Abstract
We reviewed the status of orthodox seed collections of globally threatened plants conserved in − 20 °C long-term storage at the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK in terms of their geographic and bioclimatic representativeness, taxonomic and genetic diversity, quality and physiological status. The comprehensive dataset used spans over 45 years of worldwide conservation effort across various organisations. The data provides evidence-based results and future directions for the represented globally threatened flora that are of relevance to all plant conservation and seed banking organisations across the globe. The reviewed sample includes 523 collections and represents a wide geographic range, originating from 67 countries, from all nine bio-geographic continents. The majority of collections originated from temperate climates and from habitats with no dry seasons but experiencing warm summer periods. The taxonomic composition of the collections highlighted a substantial diversity, with 303 taxa (four extinct in the wild; 56 critically endangered; 105 endangered; and 138 vulnerable) represented by 297 species, 199 genera and 74 families. Almost four fifths of the collections were harvested from wild habitats. Whilst wild-origin collections can harbour useful genes not available in the cultivated gene pool, for threatened plants both collections and taxa are likely to suffer from low genetic diversity as a low number of individual plants, populations and/or potentially viable or usable seeds were sampled at the original harvest. Large numbers of empty and infested seeds in the original harvest have significantly affected the quality of collections in terms of availability of potentially viable or usable seeds in collections. As a result, just over one third of taxa and one fifth of collections consisted of ≥ 5000 potentially viable or usable seeds. Viable seeds exhibited a sound physiological status in terms of germinability and viability at the initial round of germination tests after storage, but on average, relative germination and viability achieved were below 85%. A decline in germinability during their variable time of storage was evident for 16% of the 78 collections analysed for longevity. According to a set of criteria, suitable germination protocols for propagation of plants from seeds were identified for 165 taxa. Given the apparent differences between wild species, especially those that are rare and threatened, and domesticated crops, the quality and physiological status of reviewed collections are reasonably sound. The characteristics we observed for collections, the challenges we identified for conserving them and the germination protocols we suggested for propagation of plants from seeds have the scope to be noted, integrated and used globally across various conservation activities and policies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An estimated 345,777 species of vascular plants [lycophytes, pteridophytes and seed plants (gymnosperms and angiosperms)] are known to science, of which 332,857 species are seed plants (WCVP 2020). Of these, assessments of the proportion of species threatened with extinction can vary from ca 22% (or, one in five, Brummitt et al. 2015; RBG Kew 2016) to 36–37% (Bachman et al. 2017). While in situ conservation of species and habitats is usually considered to be optimal, the Global Strategy for Plant Conservation (GSPC), recognises the significant, complementary role of ex situ conservation to achieve its TargetsFootnote 1 8 and 9 by 2020 (CBD 2012).
Seeds represent the next generation of plants when they germinate and develop into seedlings. They are the primary propagule used for regeneration and reintroduction of plant species in ecological restoration to mitigate environmental degradation and species extinction (Broadhurst et al. 2008; León-Lobos et al. 2012; Elzenga and Bekker 2017). Conventional seed banking, whereby seeds can be preserved dried and deep frozen for many tens, if not hundreds of years, is identified as a valuable ex situ conservation tool for integrated plant conservation, both as an archive and a source of genetic variation (Gargiulo et al. 2019). It is also a practical, efficient and attractive method due to its low cost and high storage capacity. As seed banks are well placed to address both GSPC targets 8 and 9, the number of ex situ conservation facilities for wild plants has grown dramatically, and ex situ conservation of threatened plants has become a national and global priority (Li et al. 2010; CHABG 2011; León-Lobos et al. 2012; Hay and Probert 2013; Liu et al. 2018). To ensure the use of such conserved germplasm, seed collections must be of high quality and viability, and in the correct physiological state to germinate and establish seedlings (Godefroid et al. 2010; León-Lobos et al. 2012). There are more than 1750 conventional seed banks in the world and the majority focus on conserving germplasm of crop species and their closest wild relatives; while others focus on species of global or national economic importance (e.g. horticultural crops, fruits and timber species), or wild species (Hay and Probert 2013), sometimes those that are most threatened.
In response to GSPC Target 8, around half of European threatened species have been conserved ex situ in seed banks (Rivière et al. 2018); and 41% of known threatened species are conserved by botanic gardens around the world (Mounce et al. 2017). However, while reliance on conventional seed banking appears reasonable for the majority of crop wild relatives (except tropical species such as coffee, coconut, cocoa, etc.) under Target 9, Wyse et al. (2018) have shown it is unlikely to be so for achieving Target 8, particularly for threatened species, and especially when they represent tropical forest tree species. This is because of the estimated high proportions of threatened and tropical moist forest species that bear seeds that are sensitive to the drying required for conventional seed banking and cryopreservation may be the only resource to ensure the effective ex situ seed conservation of such species (Li and Pritchard 2009; Hay and Probert 2013).
The Millennium Seed Bank (MSB), managed by the Royal Botanic Gardens, Kew (RBG Kew) is the culmination of ex situ seed conservation that began at RBG Kew in the 1960s (Dickie 2018). Where possible, seeds are collected and conserved in the country of origin with duplicates being sent to RBG Kew’s − 20 °C long-term storage at the MSB. The global partnership associated with the MSB has led to the creation of an extremely valuable and rich biological resource of seed collections representing substantial taxonomic diversity, wide geographic coverage, notable uniqueness and irreplaceability, significant natural capital and population value and high-quality germplasm (Liu et al. 2018). Although gaps in coverage of threatened plants were noted for MSB germplasm, at least 10% of taxa (including species and subspecific epithets), represented by over 6703 collections (> 8% of total holdings), are either extinct, rare or vulnerable to extinction at the global and/or national scale (Liu et al. 2018). This includes at least 667 taxa, representing nearly 1600 collections, that are declared globally threatened (EW-extinct in the wild, CR-critically endangered, EN-endangered or VU-vulnerable) by the IUCN (2016). Under-representation of threatened taxa may be linked to geographic rarity, or to taxa bearing desiccation sensitive (recalcitrant) seeds, which are not suitable for preservation in conventional seed banks (Liu et al. 2018). Since the study of Liu et al. (2018), there have been new or revised global level IUCN Red List assessments and a net increase in overall collections of threatened taxa conserved at the MSB.
Restoration, revegetation and species reintroduction programmes often demand vast quantities of high quality and genetically diverse germplasm (seeds) to establish self-sustaining populations and maximise the adaptive potential of conservation efforts to current and future environmental change. To support this, and help plan future more efficient seed sampling and seed conservation activities, knowledge of population structure and seed quality, viability, germinability, and vigour are needed in addition to information on how to break seed dormancy and germinate the seeds (Mortlock 2000; Cochrane et al. 2007; Broadhurst et al. 2008; Merritt and Dixon 2011; Hay and Probert 2013). The objective of this study is to review the status of seed collections of globally threatened plants conserved in − 20 °C long-term storage at the MSB, RBG Kew in terms of their geographic and bioclimatic representativeness, taxonomic and genetic diversity, quality and physiological status. Our aim is to provide useful information to support plant conservation and research worldwide by identifying strengths and weaknesses on the status of collections and suitable germination protocols for propagation of these plants from seeds.
Materials and methods
The MSB’s Seed Bank Database (SBD) contains in-depth data on seed collections stored at the MSB, and plays a significant role in collection acquisition, curation, management, monitoring, prioritisation and reporting. Data are gathered from the point of seed collection in the field (field or passport data) and during the lifespan of seeds in storage (processing data). By matching plant names (exact match), data for collections representing globally threatened seed plants according to IUCN (2017) were extracted from SBD on 08 January 2018. It is possible that plant names on IUCN (2017) could match indirectly to MSB collections (Liu et al. 2018), but these were excluded in our review. There were a total number of 1172 collections with exact name matches representing 569 taxa (number of collections followed by taxa in brackets): EW (21, 7); CR (304, 125); EN (340, 195) and VU (507, 242). As seed germination protocols need to be identified from collections with high taxonomic certainty, only those collections with verified names and with at least one completed post-storage germination test were included in this study to establish physiological status. Consequently, the representative sample included 528 collections of 303 taxa. Seed conservation protocols related to subsequent paragraphs are discussed in Appendix 1.
Geographic and bioclimatic representativeness
Cultivated collections inherited the geographic origin of the wild plant population from which they were propagated or regenerated. To investigate any degree of geographical bias, locality data were analysed at the bio-geographic continent and country level to understand the representation of taxa across the globe. To illustrate the geographic origin of taxa according to political boundaries of countries, ISO country level data were processed and displayed in ArcGIS Desktop 10.5 (ESRI 2012). To examine the bioclimate of the habitat from which seeds were harvested, the geographic coordinates of the collections were mapped according to Köppen–Geiger climate classification (Köppen 1936).
Taxonomic and genetic diversity
To investigate any degree of taxonomic bias, the taxonomic composition of the collections (e.g. number of families, genera, species and taxa) was examined to understand the diversity of globally threatened plants represented at the MSB. We used the phylogenetic tree of Smith and Brown (2018), reduced to include only one tip for each family of angiosperms and gymnosperms, to visualise family level distribution pattern of species in the sample. Species were assigned to families following World Checklist of Vascular Plants (WCVP 2020).
Wild plants represent an extremely rich genetic resource, harbouring useful genes not available in the cultivated gene pool (Ceoloni et al. 2017). Therefore, it is important to capture wild genetic diversity within plants, especially for rare, threatened and economically important species (Neel and Ellstrand 2003; Laikre 2010). Adopting a single spatial sampling strategy and sample size for all species will likely lead to suboptimal collections with low genetic diversity, and ideal collection sizes vary widely even within genus (Hoban et al. 2020). It is important to consider population structure and adjust the sampling strategy to capture locally restricted alleles or traits, which have high conservation, ecological, or economic value (Hoban and Schlarbaum 2014). While, for example, the phylogenetic diversity of the MSB legume collections (Griffiths et al. 2015) and the genetic diversity in MSB European yew collections made in the UK (Gargiulo et al. 2019) have been assessed in separate studies, such a strategy is not currently possible for most of the MSB’s collections, as the requisite genetic data is not available for them.
In the absence of genetic analysis on the germplasm, the number of collections per taxon and the number of seeds per collection were used as surrogates for genetic diversity (Godefroid et al. 2011). Such methods only demonstrate potential or likely genetic diversity within and among collections as the breeding biology or population genetics of the germplasm have not been assessed. To ensure that representative genetic diversity is captured from a population, and to enable the use of seeds for viability monitoring, regeneration, distribution, research and conservation, it is recommended to harvest 10,000–20,000 seeds from at least 50 individual plants in one population for long-term conservation (Way 2003). To capture the genetic diversity of an individual taxon alone, at least five collections should be made, each containing 5000 seeds and originating from a different wild population (Brown and Briggs 1991; ENSCONET 2009).
To review the genetic diversity of a population captured, we inspected data on the total number of individual plants harvested to make the original collection. Data was available for 280 of the 523 collections. Where the number of plants harvested was indicated by a range (e.g. 25–50 plants), we converted it to a rounded-up middle value (in this case 38). Where a > sign had been used (e.g. > 100 plants), we converted the number to one value less than the subsequent place value for tenth, hundredth or thousandth (in this case 199). The quantity of potentially viable seeds or usable seeds conserved for collections is covered in “Quality of seeds”.
To estimate the level of genetic diversity captured for a taxon we examined data on the total number of collections and potentially viable or usable seeds currently conserved from different populations of that taxon. As we excluded collections with unverified names and/or without at least one completed post-storage germination test, this may underestimate the genetic diversity captured in the taxa. Therefore, only in this instance, for 303 of the taxa represented in the reviewed collections, we added corresponding collections (314 in total) that we excluded initially. Of the 314 collections added, seed quantity data are not available for 33 collections.
We also assigned the biological status of collections as of wild or cultivated origin by examining heredity, geographic origin, habitat, taxonomy and regeneration data. Collections originating from natural or semi-natural habitats were classified as ‘wild origin’, while those originating from cultivated habitats and propagation or regeneration activities were classified as ‘cultivated origin’ (Alercia et al. 2012).
Quality of seeds
Radiographic analysis has proved to be an effective non-destructive method of monitoring seed quality by identifying empty, insect damaged and malformed seeds and enabling the estimation of the number of potentially viable or usable seeds in a collection (Guedes et al. 2014). This technology is used at a number of wild species seed banks as an alternative to cut-testing seeds for assessing the quality of collections as well as to determine the number of seeds that need to be sown to compensate for embryoless and infested seeds when setting up germination tests.
We reviewed the quality of collections in terms of the total number of potentially viable or usable seeds as well as cumulative percentage of unusable seeds observed over time by using seed X-ray or cut-test data generated during seed cleaning and germination tests (see Appendix 1). The initial quality indicates the status of the original collections received post-cleaning but pre-banking in − 20 °C long-term storage at the MSB. The current quality indicates the status of collections at the time of review, after long-term storage at − 20 °C, and this quality varies over time (e.g. reduction of potentially viable seeds when more unusable seeds are observed over time or seeds are used for curation, conservation, education and display activities). Where the sample size was sufficient, we analysed for taxonomic patterns in the proportions of seeds lacking embryos or infested.
