler et al. 2013). To account for intra-day bee phenology, each plot was sampled during two subunits of 25 min, one before and one after 12:30, with a minimum of 120 min between the two sampling events. On structure-rich plots, which were covered by a range of different habitat components, small habitat patches (e.g. flower patches dominated by one plant species or nesting sites) were sampled for a maximum of 5 min. All encountered bee individuals were caught with an entomological net, except for those that could be determined at first sight and identified to species level on the spot. After sampling, all caught bee individuals were inspected with a field microscope or a magnifying glass and released once they had been clearly determined. Individuals that could still not be identified in the field were killed for further determination by wild bee taxonomists. To minimize collector bias and to guarantee reliable species determination, sampling was performed only by wild bee experts (see acknowledgements).
Flower resource availability
Before and during each bee survey, the vegetation of the visited area for the bee survey was visually inspected and the mean flower cover and all encountered flowering plant species that might act as feeding resources for bees were recorded. The flower cover was categorized in one of four categories: less than 1%, 1 to 10%, 11 to 40% and more than 40%. Voucher specimens of flowering plants that could not be identified to species level in the field were later determined in the laboratory.
Statistical analysis
Comparison of bee species richness and abundance between the 2 years and the three habitat types
Bee data before noon and after noon were pooled for each of the five sampling events within each year. Since all 180 sampling plots were sampled ten times in 2 years, aggregation of the data resulted in 1800 individual data points. In the next step, we separated bee data into two categories: vulnerable, extremely rare or threatened species (from here on called rare species), as classified by the Red List of Germany (Westrich et al. 2011), and non-Red List species (from here on called common species). Hence, we separately compared bee species richness and abundance for both datasets between the three habitat types and the 2 years. During the analysis of the data, we detected a strong non-linear temporal pattern in the dataset. By using the function gam of the R package mgcv (Wood 2019), we applied spatiotemporal generalized additive models (GAMs) with a negative binomial distribution following Wood (2017). This allowed us to model non-linear effects of time. The first two GAMs included either species richness or abundance as a dependent variable and an interaction between the three habitat categories (flower plantings, grassland and residual habitat elements) and year as an independent variable. To account for differing sampling dates, the day of the year (Julian date) for each sampling event was added as a thin plate spline to the model structure. To test for a spatial dependence in our data derived from the distribution of our sampling sites, we followed (Zuur et al. 2017) and fitted experimental variograms as implemented in the package geoR (Ribeiro et al. 2003). The variograms indicated spatial dependencies that were later confirmed when models with a spatial correlation structure explained a higher proportion of the total variation in our data than generalized additive mixed models with a random structure (plot nested in site). To account for this spatial dependence, we included a spatial correlation structure by adding longitudinal and latitudinal coordinates as a thin plate smooth interaction spline to the model. This allowed the model to account for spatial closeness of study sites (Wood 2017). After the running of the models, we used the package emmeans (Lenth et al. 2019) for post-hoc analyses to compare pairwise either bee species richness or abundance between the 2 years and the three habitat types. For the smooth variables, we let the package select the number of basis functions (k). Model diagnostics were performed using the function gam.check implemented in the package mgcv (Wood 2019) to ensure an adequate fit of all models. No adaptations to improve the model fit were necessary. During inspection of all GAM curves, non-overlapping confidence intervals of two curves were interpreted as significant difference (MacGregor-Fors and Payton 2013).
Correlation of species richness of bees and flowering plants
In the next step, we explored any correlations in changes in species richness in bees and flowering plants in the three habitat types. In this regard, we calculated a generalized additive mixed model with bee species richness as the dependent variable, setting flower richness in an interaction with habitat type as the independent variable. Additionally, we applied the same spatiotemporal correlation structure as described previously but added sampling year as a random factor and not as an independent variable. Subsequently, we performed post-hoc analyses for a pairwise slope comparison between habitat types by using the package emmeans (Lenth et al. 2019). As for the other models, we let the package select the number of basis functions for our smooth parameters and performed model evaluation.
Temporal patterns of bees and plants
To test for temporal patterns, data before noon and after noon were pooled for each of the five sampling events within each year. Subsequently, spatiotemporal generalized additive models were applied using the function gam (Wood 2019). In four models, flower richness, flower cover, bee richness or bee abundance were set as dependent variables. All models were implemented with a negative binomial distribution, except for the model containing cover as the depended variable, which was implemented with a beta distribution. The Julian date in combination with a factor-smooth interaction of a combination of year and habitat type was added as a thin plate spline to each model. Furthermore, the longitudinal and latitudinal centroid-coordinates of each pot were added as an interaction term to account for the spatial distribution of the data.
