Abstract
Sown, temporary field margins are a common agri-environment scheme (AES) in the Netherlands. Despite their wide application, though, there has been scarcely any long-term monitoring of the succession of invertebrates. In the field margins of 40 farms, invertebrate diversity and the abundance of three functional groups were assessed in relation to age. The diversity in terms of number of species groups was found to increase with the age of the margins. The abundance of herbivores and detritivores also showed a positive correlation with the age of the margins. However, the abundance of predators decreased with increasing age. Older margins showed a higher total vegetation cover and fewer plant species, also resulting in lower plant species evenness. We suggest several changes to the current AES regulations. For the conservation of invertebrate diversity, longer-lasting field margins are desirable. In addition, old margins are favoured by detritivores, a group that has particular difficulty finding suitable habitats in agricultural landscapes. However, such margins are less favourable from an agricultural perspective, as they appear unsuitable for high abundances of potentially useful predators and the high vegetation cover attracts many potentially harmful herbivores. To circumvent this, the AES might be extended by incorporating hay-making, which would reduce standing biomass and might lead to more predators and fewer herbivores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural landscapes in Western Europe are suffering a severe biodiversity crisis, mainly as a result of land-use intensification (Stoate et al. 2001; Robinson and Sutherland 2002; Gregory et al. 2004; Smart et al. 2006). Species richness in these landscapes is markedly improved by the presence of semi-natural landscape elements and by management of the productive fields themselves (Duelli and Obrist 2003; Gibson et al. 2007; Drapela et al. 2008). Incorporation of semi-natural habitats on arable land and adoption of agri-environmental management are therefore seen as useful ways to promote biodiversity (e.g., Whittingham 2007). Such practises are often encouraged by mandatory schemes that are subsidised by national and regional governments: agri-environment schemes (AES). Common options in current schemes include creation and management of all kinds of semi-natural areas. Frequently established semi-natural areas on arable lands are field margin habitats (e.g., De Snoo 1999; Marshall and Moonen 2002). These margins can be beneficial to biodiversity in several ways: they serve as refuge habitats for species unable to persist in intensively managed arable fields or in the declining acreage of natural habitat (Vickery et al. 2002; Marshall et al. 2006; Carvell et al. 2007; Smith et al. 2008a), provide overwintering sites for a variety of small animals (e.g., Thomas et al. 1992; Dennis et al. 1994) and may act as ecological corridors (e.g., Kohler et al. 2008).
It is not only from a conservation perspective that biodiversity in arable field margins is desirable. Because biodiversity is often positively correlated with the provision of ecosystem services (Chapin et al. 2000), it might also be beneficial to farmers (Kremen and Chaplin-Kramer 2007). Arable field margins with perennial vegetation can provide stable overwintering sites and alternative food sources for pollinators and natural enemies of pest organism (Tylianakis et al. 2004; Schmidt and Tscharntke 2005). A permanently vegetated strip can reduce erosion of the field edges and the amount of pesticides and manure drift to adjacent ditches (De Snoo and De Wit 1998). In addition, the presence of semi-natural habitats and wildlife makes the agricultural landscape more attractive, thereby possibly enhancing recreational use of the region and stimulating the local economy (Marshall and Moonen 2002).
In the Netherlands the creation of sown field margins, known as ‘fauna margins’, is a common form of subsidised AES. It is assumed that these margins provide habitat for animals in the broad sense, i.e., for birds, small mammals and invertebrates. Due to the manner in which the scheme is regulated, they are commonly installed for a period of 6 years only. As AES may not always be effective in promoting biodiversity (Kleijn et al. 2001, 2006; Kohler et al. 2007; Blomqvist et al. 2009) and often cost a considerable amount of money, it is of great importance to assess the contribution of these margins to biodiversity. Invertebrates, being a species-rich and diverse group of small animals, seem to be especially fit to use as focus group for studying the biodiversity of small landscape elements like fauna margins.