Initial quality
The initial quality of seeds in the original collections was assessed by estimating the total number of potentially viable or usable seeds (AOq, adjusted original seed quantity) using seed X-ray or cut-test data generated at the seed cleaning stage as a proportion of the original seed quantity (Oq)
where \({\text{X}}_{{\text{f}}}\) is the number of full seeds observed and \({\text{X}}_{{\text{n}}}\) is the total number of seeds that were X-rayed or cut-tested. Any significant loss of usable seeds due to empty and infested seeds in the original collections was investigated by testing the null hypothesis that there was no difference in total quantities between \({\text{O}}_{{\text{q}}}\) and \({\text{AO}}_{{\text{q}}}\), using a one-tailed paired (right) probability corresponding to t statistic using GenStat software (VSN International Ltd).
Current quality
The current quality of seeds was assessed by estimating: (1) the total number of potentially viable or usable seeds in the current collection (\({\text{AC}}_{{\text{q}}}\), adjusted current seed quantity) using seed X-ray or cut-test data generated at the seed cleaning stage as a proportion of the current seed quantity (\({\text{C}}_{{\text{q}}}\))
where \({\text{X}}_{{\text{f}}}\) is the number of full seeds observed and \({\text{X}}_{{\text{n}}}\) is the total number of seeds X-rayed or cut-tested; and (2) the cumulative percentage of unusable seeds (\({\text{CPU}}\)) observed overtime, by using seed X-ray or cut-test data generated at the seed cleaning stage and seed cut-test data from ungerminated seeds derived from all routine germination tests carried out for monitoring the viability of collections.
where \({\text{X}}_{{\text{e}}}\) is the number of empty and \({\text{X}}_{{\text{i}}}\) is the number of infested seeds observed, and \({\text{X}}_{{\text{n}}}\) is the total number of seeds that were X-rayed or cut-tested, and where \({\text{G}}_{{\text{e}}}\) is number of empty and \({\text{G}}_{{\text{i}}}\) is the number of infested ungerminated seeds observed and \({\text{G}}_{{\text{n}}}\) is the total number of seeds sown, all for a germination test. The average values of CPU were estimated for families with at least five different genera, genera with at least five different species and species with at least five different collections.
Physiological status of seeds
The physiological status of seeds reflects their capacity to germinate, highlighting the true quality of collections. Seed germination tests are the most useful way of monitoring the physiological status of seeds over time in long-term storage as well as enable the development of seed germination protocols for the propagation of taxa from seeds. At the MSB, the standard is that there should be 95% certainty that the lower bound of germinability of collection is at least 75%, hence the expected overall germinability must be 85% or more. Anything less will lead to management decisions being taken to either regenerate and/or recollect the taxon concerned. Seed germination protocols used at the MSB are standardised with conditions and/or treatments to break more abundant seed dormancies. Details of germination test protocols are described in Appendix 1. We determined the physiological status of seeds from the results of their germination tests carried out after storage in − 20 °C at the MSB (post-storage tests). The initial round of germination tests was used to analyse the relative percentages of seed germination (\({\text{RG}}\)) and viability (\({\text{RV}}){ }\) and seed vigour (\({\text{R}},\) the index of germination rate or speed). Both initial and most recent retest rounds of germination tests were used to assess the longevity of collections in long-term storage, in terms of germinability.
Seed germination, viability and vigour
The true quality of mature seeds is usually reflected in the results of germination tests when seeds are exposed to optimum conditions (Godefroid et al. 2010). If the germination conditions are not optimal or incubation periods are extended, there is a risk that some viable seeds might die during the germination test (Hay and Probert 2013). Occasions may well arise when the results of germination tests could be misleading due to application of ineffective or partially effective dormancy-breaking treatments (Ellis et al. 1985). Also, when seeds are stored, they deteriorate with time losing their fitness due to aging prior to mortality (Walters et al. 2010). This may result in some seeds losing their ability to germinate or to produce healthy radicles which can grow into healthy or normal seedlings and plants. Therefore, it is critical to identify emergence of healthy radicles versus seeds developing abnormal seedlings (e.g. cotyledons produced without a radicle, indicative of accumulating genetic damage in ageing stored seeds) during seed germination tests (e.g. Roberts 1978). Abnormal seedlings are those that are not considered capable of continued growth and development due to damage, deformation or decay. Their numbers are excluded from RG calculation but included in RV calculation as they indicate a certain, minimal degree of seed viability.
A subjective measure of the viability of a collection at the end of a germination test is often calculated by including the cut-test results of ungerminated seeds (Crawford et al. 2007; Godefroid et al. 2010). As viable seeds may have died during the incubation period, ungerminated seeds that are firm and appear fresh are considered as an indication of potentially viable dormant seeds, but not an indication of overall viability at the start of the germination test (Crawford et al. 2007).
To minimise the incorrect estimation of low germination percentages, especially for taxa which are known to produce many embryoless seeds, the original number of seeds sown (\({\text{G}}_{{\text{s}}}\)) was adjusted to reflect the true number of potentially viable seeds sown (with embryos) by discounting any empty (\({\text{G}}_{{\text{e}}}\)) and infested (\({\text{G}}_{{\text{i}}}\)) ungerminated seeds (de Santana et al. 2018). For each collection, the following variables related to seed germination were calculated.
where \({\text{G}}_{{\text{g}}}\) and \({\text{G}}_{{\text{f}}}\) are respectively the total number of germinated seeds with healthy radicles and ungerminated seeds which appear fresh, \({\text{G}}_{{\text{a}}}\) is the total number of germinated seeds that produce abnormal seedlings, \({\text{t}}\) is the time from start of the germination period in days and \({\text{g}}\) is the number of newly germinated seeds at time t (Soltani et al. 2015).
The test with > 9 true seeds sown (with embryos) and yielding the highest \({\text{RG}}\) followed by the highest \({\text{RV}}\) was used to describe the initial physiological status of a collection (\({\text{RG}}\), \({\text{RV}}\) and \({\text{R}}\)). If more than one test yielded equally high \({\text{RG}}\) and RV for a collection, the test with highest number of true seeds sown or the test where seeds germinated within the shortest period of time, as indicated by \({\text{R}}\), was used.
Seed longevity
Based on the availability of data the following variables were estimated and analysed to understand the longevity of collections: (1) true age of collections from the year seeds were harvested to current; (2) number of years seeds were being stored in − 20 °C long-term storage at the MSB; (3) any significant loss in \({\text{RG}}\) since the collections were first placed in − 20 °C long-term storage at the MSB. The latter was calculated by comparing the highest \({\text{RG}}\) achieved for the initial round of germination tests with that of the most recent retest. The null hypothesis that both these values are the same was tested using the value of normal deviate Z from a two-tailed test according to Ellis et al. (1985), where a correction factor is applied to enable the normal distribution to be used in the analysis:
where \({\text{p}}_{1}\) is \((100 \times {\text{g}}_{1} {/}({\text{n}}_{1 } - 0.5))\), \({\text{p}}_{2}\) is \((100 \times {\text{g}}_{2} {/}({\text{n}}_{2 } + 0.5))\), \({\overline{\text{p}}}\) is the mean value of \({\text{p}}_{1}\) and \({\text{p}}_{2}\), and \({\text{g}}_{1} { }\) and \({\text{g}}_{2}\) are number of germinated seeds, and n1 and n2 are number of true seeds sown (with embryos) in initial and most recent retest rounds of germination tests respectively; and (4) correlation between the number of years that seeds have been stored in − 20 °C long-term storage at the MSB and the change in RG during this period (the difference in the highest RG achieved for the most recent retest when compared to that of initial round of germination tests after storage). A sample Pearson correlation coefficient, r, was calculated to examine any linear correlation between these variables using GenStat software (VSN International Ltd).
Seed germination protocols
Suitable germination protocols for a taxon were chosen from post-storage initial germination tests, across collections if represented by more than one collection, where the number of true seeds sown (with embryos) was > 9 and where \({\text{RG}}\) was at least 70%. The aim was to identify the best germination test where: seeds were exposed to a single constant or alternate incubation temperature; no dormancy breaking conditions and/or treatments were used; the highest \({\text{RG}}\) followed by the highest \({\text{RV}}\) were achieved; and seeds germinated within the shortest period of time as indicated by R.
Results
The MSB is a duplicate storage facility for conserving a portion of the original harvest for 61% of the 523 collections reviewed. The other portion was conserved in the country of origin and/or elsewhere. The proportionate division of the share to MSB is unknown but is generally 50%. Where a portion is not stored in the country of origin, it is usually because, either suitable facilities were not available in-country, or the collection was regarded as being too low in seed number to be split, without potentially compromising the population genetic diversity represented in each sub-sample.
Geographic and bioclimatic representativeness
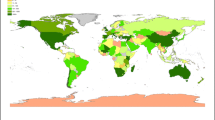
The geographic origin of the collections covered all nine bio-geographic continents and represented 67 countries. Some taxa were collected from more than one continent and/or country (Fig. 1). Fifteen collections (~ 3%) representing 15 taxa had unknown geographic origins. Most taxa and collections originate from Africa and the least from the Pacific, Asia-Tropical and Antarctic (Fig. 2). Countries where > 10 globally threatened taxa were represented (number of taxa followed by collections in brackets): Madagascar (27, 35); Chile (17, 26); Saint Helena, Ascension and Tristan da Cunha (16, 30); Italy (15, 21); South Africa (14, 18); Australia (13, 39); Tanzania (13, 27); Kenya (13, 14); USA (12, 27); Mauritius (12, 15); Georgia (11, 12); and Namibia (11, 11).
The geographic origin of globally threatened plants conserved at the MSB, RBG Kew. The representative sample included 523 collections from 303 seed plant taxa. Cultivated collections inherited the geographic origin of the wild plant population from which they were propagated or regenerated. Total number of taxa are shown according to different size classes. Some taxa originated from more than one continent and/or country. Countries that are not shown on the map are (number of taxa in brackets): Bermuda (5); British Virgin Islands (1); Cayman Islands (3); Falkland Islands (5); Mauritius (12); Saint Helena, Ascension and Tristan da Cunha (16); and Turks and Caicos Islands (6)
Köppen–Geiger climate for habitats where the wild-collected seeds were harvested was mapped for 350 collections representing 207 taxa (Table 1; Fig. 3). Seeds were collected from all five Köppen–Geiger climate groups representing 21 climate zones out of 33, with majority of collections and taxa (in brackets, respectively) originated from temperate climate (184, 90) followed by tropical (84, 64), arid (50, 40), cold continental (26, 16) and polar (6, 3). Regarding the seasonal precipitation level of these habitats, the majority of collections (in brackets followed by number of taxa) originated from habitats with no dry season (104, 38) followed by those with a dry summer (88, 67), wet savanna (50, 34), dry winter (45, 35), unknown precipitation level (23, 13), monsoon (15, 15), dry savanna (15, 15), tundra (6, 3) and rainforest (4, 3). In terms of the level of heat, the majority of collections and taxa (in brackets, respectively) were collected from habitats experiencing a warm summer (133, 59) followed by unknown heat level (90, 70), hot summer (69, 49), cold (33, 26), hot (17, 15) and cold summer (8, 4). Some taxa were collected from more than one climate zone.
Köppen–Geiger climate of wild habitats from where seeds were harvested for globally threatened plants conserved at the MSB, RBG Kew. Sample included 350 collections representing 207 taxa. Refer to Table 1 for climate abbreviations
Any bias in geographic or bio-climatic representation would be no surprise and probably expected. Early, pre-MSB seed collecting expeditions for the Kew seed bank focused on the UK and Europe, especially the Mediterranean region; and for much of the MSB Project (2000–2010) the focus was on dryland areas in a number of countries and regions.
Taxonomic and genetic diversity
There were 303 taxa represented by 39 gymnosperms and 264 angiosperms from 74 families, 199 genera and 297 species. The IUCN (2017) global conservation status for these taxa were (number of taxa in brackets): EW (4); CR (56); EN (105); and VU (138). The distribution of the number of globally threatened species conserved among seed plant families is illustrated in Fig. 4. Although there are gaps in the distribution, species occurred throughout the phylogenetic tree. Families with at least 10 taxa represented were (number of taxa in brackets): Fabaceae or Leguminosae (37); Pinaceae (21); Asteraceae or Compositae (18), Cupressaceae (16), Cactaceae (14); and Rubiaceae (10).
Phylogenetic distribution of globally threatened plants conserved at the MSB, RBG Kew. The phylogenetic tree is a version of Smith and Brown (2018) reduced to include only one tip for each family of angiosperms and gymnosperms. Species were assigned to families following WCVP (2020). Bar charts in the middle-grey area indicate the number of species represented for each family. Scale is given with a middle-dotted line which indicates representation of 20 species
About 79% of collections originated from natural or semi-natural habitats (wild origin) and 21% from either cultivated habitat (e.g. orchards, home gardens and botanic gardens) or propagation/regeneration activities in the UK or elsewhere (cultivated origin). The total number of individual plants sampled to make the original collection varied from one to > 1000 plants: 50 to > 1000 (26%); 10–49 (33%); and < 10 (41%). The total number of collections conserved for a taxon from different populations varied from one to 31: one collection (52%); 2–4 collections (35%); 5–10 collections (10%); and 11–31 collections (3%). The total number of potentially viable or usable seeds currently conserved for a taxon varied from 12 to 1,972,356 seeds: < 501 (10%); 501–1000 (16%); 1001–1500 (9%); 2001–2500 and 1501–2000 (8% each); 2501–5000 (13%); 5001–10,000 (11%); and > 10,000 (25%).