To explore whether annual and perennial plant species showed differences in flowering phenology, we calculated similar temporal models to those for plant richness and flower cover. However, this time, the data were divided between annual and perennial (including biennial) plants and the Julian date was set in a factor-smooth interaction with growth type (annual/perennial). As for the other models, we let the package select the number of basis functions for our smooth parameters and performed model evaluation.
Compositional heterogeneity of bees and plants
Compositional heterogeneity of bee communities in flower plantings was compared between the 2 years by using the vegan package (Oksanen et al. 2019). We used the vegdist function to calculate the Bray–Curtis dissimilarity between individual sampling plots and the betadisper function to calculate the dispersion of each group. Subsequently, the TukeyHSD function was used to compare the compositional heterogeneity between the first and the second years. The same analysis was performed to compare the compositional heterogeneity of plant communities between the three habitat types.
Results
During the 2 years of sampling, we recorded a total of 60,335 bee individuals belonging to 351 bee species, representing 59% of all known German bee species (Westrich 2018). In flower plantings, a total of 25,507 bee individuals were recorded from 279 species. Regarding rare species, we recorded a total of 11,471 individuals from 134 species (Table S2).
Comparisons between habitat types and years
In flower plantings, both the species richness and abundance of rare and common bees significantly increased from the first to the second year (Fig. 3a, b). In grasslands, only bee species richness of common bees increased from 2018 to 2019, whereas species richness of rare bees did not change between the 2 years. Over all three habitat types, the highest species richness of rare bees was found in grassland in the first year and in flower plantings in the second year (Table S4). In the first year, the bee abundance of common and rare species was similar in all habitat types but, in the following year (2019), the abundance in flower plantings exceeded those in grassland and residual habitats, making flower plantings generally the habitat type with the highest bee abundance.
Comparison of bee richness and abundance between the 2 years and three habitat types for common (a) and rare (b) species. Predicted values with 95% confidence intervals derived from the calculated GAM models. Bars with asterisks indicate significant differences: *p < 0.05, ***p < 0.001. Coefficients of the underlying GAMs with post-hoc comparisons, including comparisons between habitat types, are given in the Supplementary Material (Table S3, S4)
Temporal patterns of plants
From early to mid-season of the second year, the flower richness in flower plantings was similar to that of the first year but, after the partial mowing event, kept increasing until the end of the season (Fig. 4a). This resulted in a significantly higher plant species richness in the late season of the second year than in the first year.
Temporal patterns of flower richness (a), flower cover (b), bee richness (c) and bee abundance (d) in flower plantings for the first and the second years. Shaded areas represent 95% confidence intervals. The grey line indicates the partial mowing event in 2019. Complete estimate values of the underlying GAMs are given in the Supplementary Material (Table S5)
In both years, the flower cover in flower plantings reached similar maxima around the middle of June during a particularly abundant bloom of Anthemis tinctoria (Fig. 4b). Whereas maximum cover occurred as a short peak in the first year, a high cover of blooming plants was retained over a longer period after the partial mowing event in the second year. The temporal distribution of flower cover and flower richness in grassland and residual habitats showed no strong divergences between the 2 years (see Supplementary Material Fig. S1).
In flower plantings, perennial plants strongly increased total flower richness during the late season in both years (Fig. 5a, b). Remarkably, in the first year, the richness of perennial and annual plants showed a strong complimentary temporal pattern with more annual plants from the early to the mid-season and more perennial plants from the mid to the late season. This resulted in overall more stable floral resources throughout the season (compare to Fig. 4a, b). In the second year, annual plants appeared to decrease, whereas perennial plants increased in dominance and retained a high flower cover from the mid to the late season. The plants Medicago sativa, Trifolium pratense and Achillea millefolium showed particularly high flower cover in the late season (Supplementary Material Table S8 presents those plants producing the highest flower cover in each sampling event).