The age of such margins might be expected to be a leading factor in invertebrate occurrence, with older margins having a greater chance of invertebrate colonisation (Corbet 1995). However, only a limited number of papers have been published on the development of invertebrate communities in field margins after initial establishment (more papers have been published on plant succession, e.g., Kleijn et al. 1998; Critchley et al. 2006; Manhoudt et al. 2007; Musters et al. 2009). Most of them found in increase with age of the margins (Denys and Tscharntke 2002; Olson and Wäckers 2007; Frank and Reichhart 2004; Woodcock et al. 2008; Musters et al. 2009), although Woodcock et al. (2008) found predatory beetles to peak in the second year after establishment and to decrease in 2 year thereafter. However, none of these studies deal with a broad range of invertebrate groups and only Musters et al. (2009) and Denys and Tscharntke (2002) discuss patterns over a considerable period of time. To gain more insight into the development of invertebrate groups in field margins, and especially the patterns for distinct functional groups, we performed an inventory on their diversity and abundance in a large number of these margins in the province of Zeeland, the Netherlands. We formulated two research objectives: (1) How does the number of invertebrate taxa in these strips relate to the age of the margin? (2) How is the abundance of three functional feeding groups—predators, herbivores and detritivores—related to the age of the margin? From the literature cited above, we expected that the field margins would become more species rich with age and that invertebrates would become more abundant. The second question is of major importance, as two of these functional groups may have a direct impact on farming practice: predators that function as enemies of pest organisms and herbivores that might be damaging to crops. It is however possible that the two groups affect each other, resulting in unexpected changes in abundance (Corbet 1995).
Methods
Field sites and AES regulations
All field margins were in the province of Zeeland, in the south-westernmost part of the Netherlands (3°10′–4°20′ eastern longitude and 51°10′–52°00′ northern latitude; Fig. 1). This province is made up by five areas of land in the marine clay district separated by strands of the Scheldt River estuary. By selecting farms only in this province, we aimed to minimise the influence of differences in soil or landscape context. For our study we selected 40 arable farms with sown field margins. On most farms two margins were chosen, resulting in 2006 in 64 and in 2007 in 69 margins that were inventoried. These margins were always on the edge of the arable land, often adjacent to a ditch.
All the selected farms had contracts under the AES ‘fauna margin’ scheme and all the farmers were participating in local agri-environmental farmer collectives. Under this particular scheme, farmers are under a contractual obligation to establish an arable field margin at least 6 m wide and 50 m long and maintain it for at least 6 years. However, some farmers had implemented this scheme on an already existing margin. Others did not change their management of the margin after 6 years. All of these margins were not fertilized and not treated with pesticides for a long time. This provided us with a broader range in margin ages; from first-season margins (referred to in this paper as ‘age 1’) to margins in their eleventh season (see Table 2 for the number of samples per age class). The margins were sown either with a flower mixture (98 margins, comprising indigenous species, exotics and cultivars, e.g., Cichorium intybus, Chrysanthemum segetum, Centaurea cyanus, Helianthus annuus, Leucanthemeum vulgare, Malva spp., Papaver spp., Phacelia tanacetifolia, Silene spp., Trifolium spp., Sinapis alba and Tripleurospermum maritimum), or with a grass mixture (35 margins, consisting predominantly of Festuca arundinacea, Poa pratensis, Dactylis glomerata and Phleum pratense). One mowing event per year is regularly done, but the removal of cuttings is not required and consequentially almost never done. The application of manure or pesticides on the margin is prohibited, but targeted local removal of Rumex obtusifolius and Cirsium arvense with herbicides is allowed.
Invertebrate sampling and counting
To collect ground-dwelling invertebrates we used pitfall traps. In the middle of each margin and at least 10 m from field corners or disturbances such as tyre tracks, four pitfall traps were installed spaced 10 m apart. These traps had a diameter of 11 cm, were 7 cm deep and were partly filled with a 1:1 mixture of water and ethylene glycol. A plastic cover was placed above each trap to keep out rainwater. In both years, all the traps were operational for exactly 7 days at the end of June and the beginning of July (weeks 26–27), just before the mowing takes place (Noordijk et al. 2010).