Quality of seeds
Due to the availability of seed X-ray and cut-test data, adjusted seed quantities (the quantity of potentially viable seeds or usable seeds) for original and current collections were estimated for only 510 of the collections.
Initial quality
The estimated original seed quantity (\({\text{O}}_{{\text{q}}}\)) ranged from 39 to 2,012,812 seeds and the adjusted original seed quantity (\({\text{AO}}_{{\text{q}}}\)) ranged from 31 to 1,972,556 seeds. About 39% of the original collections consisted of all potentially viable or usable seeds but for the rest, the quantity was reduced by 2% to 90% due to empty and infested seeds (Figs. 5, 6). As a result, original collections with > 2500 seeds were reduced by 17% (by 36 from 216 collections), those with 2001–2500 seeds increased by 16% (by 5 from 31 collections), collections with 1501–2000 seeds were reduced by 19% (by 10 from 53 collections), and those with ≤ 1500 seeds were increased by 20% (by 41 from 210 collections). Therefore, about 21% of the original collections contain > 5000 and 18% contain < 501, potentially viable or usable seeds. The pairwise comparison of collections for their total quantities of \({\text{O}}_{{\text{q}}}\) and \({\text{AO}}_{{\text{q}}}\) confirmed a significant decline in the number of potentially viable or usable seeds due to empty and infested seeds in the original harvest (results of one-tailed paired t-test: t = 4.20 on 509 d.f. and probability < 0.001 at 95% confidence level).
Current quality
At the time of this review the estimated adjusted current seed quantity (\({\text{AC}}_{{\text{q}}} )\) ranged from 31 to 1,972,356 seeds. About 21% of the current collections contain > 5000 and 19% contain < 501, potentially viable or usable seeds (Fig. 6). The estimated cumulative percentage of unusable seeds observed overtime (\({\text{CPU}}\), sample size = 508 collections), ranged from zero to 85%. About 24% collections consisted of all potentially viable or usable seeds (CPU = 0%), for 36% of collections the \({\text{CPU}}\) was between 1 and 10%, for 29% of collections it was between 11 and 50, and for 11% of collections it was > 50% (Fig. 7).
Average CPU values were estimated for 11 families, eight genera and 15 species (Table 2). Collections from four families (Cupressaceae, Pinaceae, Apiaceae and Asteraceae or Compositae), three genera (Abies, Pinus and Dalbergia) and four species (Abies fraseri, Callitris oblonga, Pinus tecunumanii and Widdringtonia whytei) appeared to consist of 20% or more unusable seeds.
Physiological status of seeds
To estimate the physiological status of seeds, post-storage germination data for a total number of 1099 initial tests across 523 collections and a total number of 140 most recent retests across 78 collections were analysed. For initial tests the true number of seeds sown (with embryos) ranged from 10 to 298 for 84% of tests and was < 10 for 16% of tests, and for retests the true number of seeds sown (with embryos) ranged from 10 to 75 for 96% of tests and was < 10 for 4% of tests.
Seed germination, viability and vigour
The relative germination (\({\text{RG}})\) and viability (\({\text{RG}})\) for post-storage initial germination tests ranged from zero to 100% with an average of 70% and 79% respectively for collections and 67% and 77% respectively for taxa (Fig. 8). About 57% and 66% of collections and 56% and 66% of taxa respectively showed high \({\text{RG}}\) and \({\text{RV}}\) (> 80%). By comparison, 28% and 20% of collections and 32% and 22% of taxa respectively showed low \({\text{RG}}\) and \({\text{RV}}\) ≤ 50%. About 9% of collections (~ 12% of taxa) showed 0% RG and about 4% collections (5% of taxa) showed both 0% \({\text{RG}}\) and \({\text{RV}}\).
The index of germination rate or speed \(\left( {\text{R}} \right)\) was calculated for 497 collections, representing 295 taxa. \({\text{R}}\) ranged from zero to 405 days with an average of 25 days (Fig. 9). About 62% of collections germinated within three weeks of sowing on media and exposure to an incubation temperature, while ~ 11% of collections continued beyond the expected six-week period for germination (range from 45 to 405 days).
Seed longevity
The date of seed harvested was available for 496 collections. The true age of collections ranged from three to 46 years (Fig. 10a): the majority were aged either 6–10 years (35%) or 11–15 years (~ 35%), followed by 1–5 years (12%). Collections have been stored in − 20 °C long-term storage at the MSB for 1–45 years (Fig. 10b): 1–15 years (91%); 16–30 years (~ 8%); and > 30 years (~ 1%).
Only 78 collections (nine gymnosperms and 69 angiosperms) were retested over time (1st retest, 2nd retest, 3rd retest, etc.): 51 collections up to 1st; 19 up to 2nd; six up to 3rd; and two up to 4th. These collections are represented by 31 families, 47 genera and 52 taxa. For these collections the period between initial and the most recent retest ranged from three to 36 years: 1–5 years (6%); 6–10 years (41%); 11–15 years (21%); 16–20 years (18%); and > 20 years (14%).
On average, retested collections achieved 81% \({\text{RG}}\) at initial test, which decreased to 74% at most recent retest. About 80% of collections achieved \({\text{RG}}\) > 70% for their initial test but the percentage of collections decreased to ~ 65% in retests and conversely, 16% of collections achieved \({\text{RG}}\) ≤ 50% for their initial tests but the percentage of collections increased up to ~ 24% in retests (Fig. 11).
The comparison of \({\text{RG}}\) between initial and the most recent retest revealed that it remained the same for 20 collections (\({\text{RG}}\) was 100% for 14 collections, 98% for two collections, 14% for one collection and 0% for three collections), increased between 2 and 56% for 22 collections (significant for four collections with Z > 1.96 and P ≤ 0.05) and decreased between 1 and 88% for 36 collections (significant for 13 collections with Z > 1.96 and P ≤ 0.05). Therefore, decline in germinability during variable time of storage was evident for 16% of the 78 collections analysed for longevity. There was no apparent correlation between the number of years that seeds were being stored and the change in RG during this period (Fig. 12: Sample Pearson correlation coefficient (r) = 0.21).
Correlation between the number of years that seeds have been stored in − 20 °C long-term storage at the MSB and the change in the relative germination percentage (RG) during this period (difference in the highest RG achieved for the most recent retest when compared to that of initial round of germination tests after storage). To handle both negative (decrease) and positive (increase) values for change in RG, prior to log transformation a constant value of 100 is added to all data. Sample Pearson correlation coefficient on semi logarithmic transformed data (r) = 0.21
Seed germination protocols
The best germination protocols, with at least 70% \({\text{RG}}\), identified from post-storage initial germination tests for 165 taxa from 50 families are given in Appendix 2. The germination protocols of a further 19 taxa with at least 70% \({\text{RG}}\) were excluded due to the low number of true seeds sown (with embryos). The incubation temperature used for initial tests was either constant (77%), alternate (19%) or a combination of constant and alternate (4%). Overall, 43% of post-storage initial tests were set up with a dormancy breaking condition and/or treatment (see Appendix 1). Single temperatures suitable for the germination of non-dormant seeds were applied in 89% of tests (medium temperatures 57%; high temperatures 31%; and low temperatures 1%). Temperatures suitable for breaking seed dormancy were applied to 11% of tests (cold stratification 8%; move-along three or more different temperatures 2%; combined stratification 0.7%; and warm stratification 0.3%). About 32% of tests were setup with one or more dormancy breaking treatment (scarification 10%; surgical 8%; use of gibberellic acid in germination medium 7%; mechanical manipulation 6%; and application of after ripening, use of nitric acid in germination medium or soaking seeds in smoke solution < 1% each). Non-dormancy breaking methods such as use of 24 h of dark photoperiod and/or anaerobic conditions were limited to < 1% of tests.
Discussion
Pre-MSB seed collecting expeditions for the Kew seed bank focused on the UK and Europe, especially the Mediterranean region. The MSB Project (2000–2010) conservation effort was initially focused on drylands as species adapted to hot, dry environments may have evolved longer lifespans in the dry state and many produce orthodox seeds and so are suited to conservation in seed banks (Li and Pritchard 2009). The world’s drylands are also home to an immense variety of plant life, support approximately one-fifth of the world’s population (far more than the tropical rain forests), as well as 50% of the world’s livestock, and provide forage for both domestic animals and wildlife (van Slageren 2003). Drylands are identified as among the most threatened environments on Earth, with large areas being lost due to desertification each year (van Slageren 2003). The breadth and depth of species coverage and their genetic diversity conserved at the MSB are likely influenced by the nature of different funding models and objectives of different conservation projects.
The collections reviewed originated from a wide geographic range. Although, given the overall collecting bias and the consequent expectation that many species collected would be from habitats with a more or less arid climate and limited seasonal precipitation, in fact the majority of threatened species have been collected from temperate climates from habitats with no dry seasons but experiencing warm summer periods. As expected, our sample included only a few species that originated from tropical habitats, as the majority of species from these habitats bear seeds that could be extremely short lived or recalcitrant, with cryopreservation possibly the only means to ensure their effective ex situ seed conservation (Li and Pritchard 2009; Hay and Probert 2013).
The taxonomic composition within the sample highlighted a substantial diversity. As families with a high incidence of recalcitrant species are less likely to be conserved in conventional seed banks, we did not expect many collections and/or taxa from families such as (in brackets, total number of globally threatened taxa listed in IUCN (2017) followed by number globally threatened collections and taxa conserved at the MSB): Fagaceae (63, 0, 0); Lauraceae (223, 0, 0); Sapotaceae (252, 0, 0); Moraceae (59, 0, 0); Clusiaceae or Guttiferae (126, 0, 0); Sapindaceae (119, 1, 1) including Aceraceae (0, 0, 0); Arecaceae or Palmae (339, 7, 5); Myrtaceae (299, 0, 0); Annonaceae (193, 0, 0); Rutaceae (137, 3, 3); Anarcardiaceae (94, 11, 1); Dipterocarpaceae (407, 0, 0); Meliaceae (155, 8, 7) and Rhizophoraceae (13, 0, 0) (Dickie and Pritchard 2002).
Almost four fifths of the collections (79%) were made directly from the wild, with the assumption that they represented greater genetic resource than those from a cultivated gene pool. Nevertheless, by comparison with overall MSB holdings, which consist of almost 92% of collections with a wild genetic heritage (Liu et al. 2018), the threatened species collections appear to have relied rather more on non-wild sources. Furthermore, the majority of collections and taxa are likely to suffer from low genetic diversity, as a low number of individual plants (< 50), different populations (< 5) and/or potentially viable or usable seeds (< 5000) were sampled at the original harvest. A large proportion of empty and infested seeds in the original harvest significantly affected the quality of collections in terms of availability of viable or usable seeds. As a result, just over one third of taxa and one fifth of collections consisted of ≥ 5000 potentially viable or usable seeds and the majority were below the recommended threshold. It should be noted that for 61% of collections reviewed, only a portion of the original harvest is conserved at the MSB. Although, conserving the original harvest at multiple locations increases security for the germplasm, the total conservation effort could be undermined due to low seed numbers stored at individual locations.
Difficulties in meeting the demands for genetic diversity and seed quantity are reflected in seed collections of threatened plants conserved at the MSB and worldwide. For example, about 34% of overall MSB holdings are represented by > 5000 potentially viable or usable seeds (Liu et al. 2018), but this estimate reduces to 21% for threatened collections. Of the European threatened flora conserved among the seed banks of European Native Seed Conservation Network, only one third of taxa are represented by at least five collections, and only 23–28% of the species are represented by collections with ≥ 5000 seeds (Godefroid et al. 2011; Rivière and Müller 2017; Rivière et al. 2018). About 71% of the collections conserved for threatened species in the Australian PlantBank had < 1000 seeds (Offord et al. 2004), while 50% of the collections conserved for critically endangered taxa in the Western Australian Threatened Flora Seed Centre (WA TFSC) consisted of < 1000 seeds (Cochrane et al. 2007). Threatened taxa are often rare with fewer extant populations or have low seed production. It does, however, mean that more needs to be done to ensure the genetic coverage of ex situ conservation collections of these taxa.
Although incorporation of spatial distribution patterns and population structures to sampling strategies are recommended, at least for poorly connected or sparsely distributed plant taxa, common protocols for ex situ collections of seeds have not usually considered these characteristics (Hoban and Schlarbaum 2014). Due to lack of data on spatial distribution patterns of populations and population structure, we are unable to differentiate whether the assumed reduced genetic diversity is related to sampling strategy or species characteristics such as breeding system. Therefore, we suggest integrating such characteristics with future seed collection activities. For example, RBG, Kew launched the UK National Tree Seed Project (UKNTSP) in 2013 as an ex situ seed conservation initiative adopting a well-designed sampling strategy based on various social and ecological factors including the geographic patterns of targeted species in biogeographic zones (Kallow and Trivedi 2016). This is specifically to capture genetic variation within and among populations in order to protect against the loss of genetic variation from threats, including pests and diseases and climate change. The germplasm of British populations of European yew (Taxus baccata) conserved by this project at the MSB was found to be a representative of wild populations in terms of allelic capture, including rare and locally common variants, indicating that the sampling protocol applied is appropriate (Gargiulo et al. 2019).