Comparison of temporal patterns of flower richness and flower cover in flower plantings for the years 2018 (a) and 2019 (b) for annual and perennial plants. Shaded areas around the lines represent 95% confidence intervals. The grey line indicates the partial mowing event in 2019. Complete estimate values of the underlying GAMs are given in the Supplementary Material (Table S6)
Temporal patterns of bees
Although a strong linear relationship was observed between plant richness and bee richness in all three habitat types, it was particularly pronounced in flower plantings (Fig. 6). In accordance with this interdependence, the temporal patterns in bee abundance and richness in flower plantings appeared to follow the availability of floral resources. During late summer, the species richness and abundance of bees strongly increased from the first to the second year (Fig. 4c, d). Conversely, bee data showed similar temporal patterns from early to mid-season when compared between the 2 years. Species richness and abundance in grassland and residual habitats followed similar temporal patterns in both years (Supplementary Material Fig. S1).
Heterogeneity of bee communities
Compositional heterogeneity of the bee community in flower plantings was almost identical in both years (Fig. 7). The compositional heterogeneity of plant communities in flower plantings was lower than those in the two semi-natural habitat categories (Supplementary Material Fig. S2). In a comparison of the composition of flowering plants between habitats, flower plantings differed substantially from the two semi-natural habitat categories, which were comparably similar in this regard (Supplementary Material Fig. S3).
Compositional heterogeneity of bee communities between flower plantings did not increase from the first (2018) to the second year (2019). Boxes represent the median and 25th/75th percentile of the distances to group centroid calculated with betadisper. Whiskers extend to 1.5 times the interquartile range. Coefficients of the underlying analysis are given in the Supplementary Material (Table S9)
Discussion
In our study, flower plantings attracted more bee species and individuals in the second year after establishment, when floral resources were enhanced compared with the first year. This positive trend was mainly observed in flower plantings and not in the two other habitat types and was particularly pronounced for rare species. As hypothesized, increases in bee species richness and abundance from the first to the second year mainly happened during late summer, when a more constant bloom attributable to multi annual flower plantings and partial mowing resulted in a higher plant species richness and flower cover. Despite the locally increased diversity of bee species and enhanced floral resources in the flower plantings of the second year, the compositional heterogeneity of bee communities over all 20 sites in Germany did not increase as compared with the first year.
Bee richness and abundance depend on floral resources
The species richness of wild bees in our study was intricately linked to the flower richness in our sampling sites. Hence, the increases in species richness and abundance of bees in the second year of this study must have been caused by the observed improvements in floral resources, which attracted additional bees from the surrounding landscape (Neumüller et al. 2020). Specialized bee species are easily limited by a lack of suitable host plants (Biesmeijer et al. 2006; Weiner et al. 2014; Sutter et al. 2017). This might explain the particular enhancement of the species richness and abundance of rare bee species in the second year, concomitant with the higher richness of plants. Therefore, our results clearly show that plant species composition in seed mixtures is a critical aspect when designing flower plantings (Carvell et al. 2007).
In our study, both plant and bee communities were enhanced in the second year of the study. This suggests that multi annual flower plantings are superior to annual flower plantings, particularly as the frequent tillage of flower plantings might hinder the emergence of ground-nesting bees that build their nests on flower plantings (Ullmann et al. 2016). Furthermore, it remains unclear how the discontinuance of flower plantings will affect wild bee communities that are adapted to abundant feeding resources.
Prolonged floral resource availability through partial mowing and a mixture of annual and perennial plants
Many bee species have only one generation per year and the adult females of such species often have short life spans of only a few weeks (Westrich 2018). As a result, seasonal species turnover often accounts for a large part of total diversity in wild bee communities (Rollin et al. 2015). By covering a wider spectrum of temporal niches in the second year, the flower plantings were able to attract and feed more bee species than in the first year. This was certainly a main reason for the increased bee abundance and richness in flower plantings of the second year. Bee species that occupy a narrow temporal niche are predicted to be more vulnerable to temporal declines of floral resources (Blüthgen and Klein 2011). Hence, the species richness of the rare bees in our flower plantings probably increased strongly not only because of a higher flower resource diversity, but potentially also because the flower plantings covered temporal niches that were critical for some rare species.