From the pitfall trap samples, the individuals of following invertebrates groups were counted: Gastropoda, Opiliones, Araneae, Acarina, Lepidoptera larvae, Chilopoda, Diplopoda, Isopoda, Collembola, Staphylinidae, Coccinellidae including their larvae, Carabidae, Curculionidae, other Coleoptera, Coleoptera larvae, Cicadellidae, Heteroptera, Aphidoidea, Diptera, Formicidae, other Hymenoptera and Orthoptera. The catches from the four pitfall traps from each fauna margin were bulked and treated as a single sample. The number of groups were used as a measure for species richness. The number of individuals of Chilopoda, Araneae, Coccinellidae including larvae, carnivores Carabidae, and Staphylinidae were taken as a measure for the abundance of predators, the number of individuals of Isopoda, Diplopoda, and Collembola for the abundance of detritivores, and the number of individuals of Gastropoda, Curculionidae, Orthoptera, Cicadellidae, Heteroptera, and Aphidoidea for the abundance of herbivores.
Field margin variables
Apart from the age of the individual margins, several characteristics that might influence invertebrate community composition were measured: margin width, the seed mixture applied (grass or flower mixture) and soil nitrogen content. The last of these was characterised by determining the total nitrogen concentration of a bulked representative soil sample taken from a depth of 10 cm at five sites close to the individual pitfall traps. In addition, we measured several vegetation characteristics at the sites where invertebrate sampling was carried out. Vegetation height was measured in the winter (February) preceding invertebrate sampling and in summer at the time of sampling. This measurement was performed at five points 10 m apart by lowering a 30 cm diameter, 200 g vinyl drop disc from 2 m over a wooden rule. This method is well suited for medium to tall swards (Stewart et al. 2001). The vegetation cover was estimated in winter as well as summer. In summer, the botanical composition of the vegetation on the margin was measured in 1 by 25 m recordings. Three of the four pitfalls were along the middle axes of these recordings. Species occurrence was noted and abundance estimated using an adapted Braun-Blanquet method (Barkman et al. 1964). The total number of plant species, their evenness (obtained by dividing the Shannon index, based on estimated abundances, by the natural logarithm of the total number of species) and the number of non-sown species were incorporated in the analyses.
Analysis
The two research questions required a different approach and use of invertebrate catches. For research question 1, the total number of the aforementioned taxa were noted from the pitfall trap catches and used to analyse the richness in the fauna margins at the level of species groups. For research question 2, after summing the individuals of several taxa to analyse activity-density trends (from now on called abundance) for three functional groups: ‘predators’, ‘herbivores’ and ‘detritivores’.
Initially, stepwise multiple regressions were performed to identify the variables significantly affecting richness and functional group abundances.
Secondly, we used Hierarchical Generalised Linear Models (HGLM), a generalized mixed model procedure of GenStat 12.0, to calculate the relationship between age of the field margin and richness and functional group abundances, given the fact that we chose certain farms and years for sampling (Royle and Dorazio 2008). In our models, age of the margin and the significant variables of the first analyses were the fixed factors. Because we sampled usually two field margins per farm over 2 years, farm and year of sampling were included as random factors. All abundance measures were logarithmically transformed to get a normal distribution. However, since we did not know whether the relationship between the response variables and age was linear, we used the same models, but now with age as an ordinal factor, to estimate the means of the response variables per age category. After the transformation of the abundances, we could use the identity link function both for the fixed and the random part of the model in all cases. In case of the abundance of the detritivores, we had to regard the first and second year as one category in order to get our model converge, probably due to low detritivores abundance in the first year. In all models a constant term was estimated. The Wald test for testing the change in likelihood between the full model and the reduced model when taking out a variable was used for testing the significance of the fixed variables.
Furthermore, the correlations between the age and several site-specific variables of the margins were analysed using linear regressions and Spearman’s rank correlation tests.
Results
Taxonomic richness
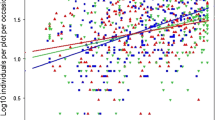
The age of the field margin was found to significantly affect the number of taxa in the field margins. The number of taxa differed significantly between years of age (Table 1A) and a clear positive relationship was found between age of the field margin and number of taxa (Table 1B; Fig. 2).