Despite the importance of and need for implementing the correct sampling strategy, limitations to the ability to capture wild genetic diversity remain. This is primarily because the seed ecology of most wild plant species including their distribution patterns, breeding biology and population genetics remain unknown (Mortlock 2000; Merritt and Dixon 2011; Hay and Probert 2013; Teixido et al. 2017). Furthermore, the availability of funding limits the capacity for comprehensive conservation programmes to sample and conserve all suitable populations. Also, many of the perceived shortcomings of seed banking of wild plants may arise from the heterogeneity of wild plant populations. Wild plant populations tend to be heterogeneous in distribution patterns, genetic integrity, flowering and fruiting seasons (phenology), production of viable seeds and seed maturity. This impacts on the availability of mature and viable seeds for ex situ conservation and consequently on seed germinability, vigour and longevity in seed banks. Seed collection protocols have been developed to ensure the survival of the natural populations is not threatened e.g. by collecting no more than 20% of the mature seeds available on the day of collection (Way 2003), or 5% of the reproductive material on each plant for threatened species (Offord et al. 2004). As the taxa that are identified as globally threatened are often characterised by small and fragmented populations, collection sizes are necessarily small. To increase the overall seed resource, multi-year sampling is often used, but individual collections tend to consist of small seed quantities (Cochrane et al. 2007). Regeneration of seeds in purpose-built facilities is an alternative method to increase the amount of seed of a species required for in situ conservation needs, this is, however, beyond the scope of most wild species seed banks.
Some taxa appear to produce naturally critically high proportions of non-viable seeds, though in most cases the relative contribution of genetic and environmental factors to this is unknown. Thus, they have a very low potential for regeneration from seed, necessitating specific in situ and ex situ conservation strategies to avoid biodiversity loss (Godefroid et al. 2011). For example, Dayrell et al. (2016) found in the megadiverse heterogeneous grasslands of the campo rupestre, that at least half of the seeds produced by 46% of the 83 populations consisted of different species were embryo-less and/or non-viable, suggesting phylogeny is related to seed viability percentages. Utah juniper (Juniperus osteosperma) is one of many plant species that produce large numbers of fruits containing parthenocarpic or otherwise empty or unviable seeds (Fuentes and Schupp 1998). Poor germination of seeds is a common occurrence in the Umbelliferae (Apiaceae), the most probable cause being the presence of non-viable seeds with no embryo as a result of the Lygus bug feeding on developing seeds (Robinson 1954). Plant families such as Asteraceae (Compositae), Cyperaceae and Poaceae are known to produce many empty seeds while Fabaceae (Leguminosae) seeds often suffer insect damage (Way and Gold 2008). As a result, achieving ideal seed quantities is often impossible when conserving wild plants of threatened taxa. About 21% of the collections reviewed belonged to plant families that are mentioned above. We identified four families (including Asteraceae or Compositae and Apiaceae stated above), three genera and four species which are likely to produce 20% or more unusable seeds. Legumes seem to have a low quantity (7%) of unusable seeds in their collections, however, estimates were made after seed cleaning, and as legumes have relatively larger seeds, empty and infested seeds are more likely to be identified and removed during the cleaning process. Our sample included collections from the families Cyperaceae and Poaceae, but the number of genera presented from each family was insufficient to derive any conclusion.
The propagation of plants from seeds is a viable, inexpensive and generally effective method in conservation activities (Cerabolini et al. 2004; Cirak 2007). Viable seeds in the collections reviewed exhibited a sound physiological status in terms of germinability, viability and vigour at initial round of germination tests after long-term storage. We identified suitable germination protocols for 165 taxa from tests where the number of true seeds sown (with embryos) was > 9 and relative germination was at least 70%.
The percentages of collections achieving > 80% relative germination (57%) and viability (66%) were respectively 4% and 10% less than the estimates reported for all MSB holdings in Liu et al. (2018). On average, relative germination percentages achieved by collection (RG = 70%) and taxa (RG = 67%) were 15–18% below the expected 85% germinability and 8–11% above the average estimated for threatened species of the Belgian flora (RG = 59%), conserved in in long-term storage at the National Botanic Gardens of Belgium, where 59% of collections require regeneration or recollection (Godefroid et al. 2010). The low germination percentage reported for the Belgian flora is related to mouldiness of seeds during the germination process and the authors reported that reducing fungal proliferation by surface-sterilisation of the seeds could improve results.
About 4% of the reviewed collections (5% of taxa) showed both 0% \({\text{RG}}\) and \({\text{RV}}\). As \({\text{RV}}\) was an estimate calculated using cut-test results of non-germinated seeds, collections with both 0% RG and \({\text{RV }}\) will be further assessed using additional germination test conditions and/or treatments or tri-phenyl tetrazolium chloride stain (see Appendix 1).
Germinability alone does not reflect the viability of a collection. The 85% germinability threshold, an estimate derived from agricultural settings, is a very high threshold for wild species. Even in their natural habitats some taxa may not achieve germination percentage as high as 85%. Viable seeds may also fail to germinate because of quiescence and/or dormancy. Quiescence is a state of suspended growth of the embryo of non-dormant seeds when minimum requirements for germination are lacking e.g. water, temperature, gasses, and light. Dormancy, a state in which seeds are prevented from germinating even under environmental conditions normally favorable for germination, is determined by morphological and physiological properties of the seed. Both quiescence and dormancy must be relieved for seeds to germinate and establish seedlings. Dormancy may be a main determinant of a species’ distribution, ensuring germination occurs under appropriate seasonal conditions, thereby reducing extinction risk and providing the opportunity for subsequent adaptive divergence (Willis et al. 2014). However, an inability to break seed dormancy is an obstacle preventing dormant but healthy seeds from germinating (Merritt and Dixon 2011). About 28% of seed collections belonging to threatened Belgian flora appeared to exhibit some degree of dormancy, with the majority being non-dormant (Godefroid et al. 2010). The authors highlighted that while dry seed storage may induce secondary dormancy for some species it may also break dormancy through after-ripening process for other species (especially non-deep physiological dormancy). Examining seed dormancy is beyond the scope of our review but at the MSB, seed germination protocols are standardised to overcome the most common seed dormancies (Appendix 1).
Decline in germinability was evident for at least 16% of collections during variable times of storage. A previous analysis of germination data for collections stored at the MSB for over 20 years, has shown no significant reduction in viability during this period in 86% of 2388 collections concluding that seed drying (15% Relative Humidity and 15 °C) and storage (− 20 °C) conditions used at the MSB are suitable for long-term storage of orthodox seeds (Probert 2003). Most of the seed collections held by the WA TFSC in short (< 5 years) and medium (5–12 years) term storage maintained their viability and declines in germination were only evident for a small number of collections, representing 10 taxa, stored in medium term conditions (Crawford et al. 2007). Many of the above declines were collection-specific and not reproduced by other collections of the same taxon. In the Australian PlantBank, seed viability assessments for the collections belonging to threatened species indicated that nearly 56% of collections older than two years had a viability > 80% indicating that the remaining 44% of the accessions required recollecting (Offord et al. 2004).
An analysis of longevity for about 42,000 orthodox seed accessions (Walters et al. 2005) highlighted that some plant families had characteristically short-lived (e.g. Apiaceae and Brassicaceae) or long-lived (e.g. Malvaceae and Chenopodiaceae) seeds, and seeds from species originating from particular localities had characteristically short (e.g. Europe) or long (e.g. South Asia and Australia) shelf lives. The effect of seed traits and environmental conditions at the site of collection on seed longevity was explored for 195 species stored at the MSB by ageing seeds at elevated temperatures and relative humidity (Probert et al. 2009). Although the causes for seed death in seed banks and rapid-ageing conditions may not be the same, Probert et al. (2009) suggested that the apparent short-lived nature of endospermic seeds from cool wet environments may have implication for re-collection and re-testing strategies in ex situ conservation. There is evidence that some species produce orthodox seeds of short longevity in dry storage (Walters et al. 2005). Understanding species’ differences in longevity is crucial for the effective management of collections in seed banks because it underpins the selection of viability monitoring periods and hence regeneration or re-collection strategies (Probert et al. 2009).
Wild-species seed banks have been criticized for having seed holdings that are insufficient to provide for the needs of large-scale restoration projects and for lacking documentation or knowledge of seed quality measures (e.g. germinability, viability and vigour), seed dormancy breaking procedures and germination protocols. The purpose of wild-species seed banks is to preserve enough genetic diversity to prevent species and populations from extinction. Restoration guidelines strongly recommend using local seed sources to maximize local adaptation and prevent outbreeding depression, but there are situations where highly modified landscapes restrict the choice of seed sampling areas to small remnants where limited, poor quality seeds are available, and where harvesting impacts may be high (Broadhurst et al. 2008). Wild-species seed banks are not a suitable seed source for large-scale restoration and species reintroduction programs unless seeds are sampled specifically for this purpose. Our review goes some way to answer this criticism and to increase understanding of the extent to which these concerns are valid for globally threatened seed collections conserved ex situ at the MSB.
It is hard to determine specific standards for the conservation of seeds from wild plant species, and most of the theory is derived from studies on crops where plants have been bred to have higher and more uniform seed production and germination (Hay and Probert 2013). Given the apparent differences between wild species, especially rare and threatened, and domesticated crops, the quality and physiological status of reviewed collections are reasonably sound. The seed conservation protocols used at the MSB (for acquisition, drying, cleaning, storage, viability monitoring, regeneration, propagation, duplication, distribution, documentation, etc.) follow international genebank standards (FAO/IPGRI 1994; FAO 2013) as well as RBG Kew’s own experience in seed banking for over 50 years. As a result, the MSB produced its own seed conservation standards for use with seed banking of wild species. These are widely used across the MSB Partnership (MSBP) to ensure that high quality material is stored throughout the MSBP (Breman and Way 2018). In addition, technical information sheets produced by MSB, covering various aspects of seed conservation practice are published at https://www.kew.org/science/collections/seed-collection/millennium-seed-bank-resources.
These protocols are established to ensure that viable and mature seeds are collected, collections are sufficiently dried, processed and stored according to gene bank standards, the quality of seeds is assessed using several parameters, viability and longevity of seeds are monitored routinely through germination tests, and seed dormancy issues are handled by applying standardised seed-dormancy breaking protocols (see Appendix 1). Over time, adjustments for these protocols are necessary to address challenges in meeting the demands for genetic diversity, quality and germinability. Research is needed to investigate the causes for low genetic diversity or quality (e.g. population, collection or taxon specific, seed trait, climate at origin, etc.) and to develop germination protocols for taxa that achieved relative germination below 70%. It would also be interesting to undertake a similar review across the MSBP to help plan future conservation activities. For now, this review provides a comprehensive background to underpin future research in seed ecology and germination protocols for propagation of plants from seeds in various conservation activities. A summary of the review is also provided as supplementary material.
Implications for practice
Our review was based on a comprehensive and empirical dataset spanning over 45 years of worldwide conservation effort across various organisations. The data provides evidence-based results and future directions for the represented globally threatened flora that are of relevance to all plant conservation and seed banking organisations across the globe. The importance of these collections in the face of threats to global plant diversity cannot be overstressed. The characteristics we observed for collections (geographic and bioclimatic representation, taxonomic and genetic diversity and physiological status), the challenges we identified for conserving them and germination protocols we suggested for propagation of plants from seeds have the scope to be noted, integrated and used globally across various conservation activities and policies.
Crop germplasm has been conserved in seed banks for over 60 years to preserve crop diversity useful to future agriculture. Wild species seed banks are adapting this technology with a relatively short period of experience, whilst working with more variable and unknown parameters associated with wild species and gradually creating their own best practice. Capturing and maintaining accurate and comprehensive data from the point of harvesting seeds from habitats and during their life cycle in seed banks is essential for effective management of wild species, through understanding their geographic and bioclimatic origin, taxonomic identity, quality and physiological status.
There is no single seed sampling strategy which will equally capture genetic diversity of different wild plant species. However, representation of wild genetic diversity can be improved by adjusting individual sampling events according to spatial distribution pattern, population structure and reproductive strategy of the targeted species. It should be noted that seed ecology of many wild plants is unknown, and some species naturally fail to germinate successfully in their natural habitats or produce a large proportion of empty or embryoless seeds. These characteristics may be genetically bound and inherited at various taxonomic levels or influenced by the natural surroundings. To understand such correlations, it is also important to differentiate quantities of potentially usable and unusable seeds according to collections and subsequently under families, genera, species, etc. Establishing standardized seed germination protocols improves the viability assessment process and well-designed germination data recording facilitates effective data analysis to identify suitable germination protocols and to monitor viability and longevity of collections. There is a need for local, national and global conservation policy makers and professionals to focus on the quality and physiological status of germplasm conserved ex situ, and to fund comprehensive and extended seed sampling activities to capture local, national, regional and global genetic diversity. We must close the gap between seed ecology and seed banking for wild-origin collections, especially in relation to seed storage behavior and suitable measures to conserve short-lived and recalcitrant seeds.