In the first year of the study, the flower cover of the plantings showed a pronounced peak in the middle of the season but was much lower before this time point and showed a severe drop after it. In order to prolong the blooming in our flower plantings, half of the area of each flower planting plot was mown in the mid-season of the second year. The partial mowing appeared to be successful; in the second year, flower cover was significantly higher towards the end of the season. During the mowing event and shortly afterwards, the unmown part of the plot was still in full bloom and thus compensated for the loss of floral resources on the other half of the plot. The cut part of the flower plantings bloomed again a few weeks after the mowing event, resulting in high flower cover and richness (Fig. 1C). A second bloom after cutting was predominantly observed in perennial plants and not in annual ones. In addition, we observed that in flower plantings an increase in richness occurred at the end of the season and that this increase was partly attributable to late-flowering plant species. Partial mowing in the second year of this study resulted in more constant floral resources being available towards the end of the season. This time of the year was previously described as a resource bottleneck for bees (Timberlake et al. 2021). Concordantly with floral resources, increases in bee species richness and abundance from the first to the second year were also noted after the partial mowing event. As mowing was only performed in the second year, the positive effects on flower plantings might also be explained by succession dynamics from the first to the second year. Nevertheless, the finding that increases in bees and plants almost exclusively occurred a few weeks after the partial mowing event, together with our personal observation (Fig. 1C), provides strong indications that mowing plays an important role.
In addition to the positive effects of mowing, perennial plants provided stable floral resources even in the first year of our study. Against our expectations, many perennial plants bloomed in the first year, probably because the seed mixtures were sown early (autumn or early spring). When the richness of annual plants declined during the mid-season of the first year, perennial plant richness persisted until the end of the season. Our results are in agreement with the recommendations by Williams et al. (2015) that perennial plants will enhance flower plantings that are designed to exist for 1 year only. Hence, the inclusion of a balanced mixture of annual and perennial plants is a good option for increasing the ecological value of seed mixtures used in agri-environmental schemes that are often dominated by annual plants.
Compositional heterogeneity in bees depends on variation in flowering plants
Even though plant species richness in flower plantings increased in the second year of our study, the mean compositional heterogeneity of the plant and bee communities over all study sites stayed almost the same, as compared with the first year (Neumüller et al. 2020). Hence, the previously observed reduced grade of heterogeneity on flower plantings (Neumüller et al. 2020) did not increase from the first to the second year. Although the composition of the seed mixtures was adapted for the various geographical sites, most annual and perennial plant species were the same for all sites. Nevertheless, the species composition of the flower plantings differed substantially from those in the two semi-natural habitats and, in both years, the compositional heterogeneity of plants was lower on flower plantings than on the two other habitat types. The strong habitat specialization of many wild bee species has been argued to drive the species turnover of wild bee communities (Rubene et al. 2015; Scheuchl and Willner 2016; Westrich 2018; Neumüller et al. 2020). Therefore, the reduced compositional heterogeneity of plants was probably the main cause for the reduced compositional heterogeneity of bees in the flower plantings.
Conclusion
Based on the results of our study, we conclude that the diversity of food plants in flower plantings is an important factor for promoting diverse bee communities. Selecting local plant species that are not common in an agricultural landscape can increase the potential for supporting rare and specialized bee species. By the constant offering of flowers throughout the season, flower plantings can compensate for shortages of feeding resources in a landscape and thus support bees with a differing phenology. In annual flower plantings, this can be achieved by mixing annual and perennial plant species. Partial mowing of areas of flower plantings during mid-season is a method for extending the blooming period in flower plantings further into the late season. As our seed mixture attracted large numbers of rare species, it seems to have been successful in conserving wild bee diversity. Moreover, common pollinators profited and together with rare species probably increased pollination success in the surroundings. The compositional heterogeneity over all study plots did not increase from the first to the second year. Hence, we recommend altering seed mixtures according to the climatic conditions or geographic region in which they are applied, especially when the aim is to promote locally endangered and rare bee species.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Codes of our statistical analyses will be provided upon request.