Mean number of taxonomic invertebrate groups (±SE) per age of field margin category. Estimated means and standard errors are based on the HGLM model with age as categorical variable. Trend is based on the same model with age as scale variable. Trend is significantly different from zero (Table 1B)
Abundance of functional groups
In total, 34,038 predator, 11,305 herbivore and 10,720 detritivore individuals were caught with the pitfall traps. Predator abundance was significantly affected by the age category of the field margin (Table 1A); the abundance of predators decreased with increasing age of the margin (Table 1B; Fig. 3). Herbivore abundance was significantly related to vegetation cover in summer, margin width and age category (Table 1A). A positive relationship with the age of the margin was found (Table 1B; Fig. 3). Detritivore abundance was not affected by age category (Table 1A), but a clear positive correlation between age of the margin and detritivore abundance was found (Table 1B; Fig. 3).
Mean number of individuals of predators, herbivores and detritivores (±SE) per age of field margin category. Estimated means and standard errors are based on the HGLM model of the Ln-transformed abundance data after correcting for other significant factors and with age as categorical variable. Trends are based on the same model with age as scale variable. All trends are significantly different from zero (Table 1B)
Field margin variables
Several site-specific variables showed significant relationships with the age of field margins (Table 2): we found a decrease in the number of plant species (t = −5.585, P < 0.001) and in their evenness (t = −2.651, P < 0.001), the latter indicating that the vegetation is moving towards dominance by certain species. The vegetation cover in summer increased (R = 0.521, P < 0.001). No trends could be detected for nutrient richness, vegetation height in summer and winter, and vegetation cover in winter.
Discussion
Invertebrate richness and abundances
Our results show that the richness of species groups increased with increasing age of the field margins and that this trend was consistent during the first 11 years. This represents an important finding, indicating the conservation value of long-lasting semi-natural elements in agricultural areas. To our knowledge, this is the first time that such a pattern has been described for field margins for a broad range of invertebrates and over a considerable period of time. It is not surprising that there is a slow but steady increase in richness, because the small margins have to be colonised by small invertebrates moving through a hostile environment (Steffan-Dewenter and Tscharntke 1999; Öckinger and Smith 2007; Kohler et al. 2008), and similar patterns of increasing diversity have been described for other habitats (Mook 1971; Judd and Mason 1995; Desender et al. 2006; Cameron and Bayne 2009). Increasing functional diversity in species communities will lead to a greater variety of ecosystem processes (Naeem et al. 1994; Tilman et al. 1996; Heemsbergen et al. 2004) and with time, therefore, margins left on their own may develop towards more natural ecosystems.
Predators form an important aspect of our study, as some of these invertebrates are beneficial to farmers because of their potential as pest control (Carter and Rypstra 1995; Obrycki and Kring 1998; Collins et al. 2002). Predator abundance decreased with progressing age of the margins (in contrast to Denys and Tscharntke 2002, but in line with Woodcock et al. 2008), due probably to the vegetation developing from a recently sown, open situation to higher standing biomass and a denser sward, although in our analyses this development was only expressed by a significant effect of age (Noordijk et al. 2010). Ground-dwelling predatory invertebrates often depend on open, sun-lit places where they can easily move to find prey (Harvey et al. 2008). Those species potentially invading the arable fields have a particular preference for the open vegetation in the margins, as this is quite similar to conditions in the fields themselves (Samu and Szinetar 2002). Consequently, young margins appear to provide the best conditions for providing pest-control services. On the other hand, it has been shown that high vegetation cover in winter provides most opportunities for predators to hide during this period (e.g., Dennis et al. 1994; Collins et al. 2003).
We found herbivore abundance to be favoured by the width of the margin, but most significantly by the age of field margin and vegetation cover in summer (see also Meek et al. 2002; Harvey et al. 2008). This latter relationship can be explained by more plant biomass being available to provide food for more individuals (e.g., McFarlin et al. 2008) and more shelter against predators, which appear to be less abundant in these situations anyway (this study; Harvey et al. 2008). More than half the herbivores counted were Gastropoda, but Cicadellidae and Aphidoidea were also caught in high numbers. All these groups include polyphagous species, which may be damaging to crops and thus result in economic loss to farmers (Glen and Moens 2002; Nickel 2003; Van Emden and Harrington 2007).