Notes
Target 8: at least 75% of threatened plant species conserved in ex situ collections, preferably in the country of origin, and at least 20% available for recovery and restoration programs; and Target 9: 70% of the genetic diversity of crops including their wild relatives and other socio-economically valuable plant species conserved, while respecting, preserving and maintaining associated indigenous and local knowledge.
References
Alercia A, Diulgheroff S, Mackay M (2012) FAO/Bioversity Multi-Crop Passport Descriptors V.2 [MCPD V.2]. Food and Agriculture Organization of the United Nations (FAO), Rome Italy and Bioversity International, Rome, Italy, 11pp https://www.bioversityinternational.org/e-library/publications/detail/faobioversity-multi-crop-passport-descriptors-v2-mcpd-v2/
Bachman SP, Nic Lughadha EM, Rivers MC (2017) Quantifying progress toward a conservation assessment for all plants. Conserv Pract Policy 32(3):516–524. https://doi.org/10.1111/cobi.13071
Breman E, Way M (2018) Safe for the future: seed conservation standards developed for the Millennium Seed Bank Partnership. In: MNHN (ed.) EuroGard VII Congress Paris, France: The European Botanic Gardens Consortium, pp 267–274
Broadhurst LM, Lowe A, Coates DJ, Cunningham SA, McDonald M, Vesk PA, Yates C (2008) Seed supply for broadscale restoration: maximizing evolutionary potential. Evol Appl 1:587–597
Brown AHD, Briggs JD (1991) Sampling strategies for genetic variation in ex situ collections of endangered plant species. In: Falk DA, Holsinger KE (eds) Genetic and conservation of rare plants. Oxford University Press, New York, pp 99–122
Brummitt NA, Bachman SP, Griffiths-Lee J, Lutz M et al. (2015) Green plants in the red: a baseline global assessment for the IUCN sampled red list index for plants. PLoS ONE. https://doi.org/10.1371/journal.pone.0135152
CBD (2012) Global strategy for plant conservation: 2011–2020. Botanic Gardens Conservation International, Richmond, p 37
Ceoloni C, Kuzmanović L, Ruggeri R, Rossini F, Forte P, Cuccurullo A, Bitti A (2017) Harnessing genetic diversity of wild gene pools to enhance wheat crop production and sustainability: challenges and opportunities. Diversity 9(4):55. https://doi.org/10.3390/d9040055
Cerabolini B, De Andreis R, Ceriani RM, Pierce S, Raimondi B (2004) Seed germination and conservation of endangered species from the Italian Alps: Physoplexis comosa and Primula glaucescens. Biol Conserv 117:351–356. https://doi.org/10.1016/j.biocon.2003.12.011
CHABG (2011) Safeguarding Australia’s flora through a national network of native plant seed banks: business plan 2011–2020. The Council of Heads of Australian Botanic Gardens Inc., Canberra, p 23
Cirak C (2007) Seed germination protocols for ex situ conservtion of some Hypericum species from Turkey. Am J Plant Physiol 2(5):287–294
Cochrane JA, Crawford AD, Monks LT (2007) The significance of ex situ seed conservation to reintroduction of threatened plants. Aust J Bot 55(3):356–361. https://doi.org/10.1071/BT06173
Crawford AD, Steadman KJ, Plummer JA, Cochran A, Probert RJ (2007) Analysis of seed-bank data confirms suitability of international seed-storage standards for the Australian flora. Aust J bot 55(1):18–29. https://doi.org/10.1071/BT06038
Dayrell RLC, Garcia QS, Negreiros D, Baskin CC, Baskin JM, Silveira FAO (2016) Phylogeny strongly drives seed dormancy and quality in a climatically buffered hotspot for plant endemism. Ann Bot 119:267–277. https://doi.org/10.1093/aob/mcw163
de Santana DG, Carvalho FJ, Toorop P (2018) How to analyze germination of species with empty seeds using contemporary statistical methods? Acta Bot Bras 32(2):271–278. https://doi.org/10.1590/0102-33062017abb0322
Dickie JB, Pritchard HW (2002) Systematic and evolutionary aspects of desiccation tolerance in seeds. In: Black M, Pritchard HW (eds) Desiccation and survival in plants drying without dying. CAB International, Wallingford, pp 239–260
Dickie JB (2018) Conserving seeds of wild species in the Millennium Seed Bank: 'one size does not fit all'. Theorien de Lebendsammlung; Pflanzen, Mikroben und Tiere als Biofakte in Genbanken. NC Karafyllis. Freiburg/Munich, Karl Alber, pp 341–360
Ellis RH, Hong TD, Roberts EH (1985) Handbook of seed technology for genebanks, vol 1. Principle and methodology, International Board for Plant Genetic Resources, Rome, p 210
Elzenga JTM, Bekker RM (2017) Seed germination: ecological aspects-special issue editorial. Plant Biol 19:3–5. https://doi.org/10.1111/plb.12522
ENSCONET (2009) ENSCONET Seed collecting manual for wild species. European Native Seed Conservation Network
ESRI (2012) ArcGIS Desktop 10.5. Version 10.5.0.6491
FAO/IPGRI (1994) Genebank standards. Food and Agriculture Organization of the United Nations, Rome, International Plant Genetic Resources Institute, Rome
FAO (2013) Draft genebank standards for plant genetic resources for food and agriculture. https://www.fao.org/docrep/meeting/027/mf804e.pdf
Fuentes M, Schupp EW (1998) Empty seeds reduce seed predation by birds in Juniperus osteosperma. Evol Ecol 12:823–827
Gargiulo R, Saubin M, Rizzuto G, West B, Fay MF, Kallow S, Trivedi C (2019) Genetic diversity in British populations of Taxus baccata L.: Is the seedbank collection representative of the genetic variation in the wild? Biol Conserv 233:289–297
Godefroid S, Van de Vyver A, Vanderborght T (2010) Germination capacity and viability of threatened species collections in seed banks. Biodivers Conserv 19:1365–1383. https://doi.org/10.1007/s10531-009-9767-3
Godefroid S, Rivière S, Waldren S, Boretos N, Eastwood R, Vanderborght T (2011) To what extent are threatened European plant species conserved in seed banks? Biodivers Conserv 144(5):1494–1498
Griffiths KE, Balding ST, Dickie JB, Lewis GP, Pearce TR, Grenyer R (2015) Maximizing the phylogenetic diversity of seed banks. Conserv Biol 29(2):370–381
Guedes RS, Gomes-Junior FG, Marcos-Filho J, Alves EU (2014) Evaluating seed quality of Amburana cearensis using X-ray scanning. Seed Technol 36(1):41–49
Hay FR, Smith RD (2003) Seed Maturity: when to collect seeds from wild Plants. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ (eds) Seed conservation: turning science into practice. Royal Botanic Gardens, Kew, pp 97–133
Hay FR, Probert RJ (2013) Advances in seed conservation of wild plant species: a review of recent research. Conserv Physiol 1:1–11. https://doi.org/10.1093/conphys/cot030
Hoban S, Schlarbaum S (2014) Optimal sampling of seeds from plant populations for ex-situ conservation of genetic biodiversity, considering realistic population structure. Biol Conserv 177:90–99. https://doi.org/10.1016/j.biocon.2014.06.014
Hoban S, Callicrate T, Clark J, Deans S, Dosmann M, Fant J, Gailing O, Havens K, Hipp AL, Kadav P, Kramer AT, Lobdell M, Magellan T, Meerow AW, Meyer A, Pooler M, Sanchez V, Spence E, Thompson P, Toppila R, Walsh S, Westwood M, Wood J, Griffith MP (2020) Taxonomic similarity does not predict necessary sample size for ex situ conservation: a comparison among five genera. Proc R Soc B 287:20200102. https://doi.org/10.1098/rspb.2020.0102
IUCN (2016) The IUCN Red List of Threatened Species. Version 2016-3 https://www.iucnredlist.org
IUCN (2017) The IUCN Red List of Threatened Species. Version 2017-3 https://www.iucnredlist.org. Accessed January 2018
Kallow S, Trivedi C (2016) Collecting Genetic Variation on a Small Island. In Proceedings of Workshop on Gene Conservation of Tree Species—banking on the Future, Chicago, IL, pp 129–136
Köppen W (1936) Das geographisca system der Klimate. In: Köppen W, Geiger G (eds) Handbuch der Klimatologie. 1. C. Gebr, Borntraeger, p 44
Laikre L (2010) Genetic diversity is overlooked in international conservation policy implementation. Conserv Genet 11:349–354. https://doi.org/10.1007/s10592-009-0037-4
León-Lobos P, Wayc M, Arandad PD, Lima-Juniore M (2012) The role of ex situ seed banks in the conservation of plant diversity and in ecological restoration in Latin America. Plant Ecol Divers 5:245–258
Li DZ, Pritchard HW (2009) The science and economics of ex situ plant conservation. Trends Plant Sci 14:614–621
Li D, Yang X, Wang Y, Cai J (2010) The germplasm bank of wild species. Southwest China BCAS 24(4):264–267
Liu U, Dickie JB (2017) Managing ex situ collections of wild species' seeds: use of biodiversity informatics in the Millennium Seed Bank to address challenges. Biodivers Inf Sci Stand 1:e20197. https://doi.org/10.3897/tdwgproceedings.1.20197
Liu U, Breman E, Cossu TA, Kenney S (2018) The conservation value of germplasm stored at the Millennium Seed Bank, Royal Botanic Gardens, Kew. UK Biodivers Conserv 27(6):1347–1386. https://doi.org/10.1007/s10531-018-1497-y
Merritt DJ, Dixon KW (2011) Restoration seed banks—a matter of scale. Science 332(6028):424–425. https://doi.org/10.1126/science.1203083
Mortlock W (2000) Local seed for revegetation. Where will all that seed come from? Ecol Manage Restor 1(2):93–101
Mounce R, Smith P, Brockington S (2017) Ex situ conservation of plant diversity in the world’s botanic gardens. Nat Plants 3:795–802. https://doi.org/10.1038/s41477-017-0019-3
Neel MC, Ellstrand NC (2003) Conservation of genetic diversity in the endangered plant Eriogonum ovalifolium var. vineum (Polygonaceae). Conserv Genet 4:337–352
Offord CA, McKensy ML, Cuneo PV (2004) Critical review of threatened species collections in the New South Wales Seedbank: implications for ex situ conservation of biodiversity. Pacif Conserv Biol 10(4):221–236
Probert RJ (2003) Seed viability under ambient conditions, and the importance of drying. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ (eds) Seed conservation: turning science into practice. Royal Botanic Gardens, Kew, pp 337–365
Probert RJ, Daws MI, Hay FR (2009) Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Ann Bot 104(1):57–69. https://doi.org/10.1093/aob/mcp082
RBG Kew (2016) The state of the world’s plants report—2016. Royal Botanic Gardens, Kew, p 80
Rivière S, Müller JV (2017) Contribution of seed banks across Europe towards the 2020 Global Strategy for Plant Conservation targets, assessed through the ENSCONET database. Oryx 52(3):464–470. https://doi.org/10.1017/S0030605316001496
Rivière S, Breman E, Kiehn M, Carta A, Müller JV (2018) How to meet the 2020 GSPC target 8 in Europe: priority-setting for seed banking of native threatened plants. Biodivers Conserv 27(8):1873–1890. https://doi.org/10.1007/s10531-018-1513-2
Roberts EH (1978) Mutations during seed storage. Acta Hortic 83:279–282. https://doi.org/10.17660/ActaHortic.1978.83.36
Robinson RW (1954) Seed germination problems in the Umbelliferae. Bot Rev 20(9):531–550. https://doi.org/10.1007/BF02958802
Smith SA, Brown JW (2018) Constructing a broadly inclusive seed plant phylogeny. Am J Bot 105(3):302–314. https://doi.org/10.1002/ajb2.1019
Soltani E, Ghaderi-Far F, Baskin CC, Baskin JM (2015) Problems with using mean germination time to calculate rate of seed germination. Aust J Bot 63(8):631–635. https://doi.org/10.1071/BT15133
Teixido AL, Toorop PE, Liu U, Ribeiro GVT, Fuzessy LF, Guerra TJ, Silveira FAO (2017) Gaps in seed banking are compromising the GSPC’s Target 8 in a megadiverse country. Biodiver Conserv 26:703–716. https://doi.org/10.1007/s10531-016-1267-7
van Slageren MW (2003) The Millennium Seed Bank: building partnerships in arid regions for the conservation of wild species. J Arid Environ 54:195–201. https://doi.org/10.1006/jare.2001.0879
VSN International Ltd., 2011. GenStat Software, 14th edition. Version 14.2.0.6297
Walters C, Wheeler LM, Grotenhuis JM (2005) Longevity of seeds stored in a genebank: species characteristics. Seed Sci Res 15(1):1–20
Walters C, Ballesteros B, Vertucci VA (2010) Structural mechanics of seed deterioration: standing the test of time. Plant Sci 179(6):565–573. https://doi.org/10.1016/j.plantsci.2010.06.016
Way MJ (2003) Collecting seeds from non-domesticated plants for long-term conservation. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ (eds) Seed conservation: turning science into practice. Royal Botanic Gardens, Kew, pp 163–201
Way MJ, Gold K (2008) Assessing a potential seed collection. Technical information sheet no. 2. Royal Botanic Gradens, Kew, Millennium seed Bank Project, Wakehurst
WCVP, World Checklist of Vascular Plants (2020) Version 2.0. https://wcvp.science.kew.org/ Facilitated by the Royal Botanic Gardens, Kew. Accessed 23 April 2020
Willis CG, Baskin CC, Baskin JM, Auld JR, Lawrence Venable D, Cavender-Bares J, Donohue K, Rubio de Casas R, The NESCent Germination Working Group (2014) The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol 203:300–309. https://doi.org/10.1111/nph.12782
Wyse SV, Dickie JB, Willis KJ (2018) Seed banking not an option for many threatened plants. Nat Plants 4:848–850
Acknowledgements
We thank all the funders, members of the Millennium Seed Bank Partnership, and staff of the Royal Botanic Gardens, Kew whose support has made the Millennium Seed Bank’s collections possible. We also thank the anonymous reviewers for providing constructive comments to improve the manuscript. The Royal Botanic Gardens, Kew is part funded by Grant in Aid from DEFRA UK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Hawksworth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Ex-situ conservation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1
Following is a summary of related seed conservation protocols followed at the MSB, RBG Kew at the time of our review. References are listed under the main text.