References
Agrarmarkt Austria (2021) Österreichisches Programm zur Förderung einer umweltgerechten, extensiven und den natürlichen Lebensraum schützenden Landwirtschaft. Agrarmarkt Austria, Wien
Ahrenfeldt EJ, Kollmann J, Madsen HB, Skov-Petersen H, Sigsgaard L (2019) Generalist solitary ground-nesting bees dominate diversity survey in intensively managed agricultural land. J Melittol. https://doi.org/10.17161/jom.v0i82.7057
Albrecht M, Kleijn D, Williams NM et al (2020) The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: a quantitative synthesis. Ecol Lett 23:1488–1498. https://doi.org/10.1111/ele.13576
Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R (2013a) Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc Natl Acad Sci USA 110:4656–4660. https://doi.org/10.1073/pnas.1218503110
Bartomeus I, Park MG, Gibbs J, Danforth BN, Lakso AN, Winfree R (2013b) Biodiversity ensures plant-pollinator phenological synchrony against climate change. Ecol Lett 16:1331–1338. https://doi.org/10.1111/ele.12170
Batáry P, Dicks LV, Kleijn D, Sutherland WJ (2015) The role of agri-environment schemes in conservation and environmental management. Conserv Biol 29:1006–1016. https://doi.org/10.1111/cobi.12536
Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354. https://doi.org/10.1126/science.1127863
BKG (2013) Digitales Geländemodell Gitterweite 200 m. http://www.geodatenzentrum.de/geodaten/gdz_rahmen.gdz_div?gdz_spr=deu&gdz_akt_zeile=5&gdz_anz_zeile=1&gdz_unt_zeile=3&gdz_user_id=0. Accessed 21 May 2019
Blaauw BR, Isaacs R (2014) Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J Appl Ecol 51:890–898. https://doi.org/10.1111/1365-2664.12257
Blüthgen N, Klein AM (2011) Functional complementarity and specialisation: the role of biodiversity in plant-pollinator interactions. Basic Appl Ecol 12:282–291. https://doi.org/10.1016/j.baae.2010.11.001
Bommarco R, Marini L, Vaissière BE (2012) Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 169:1025–1032. https://doi.org/10.1007/s00442-012-2271-6
Brittain CA, Vighi M, Bommarco R, Settele J, Potts SG (2010) Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl Ecol 11:106–115. https://doi.org/10.1016/j.baae.2009.11.007
Campbell AJ, Wilby A, Sutton P, Wäckers FL (2017) Do sown flower strips boost wild pollinator abundance and pollination services in a spring-flowering crop? A case study from UK cider apple orchards. Agric Ecosyst Environ 239:20–29. https://doi.org/10.1016/j.agee.2017.01.005
Carvalheiro LG, Seymour CL, Nicolson SW, Veldtman R (2012) Creating patches of native flowers facilitates crop pollination in large agricultural fields: mango as a case study. J Appl Ecol 49:1373–1383. https://doi.org/10.1111/j.1365-2664.2012.02217.x
Carvell C, Meek WR, Pywell RF, Goulson D, Nowakowski M (2007) Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. J Appl Ecol 44:29–40. https://doi.org/10.1111/j.1365-2664.2006.01249.x
Chopra SS, Bakshi BR, Khanna V (2015) Economic dependence of U.S. industrial sectors on animal-mediated pollination service. Environ Sci Technol 49:14441–14451. https://doi.org/10.1021/acs.est.5b03788
Crowther LI, Gilbert F (2020) The effect of agri-environment schemes on bees on Shropshire farms. J Nat Conserv. https://doi.org/10.1016/j.jnc.2020.125895
Danforth BN, Minckley RL, Neff JL (2019) The solitary bees: biology, evolution, conservation. Princeton University Press, Oxfordshire
DeGomez T (2002) Perennials for Northern Arizona above 6000 Foot Elevations. Univ. Arizona Coop. Ext. Publ. 1–12
Eichenberg D, Bowler DE, Bonn A, Bruelheide H, Grescho V, Harter D, Jandt U, May R, Winter M, Jansen F (2021) Widespread decline in Central European plant diversity across six decades. Glob Chang Biol 27:1097–1110. https://doi.org/10.1111/gcb.15447
García-Sogo B, Pineda B, Castelblanque L, Antón T, Medina M, Roque E, Torresi C, Beltrán JP, Moreno V, Cañas LA (2010) Efficient transformation of Kalanchoe blossfeldiana and production of male-sterile plants by engineered anther ablation. Plant Cell Rep 29:61–77. https://doi.org/10.1007/s00299-009-0798-8
Grab H, Branstetter MG, Amon N, Urban-Mead KR, Park MG, Gibbs J, Blitzer EJ, Poveda K, Loeb G, Danforth BN (2019) Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 363:282–284. https://doi.org/10.1126/science.aat6016