The abundance of detritivores increased with the age of the margins. This is not surprising, given the build-up of a substantial surface litter layer (especially because no cuttings were removed from the margins after mowing, Noordijk et al. 2010), on which these animals depend for food (Smith et al. 2008a). A well-developed detritivore assemblage is essential for decomposition and enhancement of soil structure (Ekschmitt and Griffiths 1998), thus promoting healthier soils. In addition, this invertebrate group in particular represents species unable to persist in arable fields, as a litter layer is generally absent there (Smith et al. 2008b). Old field margins with high standing biomass will therefore represent true refuge habitats for these invertebrates.
One should bear in mind that vegetation structure and/or density at ground level might affect the activity-density of invertebrates and therefore pitfall trap catches (Greenslade 1964; Thomas et al. 2006), implying certain limitations on interpretation of our results. Moreover, different species groups may have very different activity patterns that could be affected differently by vegetation, for example, Gastropods versus Carabids. And our pitfalls were only open during 1 week each year, making the catches potentially vulnerable to uncommon weather conditions. However, we think that this will have hardly any effect on our richness analyses, as these are based only on the presence of a particular group, and not on its abundance. If it did have any effect, the already significant trend would likely be stronger, since there may especially be undersampling in the older margins with denser vegetation. For predator abundances, though, caution may be in order. On the other hand, the increasing abundance of herbivores with increasing vegetation cover might have been underestimated, so our recommendations concerning management of these margins for agricultural benefits (see below) therefore remain sound and grounded in empirical findings.
Pitfalls do not catch all invertebrates (Thomas and Marshall 1999). Flying insects, for example, are missed and of these many are also predators or parasitoids that may be beneficial to farmers. Therefore, our results cannot be generalised to all predators, herbivores or detritivores that occur in field margins.
Management recommendations
We recommend creating field margins for ‘as long as possible’ at the same location, as this will increase the number of taxonomic groups present in these structures, thereby promoting a variety of functions, leading to healthy ecological systems (Brussaard et al. 2007). In contrast, most agri-environmental schemes last only for a limited number of years (Kleijn et al. 2006), a situation that needs to be changed if better conservation results are to be achieved. However, old margins where no plant biomass is removed provide habitat for many herbivores and may also lead to less suitable situations for predators. To benefit farmers, then, these margins need to be managed differently. Since scarification, in particular, can be detrimental to many soil and ground-dwelling organisms (Smith et al. 2008b), re-establishing margins will not be the best option. An alternative is to introduce a hay-making management regime, with the vegetation being cut once a year, for example (Hovd and Skogen 2005; De Cauwer et al. 2005; Manhoudt et al. 2007). Margins can then still be established to last for a long time, but with plant biomass now being removed and vegetation succession set-back, thus providing less suitable conditions for high herbivore abundances while probably promoting predators. In addition, margins managed for hay-making will have fewer noxious weeds (De Cauwer et al. 2008), but greater plant diversity (Schaffers 2002; Musters et al. 2009; Blomqvist et al. 2009), which might in turn permit higher invertebrate diversity (Thomas and Marshall 1999; Asteraki et al. 2004) and more flower-visiting insects (Noordijk et al. 2009). The actual effect of hay-making on invertebrate species richness in arable field margins needs further study. As the possibilities for overwintering invertebrates increases with vegetation cover in winter, in the case of a hay-making management regime we recommend mowing the margins not too late in autumn (and preferably in late summer), permitting a certain amount of subsequent re-growth and thus providing sufficient overwintering opportunities.