Collecting
Seeds are collected following national and international regulations. Where possible, good quality mature seed collections are made at least from 50 individual plants in a single population without taking more than 20% of the mature seeds available on the day of sampling and without compromising the long-term survival of the source population.
To maximise the quality of seeds, collections needs to be made at the optimum stage of seed development (ripeness or maturity) and should be substantially free from insect damage or empty seeds (Way 2003). If seeds are harvested too early, losses may be incurred because immature seeds have not yet acquired desiccation tolerance and/or because seeds lose viability more rapidly in storage due to impaired longevity (Hay and Smith 2003). To assess the readiness of targeted plant population, seed collectors are advised to identify signs of seed maturity, for example colour, texture, odour and hardness of fruits or seeds, dehiscent fruits and seed dispersal stage. To judge the physical quality and availability of seeds, prior to collecting, cut testing a representative sample of seeds is recommended to assess the proportion of filled, empty, infested and damaged seeds in order to estimate the quantity of potentially viable or usable seeds that can be collected without overharvesting the population.
Arrival
Collections are received by the MSB following Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and plant health regulations. On arrival, collections are placed in the humidity room (if fresh and immature), initial dry room (if dry and mature), or cleaned and/or processed immediately (if wet, micro seeds or short-lived taxa).
Ripening
Collections with freshly collected immature seeds, may be placed in a tray at 60% relative humidity (RH) and 20 °C in a control atmosphere room (humidity room) for a maximum of 7–10 days before drying and/or cleaning.
Drying
Collections are dried in loose, moisture permeable cloth or paper bags placed in crates at 15% RH and 15 to 18 °C in a controlled atmosphere room (initial dry room). The time at dry room conditions is restricted, ideally, to a maximum of 6 months.
Cleaning
Collections are usually cleaned by hand sorting, sieving, rubbing, crushing, rolling and/or using an aspirator to separate empty seeds and debris from filled seeds. Wet fruits are first washed in a sieve to remove fruit pulp, then dried in humidification room for a week before transferring to dry room and clean as dry seeds. The method used to clean collections depends on morphology of fruit or seeds (e.g. pod, capsule, cone, etc.), their size, robustness, etc.
X-raying or cut-testing
Efficacy of cleaning or purity of collection is usually checked by X-ray imaging or cut-testing a randomly selected sample of 10–50 seeds and assessing the number of full, empty, and infested seeds.
Weighing and counting
Usually, five samples of 50 seeds or one sample of 250 seeds (if micro seeds) and remainder of the collection are weighted in grams up to five to seven decimal places using an electronic balance. Weights of the samples are used to calculate the weight for 1000 seeds (1000 seed weight). For majority of collections, the original seeds quantity is estimated by applying the 1000 seed weight for the weight of whole collection. Where applicable, the quantity is estimated by hand counting or using a seed counter (e.g. Contador).
Drying before long-term storage
After counting, collections are placed in crates and transferred to main dry room, controlled at 15% RH and 18 °C, for at least one month prior to long-term storage. To ensure that collections are sufficiently dry, moisture status of a sub-samples of seeds (4–8 collections per crate) are checked from every crate using a Rotronic hygrometer sensor housed in an AW-DIO water activity probe, used in conjunction with a HygroPalm 3 display unit (Rotronic Instruments UK Ltd., Crawley, UK).
Long-term storage
Each collection is usually divided to a base (long-term storage) and an active (available for curation, regeneration and distribution) portions and transferred to air-tight glass containers or foil bags and stored in main vault that operates at – 20 °C (active and base collections are stored in separate rooms). Collections with insufficient seeds (≤ 259 full seeds) or black-box collections are only stored in base. For collections that are suspected to be short-lived, management decisions are taken to duplicate within small plastic vials or foil bags in cryo storage, a vessel containing liquid nitrogen in the vapour phase at ~ − 186 °C. Cryo storage procedures are not covered in this summary.
Germination
Once the collections are stored at – 20 °C storage for at least 7 days, preferably within three months of banking, the physiological status of seeds (initial germination, viability and vigour) in terms of true quality is tested on randomly selected samples of seeds using post-storage germination tests (initial tests). Longevity of most collections are monitored through germination retests at least every 5, 10 or 20-year period (1st, 2nd, 3rd re-tests, etc.), depending on expected longevity, during their life cycle in storage. Where possible, collections that are suspected to be short-lived or show any signs of loss of viability, germination or vigour during storage are monitored more frequently, and management decisions are taken either to duplicate a part of collection in cryo storage (if not already done so and viability is still above 50%) or to regenerate seeds (if not trees) using existing seeds and/or to re-collect. Currently, collections that are stored in cryo storage are not routinely monitored but there are on-going experimental trials for comparing longevity of seeds with those stored in – 20 °C storage.
Sample size
The number of seeds used, and tests set up (10, 25 or 50 full seeds and one to five tests) for each monitoring period depend on the size of the collection and whether germination conditions and treatments are sufficiently known. Seed X-ray/cut-test data are used in germination tests to estimate the total number of seeds required to sow for compensating likely empty (embryoless) and infested seeds, if any, in the collection. For e.g. if the fraction of full seeds in the X-rayed sample is 0.8, to sow 50 good seeds, the total number of seeds required to sow is 63 seeds (50/0.8). Approximately 4–5000 germination tests were carried out yearly during 2013 to 2017 for testing initial viability alone for an average number of 2352 collections per year. Therefore, use of controlled tests or replication of tests are impractical for routine viability monitoring process in a seed bank and would compromise collection size, which is often small in conservation collections.
Germination media
Seeds are usually sown on 1% aqueous agar in plastic petri dishes, but a range of other germination substrates may also be used i.e. moist sand or filter papers, distilled or deionised water, sterile nutrients (Knudson C, Norstog, Phytamax and basic cultures), and horticulture media (mixtures of loam, peat, grit and vermiculite).
Germination requirements
Seed collections stored at the MSB represent a wide geographic origin and taxonomic diversity. Germination ecology of many of these taxa that are collected from the wild are unknown. Therefore, collection’s data (taxonomic identity, geographic origin, and month of seeds collection) and a range of other resources (RBG Kew’s Seed Information Database—https://data.kew.org/sid/ and Seed Bank Database, Global Climate Data—https://www.worldclim.org/ and mainstream literature) are used to predict environmental factors or germination conditions (e.g. temperature, precipitation, diurnal patterns of exposure to temperature or thermoperiod and light and darkness or photoperiod, availability of oxygen; e.g. aerobic or anaerobic and natural processes; e.g. wildfires) and treatments (e.g. dormancy-breaking) that trigger germination of seeds in their natural habitats (Liu and Dickie 2017). Based on the availability of data, these predictions are either based on family, genus, species, or taxa level and the closest approximate of geographic origin of collections. These are then simulated under controlled laboratory conditions in incubators with pre-conditions and/or pre-treatments, as necessary, to break quiescence and/or dormancy of seeds to stimulate germination. Pre-conditions and/or pre-treatments may be applied to seeds before they are sown and exposed to germination conditions.
Conditions
Seeds are exposed to either constant, alternate or a combination of constant and alternate temperatures during pre-conditions to break dormancy and/or incubation period for germination. To compensate photoperiod required for seed germination for majority of plant taxa, the incubators are set with a diurnal period of either 8 h light:16 h dark or 12 h light:12 h dark photoperiod. Seeds of dark-requiring species are given with a diurnal period of 0 h light:24 h dark photoperiod. The thermoperiod is maintained to 24 h period of exposure for constant temperatures and to coincide it with photoperiod for alternate temperatures (maximum temperature during light hours and minimum temperature during darkness hours).
According to germination requirement predictions, seeds are exposed to low (< 10 °C), medium (10–20 °C inclusive) or high (> 20 °C) temperature for germination. Where necessary, one of following pre-conditions is applied to break dormancy.
-
i.
cold stratification: two weeks or more of chilling seeds at ≤ 5 °C followed by a move to a higher temperature for germination.
-
ii.
warm stratification: two weeks or more of warming at ≥ 20 °C followed by a move to a lower temperature for germination.
-
iii.
combined stratification: applying a combination of cold/warm or warm/cold stratification followed by a move to a third temperature for germination.
-
iv.
move-along temperatures: by mimicking seasonal climate cycle from where the seeds shed naturally and germinate: three or more different temperatures.
Treatments
Seeds vulnerable to imbibition injury are rehydrated gently at high constant temperature (20 °C) for one day by suspending over water at 100% RH before soaking in any chemical solution. To remove contaminants, seeds may be sanitised, as required, by immersing them in a disinfection for a brief period (10% bleach or 0.5% sodium hypochlorite for five to 20 min). Where necessary, one or more of the following pre-treatments are applied to break dormancy.
-
i.
Physiological dormancy (PD) Surgical treatment (excise seed coat at both radicle tip and cotyledon ends, excise pericarp along proximal to distal ridge above embryo, excise seed coat adjacent to radicle, pierce seed coat or remove seed coat partially or completely, using a needle, file, mini saw, dental drill bar or hammer or by hand); continuous or pulse application of gibberellic acid or potassium nitrate to germination substrate; or dry after ripening, suitable for seeds originate from dry climates, by holding seeds for a period of at least two weeks at a high constant temperature often at 60% RH, before transferring to a medium for germination.
-
ii.
Physical dormancy (PY) Scarification of seeds (pierce seed coat or remove seed coat partially or completely using a needle, file, mini saw, dental drill bar or hammer or by hand); acid scarification using concentrated sulphuric acid; wet heat treatment by immersing seeds in hot water for required period of time and plunging to cold water; dry heat treatment by placing seeds in an oven for few minutes at high temperature (80 to > 100 °C); or soaking seeds in a smoke solution usually for a day. We used the term ‘surgical’ against ‘scarification’ to distinguish PD from PY treatments.
-
iii.
Mechanical dormancy Mechanical manipulation to remove covering structures including outgrowths, not seed coat, such as endocarp and/or mesocarp partially or completely by hand or using an instrument.
Duration of test
To allow seeds sufficient time for germination, tests are usually continued at least for six weeks and terminated either after all seeds are germinated or germination has ceased for at least four weeks. Period is extended as necessary if the number of germinated seeds are still increasing or seeds require prolonged periods of exposure to varied conditions (i.e. move-along temperatures) according to predicted germination requirements.
Observations and data recording
For each germination test, conditions and treatments to which seeds are exposed to are recorded in chronological order as different steps with start and end dates. Newly germinated seeds are counted and discarded at weekly intervals. Seeds which exhibit growth and differentiation by emergence of a healthy radicle ~ 2 mm in length (less for micro and dwarf seeds) are considered germinated (visible germination) and viable, those grew into abnormal seedlings are considered ungerminated but viable (e.g. cotyledons produced without a radicle). The start date of the step during which first observed germination, is considered as the starting point of germination period to calculate germination rate. If none of the seeds sown are germinated, then the starting point of germination period is considered as the date when seeds are exposed to last optimum temperature (or start day of last step).
Ungerminated seeds
After terminating a test, the viability of ungerminated seeds is checked by dissecting seeds (cut-testing), using a microscope if required, and the number of seeds which appears fresh, mouldy, empty, and infested are counted. Those ungerminated but remaining healthy and firm are considered viable (fresh), and those showing signs of decay but remain full are considered not viable (mouldy). Seeds without contents or embryo (empty) and insect damaged (infested) seeds are considered as debris and excluded from germination calculations.
Further testing
Collections with both 0% germination and viability will be further assessed using additional germination test conditions and/or treatments or tri-phenyl tetrazolium chloride stain which reacts with active respiratory dehydrogenases in living tissue producing a bright red dye in living seeds.