Haaland C, Naisbit RE, Bersier LF (2011) Sown wildflower strips for insect conservation: a review. Insect Conserv Divers 4:60–80. https://doi.org/10.1111/j.1752-4598.2010.00098.x
Hector A, Hautier Y, Saner P et al (2010) General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 91:2213–2220. https://doi.org/10.1890/09-1162.1
Hoehn P, Tscharntke T, Tylianakis JM, Steffan-Dewenter I (2008) Functional group diversity of bee pollinators increases crop yield. Proc R Soc B Biol Sci 275:2283–2291. https://doi.org/10.1098/rspb.2008.0405
Holzschuh A, Dudenhöffer JH, Tscharntke T (2012) Landscapes with wild bee habitats enhance pollination, fruit set and yield of sweet cherry. Biol Conserv 153:101–107. https://doi.org/10.1016/j.biocon.2012.04.032
Holzschuh A, Dormann CF, Tscharntke T, Steffan-Dewenter I (2013) Mass-flowering crops enhance wild bee abundance. Oecologia 172:477–484. https://doi.org/10.1007/s00442-012-2515-5
Jönsson AM, Ekroos J, Dänhardt J, Andersson GKS, Olsson O, Smith HG (2015) Sown flower strips in southern Sweden increase abundances of wild bees and hoverflies in the wider landscape. Biol Conserv 184:51–58. https://doi.org/10.1016/j.biocon.2014.12.027
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant-pollinator interactions. Annu Rev Ecol Syst 29:83–112. https://doi.org/10.1146/annurev.ecolsys.29.1.83
Kennedy CM, Lonsdorf E, Neel MC et al (2013) A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol Lett 16:584–599. https://doi.org/10.1111/ele.12082
Kirmer A, Rydgren K, Tischew S (2018) Smart management is key for successful diversification of field margins in highly productive farmland. Agric Ecosyst Environ 251:88–98. https://doi.org/10.1016/j.agee.2017.09.028
Klatt BK, Holzschuh A, Westphal C, Clough Y, Smit I, Pawelzik E, Tscharntke T (2013) Bee pollination improves crop quality, shelf life and commercial value. Proc R Soc B Biol Sci 281:20132440. https://doi.org/10.1098/rspb.2013.2440
Klatt BK, Nilsson L, Smith HG (2020) Annual flowers strips benefit bumble bee colony growth and reproduction. Biol Conserv 252:108814. https://doi.org/10.1016/j.biocon.2020.108814
Kleijn D, Winfree R, Bartomeus I et al (2015) Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat Commun 6:7414. https://doi.org/10.1038/ncomms8414
Klein AM, Steffan-Dewenter I, Tscharntke T (2003) Fruit set of highland coffee increases with the diversity of pollinating bees. Proc R Soc B Biol Sci 270:955–961. https://doi.org/10.1098/rspb.2002.2306
Knop E, Kleijn D, Herzog F, Schmid B (2006) Effectiveness of the Swiss agri-environment scheme in promoting biodiversity. J Appl Ecol 43:120–127. https://doi.org/10.1111/j.1365-2664.2005.01113.x
Krimmer E, Martin EA, Krauss J, Holzschuh A, Steffan-Dewenter I (2019) Size, age and surrounding semi-natural habitats modulate the effectiveness of flower-rich agri-environment schemes to promote pollinator visitation in crop fields. Agric Ecosyst Environ 284:106590. https://doi.org/10.1016/j.agee.2019.106590
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2019) Estimated marginal means, aka least-squares means. https://cran.r-project.org/web/packages/emmeans/emmeans.pdf
Leonhardt SD, Blüthgen N (2012) The same, but different: pollen foraging in honeybee and bumblebee colonies. Apidologie 43:449–464. https://doi.org/10.1007/s13592-011-0112-y
MacGregor-Fors I, Payton ME (2013) Contrasting diversity values: statistical inferences based on overlapping confidence intervals. PLoS ONE 8:e56794. https://doi.org/10.1371/journal.pone.0056794
Martins KT, Albert CH, Lechowicz MJ, Gonzalez A (2018) Complementary crops and landscape features sustain wild bee communities. Ecol Appl 28:1093–1105. https://doi.org/10.1002/eap.1713
Mastel K, Butz A, Schaufelberger R, Morky M, Unterseher E, Breuer J, Ott J, Zarnik S, Mann T, Walther J (2021) Informationen zu ackerbaulichen Maßnahmen in FAKT und Greening. Landwirtschaftliches Technologiezentrum Augustenberg. https://www.km-bw.de/pb/site/lel/get/params_Dattachment/3866704/Ackerbauliche%20Ma%C3%9Fnahmen%20in%20FAKT.pdf. Accessed 7 July 2021
Melathopoulos AP, Cutler GC, Tyedmers P (2015) Where is the value in valuing pollination ecosystem services to agriculture? Ecol Econ 109:59–70. https://doi.org/10.1016/j.ecolecon.2014.11.007
Neumüller U, Burger H, Krausch S, Blüthgen N, Ayasse M (2020) Interactions of local habitat type, landscape composition and flower availability moderate wild bee communities. Landsc Ecol. https://doi.org/10.1007/s10980-020-01096-4