References
Asteraki EJ, Hart BJ, Ings TC, Manley WJ (2004) Factors influencing the plant and invertebrate diversity of arable field margins. Agric Ecosyst Environ 202:219–231
Barkman JJ, Doing H, Segal S (1964) Kritische bemerkungen und vorschläge zur quantitativen Vegetationsanalyse. Acta Bot Neerl 13:394–419
Blomqvist MM, Tamis WLM, De Snoo GR (2009) No improvement of plant biodiversity in ditch banks after a decade of agri-environment schemes. Basic Appl Ecol 10:268–278
Brussaard L, De Ruiter PC, Brown GG (2007) Soil biodiversity for agricultural sustainability. Agric Ecosyst Environ 121:233–244
Cameron EK, Bayne EM (2009) Road age and its importance in earthworm invasion of northern boreal forests. J Appl Ecol 46:28–36
Carter PE, Rypstra AL (1995) Top-down effects in soybean agroecosystems: spider density affects herbivore damage. Oikos 72:433–439
Carvell C, Meek WR, Pywell RF, Goulson D, Nowakowski M (2007) Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. J Appl Ecol 44:29–44
Chapin FS, Zavaletta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Diaz S (2000) Consequences of changing biodiversity. Nature 405:234–242
Collins KL, Boatman ND, Wilcox A, Holland JM, Chaney K (2002) Influence of beetle banks on cereal aphid predation in winter wheat. Agric Ecosyst Environ 93:337–350
Collins KL, Boatman ND, Wilcox A, Holland JM (2003) Effects of different grass treatments used to create overwintering habitat for predatory arthropods on arable farmland. Agric Ecosyst Environ 96:59–67
Corbet SA (1995) Insects, plants and succession: advantages of long-term set-aside. Agric Ecosyst Environ 53:201–217
Critchley CNR, Fowbert JA, Sherwood AJ (2006) The effects of annual cultivation on plant community composition of uncropped arable field boundary strips. Agric Ecosyst Environ 113:196–205
De Cauwer B, Reheul D, D’hooghe K, Nijs I, Milbau A (2005) Evolution of the vegetation of mown field margins over their first 3 years. Agric Ecosyst Environ 109:87–96
De Cauwer B, Reheul D, Nijs I, Milbau A (2008) Management of newly established field margins on nutrient-rich soil to reduce weed spread and seed rain into adjacent crops. Weed Res 48:102–112
De Snoo GR (1999) Unsprayed field margins: effects on environment, biodiversity and agricultural practice. Landsc Urban Plan 46:151–160
De Snoo GR, De Wit PJ (1998) Buffer zones for reducing pesticide drift to ditches and risks to aquatic organisms. Ecotoxicol Environ Saf 41:112–118
Dennis P, Thomas MB, Sotherton NW (1994) Structural features of field boundaries which influence the overwintering densities of beneficial arthropod predators. J Appl Ecol 31:361–370
Denys C, Tscharntke T (2002) Plant-insect communities and predator-prey ratios in field margin strips, adjacent crop fields, and fallows. Oecologia 130:315–324
Desender K, Baert L, Maelfait J-P (2006) Evaluation of recent nature development measures in the river IJzer estuary and long-term ground beetle and spider monitoring (Coleoptera, Carabidae: Araneida). Bull Inst R Sci Nat Belg Entomol 76:103–122
Drapela T, Moser D, Zaller JG, Frank T (2008) Spider assemblages in winter oilseed rape affected by landscape and site factors. Ecography 31:254–262
Duelli P, Obrist MK (2003) Regional biodiversity in an agricultural landscape: the contribution of seminatural habitat islands. Basic Appl Ecol 4:129–138
Ekschmitt K, Griffiths BS (1998) Soil biodiversity and its implications for ecosystem functioning in a heterogeneous and variable environment. Appl Soil Ecol 10:201–215
Frank T, Reichhart B (2004) Staphylinidae and Carabidae overwintering in wheat and sown wildflower areas of different age. Bull Entomol Res 94:209–217
Gibson RH, Pearce S, Morris RJ, Symondson WOC, Memmott J (2007) Plant diversity and land use under organic and conventional agriculture: a whole-farm approach. J Appl Ecol 44:792–803
Glen DM, Moens R (2002) Agriolimacidae, Arionidae and Milacidae as pests in west European cereals. In: Barker GM (ed) Molluscs as crop pests. CABI, Wallingford, pp 271–300