Appendix 2
Successful germination protocols for 165 globally threatened seed plant taxa. Across 523 seed collections representing 303 taxa, 1099 post-storage initial germination tests were analysed. Suitable germination protocols for a taxon were chosen from tests, across collections if represented by more than one collection, where the number of true seeds sown (with embryos) > 9 and relative germination (\({\text{RG}})\) is at least 70%. Aim was to identify the best test where: seeds were exposed to a single constant or alternate incubation temperature; no dormancy breaking conditions and/or treatments were used; highest \({\text{RG}}\) followed by the highest percentage of relative viability (\({\text{RV}})\) were achieved; and seeds germinated within the shortest period of time as indicated by seed vigour (R, the index of germination rate or speed). Unless otherwise stated incubators were set with a diurnal period of either 8 h light:16 h dark or 12 h light:12 h dark photoperiod. The thermoperiod was maintained to 24 h period of exposure for constant temperatures and to coincide it with photoperiod for alternate temperatures (maximum temperature during light hours and minimum temperature during darkness hours).
Family –ACEAE | Seed plant taxa | Seed germination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Treatment (on day one, if not stated) | Condition (d, number of days) | Medium (d, number of days) | Dormancy-breaking | Number of true seeds sown (with embryos) | RG% | RV% | R (days) | |||
Condition | Treatment | |||||||||
ACANTH– | Blepharis crinita | MECC | 20 °C (7d) | 1% agar | MEC | 20 | 100 | 100 | 7 | |
ACANTH– | Blepharis kenyensis | MECC | 25 °C (7d) | 1% agar | MEC | 20 | 100 | 100 | 7 | |
ADOX– | Sambucus tigranii | REH; Day 2: STR | 25/15 °C (378d): 20/10 °C (77d) | 1% agara | GA | 47 | 79 | 81 | 143 | |
AIZO– | Antimima eendornensis | MECC | 25 °C (7d) | 1% agar | MEC | 20 | 100 | 100 | 7 | |
AIZO– | Antimima eendornensis | MECC | 25/10 °C (7d) | 1% agar | MEC | 20 | 100 | 100 | 7 | |
AIZO– | Khadia beswickii | 15 °C (21d) | 1% agar | 21 | 100 | 100 | 14 | |||
AIZO– | Lithops francisci | 20 °C (7d) | 1% agar | 20 | 100 | 100 | 7 | |||
AIZO– | Lithops werneri | MECC | 30/15 °C (91d) | 1% agar | MEC | 51 | 100 | 100 | 17 | |
ALISMAT– | Damasonium alisma | SURP | 15 °C (7d) | 1% agar | SUR | 20 | 100 | 100 | 7 | |
ALISMAT– | Damasonium polyspermum | SC | 15 °C (56d) | 1% agar | SC | 50 | 84 | 92 | 13 | |
AMARANTH– | Chenopodium helenense | Day 105: SURP | 15 °C (63d); 25/10 °C (21d); 15 °C (98d) | 1% agar (84d); 1% agara (21d); 1% agar (77d) | SUR + GA | 47 | 87 | 94 | 74 | |
AMARANTH– | Pleuropetalum darwinii | 25/10 °C (48d) | 1% agar | 23 | 100 | 100 | 14 | |||
ANACARDI– | Rhus coriaria | 1. MEC: aril removed; 2. SC (also on day 207); and 3. REH | 25/10 °C (246) | Moist sand | MEC + SC | 42 | 90 | 93 | 56 | |
API– | Berula bracteata | 25/10 °C (42d) | 1% agar | 18 | 94 | 100 | 21 | |||
API– | Berula burchellii | 25/10 °C (133d) | 1% agar | 25 | 80 | 80 | 58 | |||
API– | Ferula sadleriana | 0 °C (105d) | 1% agar | 49 | 100 | 100 | 58 | |||
AR– | Arum purpureospathum | 15 °C (49d) | 1% agar | 49 | 100 | 100 | 21 | |||
ARALI– | Aralia chinensis | 0 h light:24 h dark: 25/10 °C (84d) | 1% agar | 17 | 100 | 100 | 57 | |||
AREC– | Sabal bermudana | 30 °C (98d) | moist sand | 20 | 95 | 95 | 45 | |||
ASPARAG– | Leopoldia gussonei | 10 °C (29d) | 1% agar | 10 | 100 | 100 | 29 | |||
ASTER– | Bidens eatonii | Day 43: SURP | 30 °C (1d); 10 °C (52d) | 1% agar | SUR | 10 | 100 | 100 | 57 | |
ASTER– | Blepharispermum hirtum | SURC | 26 °C | 1% agar | SUR | 22 | 100 | 100 | ||
ASTER– | Cheirolophus junonianus | 21 °C | 1% agar | 100 | 100 | 100 | ||||
ASTER– | Commidendrum robustum | STR | 25/10 °C (28d) | 1% agar | 13 | 100 | 100 | 14 | ||
ASTER– | Cylindrocline commersonii | 25 °C (14d) | 1% agar | 21 | 100 | 100 | 14 | |||
ASTER– | Dendroseris litoralis | 15 °C (35d) | 1% agar | 11 | 100 | 100 | 9 | |||
ASTER– | Hypochaeris oligocephala | 20 °C (56d) | 1% agar | 25 | 100 | 100 | 9 | |||
ASTER– | Lamyropsis microcephala | Day 28: SURP | 15 °C (63d) | 1% agar | SUR | 17 | 82 | 82 | 13 | |
ASTER– | Pectis caymanensis var. robusta | 25 °C (49d) | 1% agara | GA | 16 | 81 | 100 | 16 | ||
ASTER– | Plagius flosculosus | 25/10 °C (84d) | 1% agar | 48 | 98 | 98 | 11 | |||
ASTER– | Psephellus erivanensis | 30/15 °C (42d) | 1% agar | 27 | 96 | 96 | 7 | |||
BEGONI– | Begonia salaziensis | 20 °C (119d) | 1% agar | 54 | 100 | 100 | 87 | |||
BEGONI– | Begonia serotina | 20 °C (70d) | 1% agar | 52 | 75 | 75 | 25 | |||
BRASSIC– | Brassica macrocarpa | 15 °C (56d) | 1% agar | 21 | 95 | 95 | 9 | |||
BRASSIC– | Cochlearia tatrae | 25/10 °C (42d) | 1% agar | 20 | 100 | 100 | 10 | |||
BRASSIC– | Erysimum contractum | 25/10 °C (301d) | 1% agar (98d); 1% agara (203d) | GA | 37 | 97 | 97 | 112 | ||
BRASSIC– | Phlebolobium maclovianum | 25/10 °C (112d) | 1% agar | 23 | 96 | 96 | 33 | |||
CACT– | Copiapoa cinerascens | 15 °C (47d) | 1% agar | 44 | 73 | 73 | 11 | |||
CACT– | Copiapoa fiedleriana | 20/10 °C (42d) | 1% agar | 18 | 78 | 89 | 14 | |||
CACT– | Copiapoa grandiflora | 15 °C (35d) | 1% agar | 20 | 100 | 100 | 14 | |||
CACT– | Copiapoa megarhiza | 15 °C (70d) | Moist sand | 46 | 100 | 100 | 20 | |||
CACT– | Eriosyce napina | 25 °C (43d) | 1% agar | 20 | 95 | 100 | 16 | |||
CACT– | Eriosyce occulta | 20 °C (28d) | 1% agar | 20 | 100 | 100 | 19 | |||
CACT– | Eriosyce simulans | 20 °C (14d) | 1% agar | 20 | 100 | 100 | 9 | |||
CACT– | Eriosyce sociabilis | 15 °C (21d) | 1% agar | 20 | 100 | 100 | 16 | |||
CACT– | Ferocactus flavovirens | 20 °C (42d) | 1% agar | 20 | 95 | 95 | 7 | |||
CACT– | Pilosocereus quadricentralis | 30 °C (35d) | 1% agar | 20 | 80 | 80 | 7 | |||
CALYCER– | Nastanthus falklandicus | 5 °C (227d); 0 °C (200d) | 1% agar | 52 | 98 | 98 | 302 | |||
CAMPANUL– | Campanula kachethica | 20 °C (35d) | 1% agar | 20 | 100 | 100 | 12 | |||
CAMPANUL– | Campanula sabatia | 15 °C (77d) | 1% agar | 25 | 92 | 92 | 13 | |||
CAMPANUL– | Musschia wollastonii | 20 °C (231d) | 1% agar | 52 | 81 | 81 | 84 | |||
CAMPANUL– | Wahlenbergia angustifolia | 25 °C (63d) | 1% agar | 16 | 100 | 100 | 15 | |||
CAPRIFOLI– | Centranthus trinervis | SURP | 15 °C (7d) | 1% agar | SUR | 20 | 100 | 100 | 7 | |
CARYOPHYLL– | Alsinidendron obovatum | 20 °C (21d) | 1% agar | 20 | 100 | 100 | 17 | |||
CARYOPHYLL– | Alsinidendron trinerve | 25/10 °C (28d) | 1% agar | 10 | 90 | 100 | 21 | |||
CARYOPHYLL– | Dianthus morisianus | 25/10 °C (28d) | 1% agar | 10 | 100 | 100 | 12 | |||
CARYOPHYLL– | Gypsophila robusta | 15 °C (77d) | 1% agar | 19 | 95 | 100 | 12 | |||
CARYOPHYLL– | Silene diclinis | 21/11 °C | 1% agar | 150 | 98 | 100 | ||||
CARYOPHYLL– | Silene hicesiae | 20 °C (14d) | 1% agar | 25 | 100 | 100 | 7 | |||
CRASSUL– | Aichryson dumosum | 20 °C (28d) | 1% agar | 59 | 93 | 93 | 8 | |||
CUPRESS– | Athrotaxis laxifolia | 20 °C (35d) | 1% agar | 10 | 90 | 90 | 8 | |||
CUPRESS– | Callitris oblonga | 15 °C (63d) | 1% agar | 13 | 100 | 100 | 28 | |||
CUPRESS– | Cupressus arizonica var. nevadensis | 20 °C (133d) | 1% agar | 21 | 95 | 95 | 57 | |||
CUPRESS– | Cupressus goveniana var. goveniana | 15 °C (70d) | 1% agar | 23 | 87 | 96 | 30 | |||
CUPRESS– | Cupressus macrocarpa | 20 °C (133d) | 1% agar | 17 | 88 | 88 | 16 | |||
CUPRESS– | Cupressus sargentii | 5 °C (126d) | 1% agar | 11 | 91 | 91 | 82 | |||
CUPRESS– | Juniperus bermudiana | 1. MECC; and 2. SURP | 20 °C (70d) | 1% agar | MEC + SUR | 20 | 70 | 70 | 24 | |
CUPRESS– | Pilgerodendron uviferum | 20 °C (35d) | 1% agar | 20 | 100 | 100 | 24 | |||
CUPRESS– | Widdringtonia whytei | 20 °C (77d) | 1% agar | 26 | 96 | 96 | 27 | |||
CYPER– | Carex bermudiana | 35/20 °C (56d) | 1% agar | 18 | 89 | 89 | 30 | |||
CYPER– | Nemum bulbostyloides | 30/15 °C (49d) | 1% agar | 20 | 85 | 85 | 21 | |||
DIOSCORE– | Dioscorea longicuspis | 25 °C (21d) | 1% agar | 10 | 100 | 100 | 15 | |||
DIOSCORE– | Dioscorea strydomiana | 15 °C (56d) | 1% agar | 13 | 85 | 85 | 20 | |||
EUPHORBI– | Euphorbia leistneri | SURP | 20 °C (147d) | 1% agar | SUR | 10 | 90 | 100 | 87 | |
EUPHORBI– | Euphorbia origanoides | 25 °C (100d) | moist sand | 21 | 100 | 100 | 20 | |||
FAB– | Acacia anegadensis | SC | 20 °C (7d) | 1% agar | SC | 20 | 100 | 100 | 3 | |
FAB– | Acacia repanda | SC | 15 °C (7d) | 1% agar | SC | 50 | 100 | 100 | 7 | |
FAB– | Ammopiptanthus nanus | SC | 20 °C (7d) | 1% agar | SC | 20 | 100 | 100 | 7 | |
FAB– | Astragalus maritimus | SC | 20 °C (7d) | 1% agar | SC | 20 | 100 | 100 | 7 | |
FAB– | Astragalus verrucosus | SC | 20 °C (7d) | 1% agar | SC | 10 | 100 | 100 | 7 | |
FAB– | Ceratonia oreothauma subsp. oreothauma | SC | 21 °C | 1% agar | SC | 20 | 100 | 100 | ||
FAB– | Dalbergia abrahamii | SC | 25 °C (7d) | 1% agar | SC | 20 | 100 | 100 | 7 | |
FAB– | Dalbergia lemurica | 25 °C (14d) | 1% agar | 13 | 77 | 77 | 11 | |||