Landwirtschaftskammer Nordrhein-Westfalen (2021) Greeningprämie. Landwirtschaftskammer Nordrhein-Westfalen. https://www.landwirtschaftskammer.de/foerderung/direktzahlungen/greeningpraemie.htm. Accessed 22 March 2021
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) Vegan: community ecology package. R package version 2.5-6. https://cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed 18 November 2020
Orr MC, Hughes AC, Chesters D, Pickering J, Zhu CD, Ascher JS (2021) Global patterns and drivers of bee distribution. Curr Biol 31:451-458.e4. https://doi.org/10.1016/j.cub.2020.10.053
Ouvrard P, Transon J, Jacquemart AL (2018) Flower-strip agri-environment schemes provide diverse and valuable summer flower resources for pollinating insects. Biodivers Conserv 27:2193–2216. https://doi.org/10.1007/s10531-018-1531-0
Pfiffner L, Cahenzli F, Steinemann B, Jamar L, Bjørn MC, Porcel M, Tasin M, Telfser J, Kelderer M, Lisek J, Sigsgaard L (2019) Design, implementation and management of perennial flower strips to promote functional agrobiodiversity in organic apple orchards: a pan-European study. Agric Ecosyst Environ 278:61–71. https://doi.org/10.1016/j.agee.2019.03.005
Potts SG, Vulliamy B, Dafni A, Neeman G, Willmer P (2003) Linking bees and flowers: How do floral communities structure pollinator communities? Ecology 84:2628–2642. https://doi.org/10.1890/02-0136
Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J, Vanbergen AJ (2016) Safeguarding pollinators and their values to human well-being. Nature 540:220–229. https://doi.org/10.1038/nature20588
Powney GD, Carvell C, Edwards M, Morris RKA, Roy HE, Woodcock BA, Isaac NJB (2019) Widespread losses of pollinating insects in Britain. Nat Commun 10:1–6. https://doi.org/10.1038/s41467-019-08974-9
Ribeiro PJJ, Christensen OF, Diggle PJ (2003) geoR and geoRglm : software for model-based geostatistics. https://www.r-project.org/nosvn/conferences/DSC-2003/Proceedings/RibeiroEtAl.pdf. Accessed 7 July 2021
Rollin O, Bretagnolle V, Fortel L, Guilbaud L, Henry M (2015) Habitat, spatial and temporal drivers of diversity patterns in a wild bee assemblage. Biodivers Conserv 24:1195–1214. https://doi.org/10.1007/s10531-014-0852-x
Rubene D, Schroeder M, Ranius T (2015) Diversity patterns of wild bees and wasps in managed boreal forests: effects of spatial structure, local habitat and surrounding landscape. Biol Conserv 184:201–208. https://doi.org/10.1016/j.biocon.2015.01.029
Rundlöf M, Lundin O, Bommarco R (2018) Annual flower strips support pollinators and potentially enhance red clover seed yield. Ecol Evol 8:7974–7985. https://doi.org/10.1002/ece3.4330
Scheper J, Reemer M, Van Kats R, Ozinga WA, Van Der Linden GTJ, Schaminée JHJ, Siepel H, Kleijn D (2014) Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in the Netherlands. Proc Natl Acad Sci USA 111:17552–17557. https://doi.org/10.1073/pnas.1412973111
Scheper J, Bommarco R, Holzschuh A, Potts SG, Riedinger V, Roberts SPM, Rundlöf M, Smith HG, Steffan-Dewenter I, Wickens JB, Wickens VJ, Kleijn D (2015) Local and landscape-level floral resources explain effects of wildflower strips on wild bees across four European countries. J Appl Ecol 52:1165–1175. https://doi.org/10.1111/1365-2664.12479
Scheuchl E, Willner W (2016) Taschenlexikon der Wildbienen Mitteleuropas. Quelle & Meyer, Wiebelsheim
Schindler M, Diestelhorst O, Härtel S, Saure C, Schanowski A, Schwenninger HR (2013) Monitoring agricultural ecosystems by using wild bees as environmental indicators. BioRisk 71:53–71. https://doi.org/10.3897/biorisk.8.3600
Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T (2002) Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83:1421–1432. https://doi.org/10.1890/0012-9658(2002)083[1421:SDEOLC]2.0.CO;2
Stoate C, Boatman ND, Borralho RJ, Carvalho CR, De Snoo GR, Eden P (2001) Ecological impacts of arable intensification in Europe. J Environ Manag 63:337–365. https://doi.org/10.1006/jema.2001.0473
Sutter L, Jeanneret P, Bartual AM, Bocci G, Albrecht M (2017) Enhancing plant diversity in agricultural landscapes promotes both rare bees and dominant crop-pollinating bees through complementary increase in key floral resources. J Appl Ecol 54:1856–1864. https://doi.org/10.1111/1365-2664.12907
Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284. https://doi.org/10.1126/science.1057544
Timberlake TP, Vaughan IP, Baude M, Memmott J (2021) Bumblebee colony density on farmland is influenced by late-summer nectar supply and garden cover. J Appl Ecol. https://doi.org/10.1111/1365-2664.13826
Ullmann KS, Meisner MH, Williams NM (2016) Impact of tillage on the crop pollinating, ground-nesting bee, Peponapis pruinosa in California. Agric Ecosyst Environ 232:240–246. https://doi.org/10.1016/j.agee.2016.08.002
von Haaren C, Reich M (2006) The German way to greenways and habitat networks. Landsc Urban Plan 76:7–22. https://doi.org/10.1016/j.landurbplan.2004.09.041
Weiner CN, Werner M, Linsenmair KE, Blüthgen N (2014) Land-use impacts on plant-pollinator networks: interaction strength and specialization predict pollinator declines. Ecology 95:466–474. https://doi.org/10.1890/13-0436.1
Westphal C, Bommarco R, Carré G et al (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78:653–671. https://doi.org/10.1890/07-1292.1
Westrich P (2018) Die Wildbienen Deutschlands. Eugen Ulmer, Stuttgart
Westrich P, Frommer U, Mandery K, Riemann H, Ruhnke H, Saure C, Voith J (2011) Rote Liste und Gesamtartenliste der Bienen (Hymenoptera, Apidae) Deutschlands. 5. Fassung, Stand Februar 2011. Bundesamt für Naturschutz, Münster
Williams NM, Ward KL, Pope N, Isaacs R, Wilson J, May EA, Ellis J, Daniels J, Pence A, Ullmann K, Peters J (2015) Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol Appl 25:2119–2131. https://doi.org/10.1890/14-1748.1
Winfree R, Reilly JR, Bartomeus I, Cariveau DP, Williams NM, Gibbs J (2018) Species turnover promotes the importance of bee diversity for crop pollination at regional scales. Science 359:791–793. https://doi.org/10.1126/science.aao2117
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. CRC Press, Boca Raton
Wood S (2019) Mixed GAM computation vehicle with automatic smoothness estimation. https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. Accessed 18 November 2020
Zattara EE, Aizen MA (2021) Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4:114–123. https://doi.org/10.1016/j.oneear.2020.12.005
Zuur AF, Ieno EN, Saveliev AA (2017) Beginner’s guide to spatial, temporal, and spatial-temporal ecological data analysis with R-INLA. Highland Statistics Ltd., Newburgh
Acknowledgements
We thank L. Woppowa (VDI e.V.) and H Seitz (VDI e.V.) for coordination of the BienABest project, R. Burger, O. Diestelhorst, M. Haider, J. C. Kornmilch, C. Saure, A. Schanowski and E. Scheuchl for conducting bee sampling and determination, and M. Weiss and all stakeholders for their role in establishing flower plantings. J. Kuppler helped us with the statistical evaluation of data and provided valuable comments on the manuscript. We are grateful to T. Jones for linguistic advice, A. Mayr for her contribution to the data handling, and to the three reviewers for their valuable comments.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project “BienABest” of the VDI Society Technologies of Life Sciences and the University of Ulm is funded by the Federal Agency for Nature Conservation (BfN) in the Federal Programme for Biological Diversity with funding from the Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU). The project is financially supported by the Ministry of the Environment, Climate Protection and the Energy Sector Baden-Württemberg, BASF SE and Bayer AG.
Author information
Authors and Affiliations
Contributions
HS, KW and MA conceived the study concept; UN and HB performed analyses and interpreted the results under the supervision of MA; UN wrote the original draft of the manuscript with contributions from HB; all authors commented on previous versions and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All persons who collected bees for this study received the necessary authorization to do so from the competent authority.
Additional information
Communicated by Jens Wolfgang Dauber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neumüller, U., Burger, H., Schwenninger, H.R. et al. Prolonged blooming season of flower plantings increases wild bee abundance and richness in agricultural landscapes. Biodivers Conserv 30, 3003–3021 (2021). https://doi.org/10.1007/s10531-021-02233-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02233-4