Greenslade PJM (1964) Pitfall trapping as a method for studying populations of Carabidae (Coleoptera). J Anim Ecol 33:301–310
Gregory RD, Noble DG, Custance J (2004) The state of play of farmland birds: population trends and conservation status of lowland farmland birds in the United Kingdom. Ibis 146:1–13
Harvey JA, Van der Putten WH, Turin H, Wagenaar R, Bezemer TM (2008) Effects of changes in plant species richness and community traits on carabid assemblages and feeding guilds. Agric Ecosyst Environ 127:100–106
Heemsbergen DA, Berg MP, Loreau M, Van Hal JR, Faber JH, Verhoef HA (2004) Biodiversity effects on soil processes explained by interspecific functional dissimilarity. Science 306:1019–1020
Hovd H, Skogen A (2005) Plant species in arable field margins and road verges of central Norway. Agric Ecosyst Environ 110:257–265
Judd KW, Mason CF (1995) Colonization of a restored landfill site by invertebrates, with particular reference to the Coleoptera. Pedobiologia 39:116–125
Kleijn D, Joenje W, Le Coeur D, Marshall EJP (1998) Similarities in vegetation development of newly established herbaceous strips along contrasting European field boundaries. Agric Ecosyst Environ 68:13–26
Kleijn D, Berendse F, Smit R, Gilissen N (2001) Agri-environment schemes do not effectively protect biodiversity in Dutch agricultural landscapes. Nature 413:723–725
Kleijn D, Baquero RA, Clough Y, Díaz M, De Esteban J, Fernández F, Gabriel D, Herzog F, Holzschuh A, Jöhl R, Knop E, Kruess A, Marshall EJP, Steffan-Dewenter I, Tscharntke T, Verhulst J, West TM, Yela JL (2006) Mixed biodiversity benefits of agri-environment schemes in five European countries. Ecol Lett 9:243–254
Kohler F, Verhulst J, Knop E, Herzog F, Kleijn D (2007) Indirect effects of grassland extensification schemes on pollinators in two contrasting European countries. Biol Conserv 135:302–307
Kohler F, Verhulst J, Van Klink R, Kleijn D (2008) At what spatial scale do high-quality habitats enhance the diversity of forbs and pollinators in intensively farmed landscapes? J Appl Ecol 45:753–762
Kremen C, Chaplin-Kramer R (2007) Insects as providers of ecosystem services: crop pollination and pest control. In: Stewart EJE, New TR, Lewis OT (eds) Insect conservation biology. CABI, Wallingford, pp 349–404
Manhoudt AGE, Visser AJ, De Snoo GR (2007) Management regimes and farming practices enhancing plant species richness on ditch banks. Agric Ecosyst Environ 119:353–358
Marshall EJP, Moonen AC (2002) Field margins in northern Europe: their functions and interactions with agriculture. Agric Ecosyst Environ 89:5–21
Marshall EJP, West TM, Kleijn D (2006) Impacts of an agri-environment field margin prescription on the flora and fauna of arable farmland in different landscapes. Agric Ecosyst Environ 113:36–44
McFarlin CR, Brewer JS, Buck TL, Pennings SC (2008) Impact of fertilization on a salt marsh food web in Georgia. Estuar Coasts 31:313–325
Meek B, Loxton D, Sparks T, Pywell R, Pickett H, Nowakowski M (2002) The effect of arable field margin composition on invertebrate biodiversity. Biol Conserv 106:259–271
Mook JH (1971) Observations on the colonization of the new IJselmeer-polders by animals. Miscellaneous Papers Landbouwhogeschool Wageningen 8:13–31
Musters CJM, Van Alebeek F, Geers RHEM, Korevaar H, Visser A, De Snoo GR (2009) Development of biodiversity in field margins recently taken out of production and adjacent ditch banks in arable areas. Agric Ecosyst Environ 129:131–139
Naeem S, Thompson LJ, Lawler SP, Lawton JH, Woodfin RM (1994) Declining biodiversity can alter the performance of ecosystems. Nature 368:734–737
Nickel H (2003) The leafhoppers and planthoppers of Germany (Hemiptera, Auchenorrhyncha). Patterns and strategies in a highly diverse group of phytophagous insects. Pensoft Series Faunistica 28. Pensoft Publishers, Sofia-Moscow
Noordijk J, Delille K, Schaffers AP, Sýkora KV (2009) Optimizing grassland management in roadside verges for flower-visiting insects. Biol Conserv 142:2095–2103