FAB– | Dalbergia neoperrieri | SC | 25 °C (35d) | 1% agar | SC | 12 | 100 | 100 | 7 | |
FAB– | Dalbergia purpurascens | SC | 20 °C (21d) | 1% agar | SC | 20 | 100 | 100 | 8 | |
FAB– | Dalbergia suaresensis | SC | 25 °C (7d) | 1% agar | SC | 10 | 100 | 100 | 7 | |
FAB– | Dalbergia xerophila | SC | 25 °C (28d) | 1% agar | SC | 50 | 90 | 90 | 7 | |
FAB– | Dorycnium spectabile | SC | 25 °C (14d) | 1% agar | SC | 20 | 100 | 100 | 7 | |
FAB– | Lamprolobium grandiflorum | SC | 25 °C (14d) | 1% agar | SC | 20 | 95 | 95 | 7 | |
FAB– | Lamprolobium grandiflorum | SC | 20 °C (14d) | 1% agar | SC | 20 | 95 | 95 | 7 | |
FAB– | Millettia micans | SC | 25 °C (7d) | 1% agar | SC | 10 | 100 | 100 | 7 | |
FAB– | Millettia taolanaroensis | SC | 25 °C (7d) | 1% agar | SC | 10 | 100 | 100 | 7 | |
FAB– | Newtonia paucijuga | SC | 20 °C (14d) | 1% agar | SC | 10 | 100 | 100 | 12 | |
FAB– | Pericopsis elata | 25 °C (14d) | moist sand | 50 | 74 | 74 | 34 | |||
FAB– | Phaseolus lignosus | SC | 25 °C | 1% agar | SC | 20 | 100 | 100 | 7 | |
FAB– | Psoralea fascicularis | Day 35: SC | 20 °C (84d) | 1% agar | SC | 49 | 100 | 100 | 14 | |
FAB– | Pultenaea pinifolia | SC | 20 °C (7d) | 1% agar | SC | 10 | 100 | 100 | 7 | |
FAB– | Swainsona murrayana | SC | 25 °C (7d) | 1% agar | SC | 20 | 100 | 100 | 7 | |
FAB– | Swainsona murrayana | SC | 20 °C (7d) | 1% agar | SC | 20 | 100 | 100 | 7 | |
FAB– | Vicia esdraelonensis | Day 11: SC | 20 °C (26d) | 1% agar | SC | 10 | 100 | 100 | 7 | |
FAB– | Vicia hyaeniscyamus | SC | 20 °C (8d) | 1% agar | SC | 10 | 100 | 100 | 8 | |
FRANKENI– | Frankenia portulacifolia | 25 °C (28d) | 1% agar | 19 | 100 | 100 | 8 | |||
GESNERI– | Saintpaulia ionantha subsp. grandifolia | 25 °C (70d) | 1% agara | GA | 40 | 90 | 92 | 19 | ||
GESNERI– | Saintpaulia ionantha subsp. grotei | 25 °C (91d) | 1% agara | GA | 44 | 93 | 93 | 22 | ||
GESNERI– | Saintpaulia ionantha subsp. ionantha | 25 °C (105d) | 1% agara | GA | 50 | 84 | 88 | 32 | ||
GESNERI– | Saintpaulia ionantha subsp. rupicola | 25 °C (154d) | 1% agar (28d); 1% agara (126d) | GA | 32 | 72 | 72 | 25 | ||
LAMI– | Origanum cordifolium | 25/10 °C (35d) | 1% agar | 50 | 100 | 100 | 17 | |||
LAMI– | Origanum ehrenbergii | 25/15 °C (7d) | 1% agar | 20 | 100 | 100 | 7 | |||
LAMI– | Origanum ehrenbergii | 15 °C (7d) | 1% agar | 20 | 100 | 100 | 7 | |||
LAMI– | Origanum ehrenbergii | 20 °C (7d) | 1% agar | 20 | 100 | 100 | 7 | |||
LAMI– | Plectranthus unguentarius | 20 °C (42d) | 1% agar | 19 | 89 | 89 | 8 | |||
LAMI– | Salvia caymanensis | 25 °C (7d) | 1% agar | 21 | 100 | 100 | 7 | |||
LILI– | Lilium rhodopeum | 5 °C (315d) | 1% agar | 19 | 74 | 89 | 79 | |||
LIN– | Linum muelleri | 20 °C (56d) | 1% agar | 18 | 100 | 100 | 16 | |||
MALV– | Dombeya acutangula | SC | 25 °C (42d) | 1% agar | SC | 47 | 100 | 100 | 7 | |
MALV– | Hibiscus fragilis | 1. STR; and 2. SC | 25 °C (7d) | 1% agar | SC | 13 | 100 | 100 | 7 | |
MALV– | Paramelhania decaryana | SC | 25 °C (35d) | 1% agar | SC | 19 | 84 | 84 | 10 | |
MELI– | Cedrela odorata | 20 °C (28d) | 1% agar | 50 | 92 | 92 | 7 | |||
MELI– | Entandrophragma utile | 25 °C (48d) | 1% agar | 51 | 92 | 94 | 14 | |||
MELI– | Khaya senegalensis | MECC | 26 °C | 1% agar | 20 | 100 | 100 | |||
NOTHOFAG– | Nothofagus glauca | 10 °C (126d) | 1% agar | 25 | 88 | 100 | 76 | |||
ORCHID– | Ansellia africana | 0 h light:24 h dark: 25 °C (60d) | Phytamax | 36 | 100 | 100 | 60 | |||
ORCHID– | Paphiopedilum stonei | 0 h light:24 h dark: 25 °C (257d) | Knudson C | 155 | 81 | 100 | 257 | |||
OROBANCH– | Agalinis kingsii | 15 °C (35d) | 1% agar | 52 | 98 | 98 | 28 | |||
PAPAVER– | Papaver roseolum | 20 °C (63d); 25/10 °C (307d); 25/10 °C (267d) | 1% agara | GA | 50 | 92 | 98 | 238 | ||
PIN– | Abies fraseri | 5 °C (28d); 20 °C (42d) | 1% agar | CS | 14 | 93 | 100 | 14 | ||
PIN– | Picea breweriana | 20 °C (77d) | 1% agar | 50 | 90 | 90 | 18 | |||
PIN– | Picea omorika | 20 °C (7d) | 1% agar | 10 | 100 | 100 | 7 | |||
PIN– | Pinus caribaea var. bahamensis | 25 °C (28d) | 1% agar | 21 | 81 | 81 | 8 | |||
PLANTAGIN– | Antirrhinum charidemi | 16 °C | 1% agar | 18 | 100 | 100 | ||||
PLANTAGIN– | Isoplexis chalcantha | 15 °C (110d); 15 °C (23d) | 1% agar | 49 | 82 | 84 | 15 | |||
PLANTAGIN– | Isoplexis isabelliana | 15 °C (42d) | 1% agar | 19 | 84 | 95 | 15 | |||
PLANTAGIN– | Linaria tonzigii | 25/10 °C (56d); 5 °C (56d) 25/10 °C (133d) | 1% agar (168d); 1% agara (77d) | COMBS | GA | 47 | 98 | 100 | 19 | |
PLANTAGIN– | Plantago robusta | 20 °C (14d) | 1% agar | 53 | 98 | 98 | 7 | |||
PLANTAGIN– | Veronica micrantha | 20 °C (20d) | 1% agar | 48 | 100 | 100 | 7 | |||
PLUMBAGIN– | Armeria sampaioi | 15/5 °C (77d) | 1% agar | 50 | 74 | 74 | 15 | |||
PLUMBAGIN– | Armeria soleirolii | 20 °C (63d) | 1% agar | 19 | 100 | 100 | 16 | |||
PLUMBAGIN– | Limonium bahamense | 15 °C (7d) | 1% agar | 20 | 100 | 100 | 7 | |||
PLUMBAGIN– | Limonium bahamense | 20 °C (7d) | 1% agar | 20 | 100 | 100 | 7 | |||
PO– | Bromus bromoideus | 15 °C (7d) | 1% agar | 19 | 100 | 100 | 7 | |||
PO– | Bromus interruptus | 15 °C (7d) | 1% agar | 50 | 100 | 100 | 4 | |||
PO– | Eragrostis episcopulus | 25/10 °C (21d) | 1% agar | 20 | 100 | 100 | 16 | |||
PO– | Eragrostis saxatilis | 30 °C (28d) | 1% agar | 19 | 100 | 100 | 7 | |||
PO– | Phalaris maderensis | 20 °C (35d) | 1% agar | 50 | 98 | 98 | 8 | |||
PO– | Sporobolus caespitosus | 25 °C (7d) | 1% agar | 10 | 90 | 90 | 7 | |||
POLYGON– | Rumex rupestris | 25/10 °C (7d) | 1% agar | 10 | 100 | 100 | 7 | |||
PRIMUL– | Lysimachia minoricensis | 25/10 °C (21d) | 1% agar | 20 | 100 | 100 | 14 | |||
PRIMUL– | Primula boveana | 25 °C (42d) | 1% agar | 20 | 95 | 95 | 8 | |||
PROTE– | Leucadendron argenteum | 25/10 °C (84d) | 1% agar | 20 | 85 | 100 | 41 | |||
PROTE– | Leucadendron discolor | 25/10 °C (70d) | 1% agar | 48 | 96 | 100 | 36 | |||
PROTE– | Protea aurea subsp. potbergensis | 20 °C (14d) | 1% agar | 10 | 100 | 100 | 14 | |||
PROTE– | Protea lanceolata | 15 °C (21d) | 1% agar | 24 | 100 | 100 | 18 | |||
RANUNCUL– | Ranunculus kykkoensis | 15 °C (35d) | 1% agar | 26 | 88 | 88 | 29 | |||
RHAMN– | Emmenosperma pancherianum | 1. STR; and 2. SC | 25 °C (28d) | 1% agar | SC | 20 | 90 | 90 | 8 | |
ROS– | Prunus korshinskyi | Day 42: MECC | 5 °C (98d) | 1% agar (42d); moist sand (56d) | MEC | 10 | 90 | 100 | 18 | |
ROS– | Pyrus korshinskyi | Day 56: SURC | 5 °C (56d); 10 °C (7d) | 1% agar | CS | SUR | 10 | 90 | 90 | 6 |
ROS– | Sorbus anglica | Day 56: SURC | 5 °C (56d); 10 °C (7d) | 1% agar | CS | SUR | 13 | 92 | 100 | 7 |
ROS– | Sorbus bristoliensis | Day 54: SURC | 5 °C (54d); 10 °C (15d) | 1% agar | CS | SUR | 53 | 89 | 89 | 15 |
ROS– | Sorbus eminens | 5 °C (56d); 10 °C (70d) | 1% agar | CS | 11 | 82 | 91 | 27 | ||
ROS– | Sorbus leptophylla | Day 56: SURC | 5 °C (56d); 10 °C (21d) | 1% agar | CS | SU | 25 | 100 | 100 | 15 |
ROS– | Sorbus wilmottiana | Day 56: SURC | 5 °C (56d); 10 °C (14d) | 1% agar | CS | SU | 10 | 90 | 90 | 14 |
RUBI– | Mussaenda monticola var. monticola | 25 °C (126d) | 1% agar | 19 | 84 | 84 | 74 | |||
SCROPHULARI– | Cromidon pusillum | 20 °C (42d); 5 °C (63d) | 1% agar | WS | 18 | 89 | 100 | 32 | ||
XANTHORRHOE– | Aloe erinacea | 25 °C (35d) | 1% agar | 19 | 100 | 100 | 7 | |||
XANTHORRHOE– | Aloe helenae | 25 °C (21d) | 1% agar | 24 | 100 | 100 | 14 | |||
XANTHORRHOE– | Aloe peglerae | 20 °C (42d) | 1% agar | 18 | 83 | 83 | 8 | |||
XANTHORRHOE– | Aloe pillansii | 20 °C (4d) | 1% agar | 20 | 100 | 100 | 4 | |||
XANTHORRHOE– | Aloe ramosissima | 20 °C (56d) | 1% agar | 17 | 94 | 94 | 7 | |||
XANTHORRHOE– | Aloe suzannae | 25 °C (42d) | 1% agar | 20 | 95 | 95 | 8 | |||
ZYGOPHYLL– | Guaiacum officinale | 30/20 °C (49d) | 1% agar | 50 | 96 | 96 | 14 | |||
ZYGOPHYLL– | Guaiacum sanctum | 30/20°Cs (28d) | 1% agar | 25 | 100 | 100 | 17 | |||
Treatment: MEC: mechanical manipulation of seeds using a scalpel or by hand and C for when covering structure, not the seed coat, was removed; SC: scarification of seeds by removing the seed coat partially with a scalpel; SUR: surgical method by removing the seed coat with a scalpel and C, for completely and P, for partially; STR: sterilisation of seeds by immersing them in 10% bleach for 5 min; and REH: rehydration of seeds by suspending them over water at 100% relative humidity at 20 °C for one day; GA: gibberellic acid in germination medium.
CS cold stratification, COMBS combined stratification, WS warm stratification.
aMedium: agar: 250 mg/l gibberellic acid in agar.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, U., Cossu, T.A., Davies, R.M. et al. Conserving orthodox seeds of globally threatened plants ex situ in the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK: the status of seed collections. Biodivers Conserv 29, 2901–2949 (2020). https://doi.org/10.1007/s10531-020-02005-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-02005-6