Noordijk J, Musters CJM, Van Dijk J, De Snoo GR (2010) Vegetation development in sown field margins and on adjacent ditch banks. Plant Ecol. doi:10.1007/s11258-010-9811-0
Obrycki JJ, Kring TJ (1998) Predaceous Coccinellidae in biological control. Annu Rev Entomol 43:295–321
Öckinger E, Smith HG (2007) Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J Appl Ecol 44:50–59
Olson DM, Wäckers FL (2007) Management of field margins to maximize multiple ecological services. J Appl Ecol 44:13–21
Robinson RA, Sutherland WJ (2002) Post-war changes in arable farming and biodiversity in Great-Britain. J Appl Ecol 39:157–176
Royle JA, Dorazio RM (2008) Hierarchical modelling and inference in ecology. Academic Press, London
Samu F, Szinetar C (2002) On the nature of agrobiont spiders. J Arachnol 30:389–402
Schaffers AP (2002) Soil, biomass, and management of semi-natural vegetation. II. Factors controlling species diversity. Plant Ecol 158:247–268
Schmidt MH, Tscharntke T (2005) The role of perennial habitats for Central European farmland spiders. Agric Ecosyst Environ 105:235–242
Smart SM, Marrs RH, Le Duc MG, Thompson K, Bunce RGH, Firbank LG, Rossall MJ (2006) Spatial relationships between intensive land cover and residual plant species diversity in temperate farmed landscapes. J Appl Ecol 43:1128–1137
Smith J, Potts S, Eggleton P (2008a) The value of sown grass margins for enhancing soil macrofaunal biodiversity in arable systems. Agric Ecosyst Environ 127:119–125
Smith J, Potts SG, Woodcock BA, Eggleton P (2008b) Can arable field margins be managed to enhance their biodiversity, conservation and functional value for soil macrofauna? J Appl Ecol 45:269–278
Steffan-Dewenter I, Tscharntke T (1999) Effects of habitat isolation on pollinator communities and seed set. Oecologia 121:432–440
Stewart KEJ, Bourn NAD, Thomas JA (2001) An evaluation of the three quick methods commonly used to assess sward height in ecology. J Appl Ecol 38:1148–1154
Stoate C, Boatman ND, Borralho RJ, Carvalho CR, De Snoo GR, Eden P (2001) Ecological impacts of arable intensification in Europe. J Environ Manag 63:337–365
Thomas CFG, Marshall EJP (1999) Arthropod abundance and diversity in differently vegetated margins of arable fields. Agric Ecosyst Environ 72:131–144
Thomas MB, Sotherton NW, Coombes DS, Wratten SD (1992) Habitat factors influencing the distribution of polyphagous predatory insects between field boundaries. Ann Appl Biol 120:197–202
Thomas CFG, Brown NJ, Kendall DA (2006) Carabid movement and vegetation density: implications for interpreting pitfall trap data from split-field trials. Agric Ecosyst Environ 113:51–61
Tilman D, Wedin D, Knops J (1996) Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379:718–720
Tylianakis JM, Didham RK, Wratten SD (2004) Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 85:658–666
Van Emden HF, Harrington R (eds) (2007) Aphids as crop pests. CABI, Wallingford
Vickery J, Carter N, Fuller RJ (2002) The potential value of managed cereal field margins as foraging habitats for farmland birds in the UK. Agric Ecosyst Environ 89:41–52
Whittingham MJ (2007) Will agri-environment schemes deliver substantial biodiversity gain, and if not why not? J Appl Ecol 44:1–5
Woodcock BA, Westbury DB, Tscheulin T, Harrison-Cripps J, Harris SJ, Ramsey AJ, Brown VK, Potts SG (2008) Effects of seed mixture and management on beetle assemblages of arable field margins. Agric Ecosyst Environ 125:246–254
Acknowledgements
We are indebted to E. Gertenaar and R. van der Poll for assistance during the fieldwork and invertebrate counting and to A.M. Lokhorst and H. Staats for input in the study design. In addition, we would like to thank all the representatives of the participating farmer collectives and all the individual farmers for their efforts in contributing to this research and allowing us to perform the field sampling. We are also grateful to N. Harle for his correction of the English. This study was financially supported by the Netherlands Organization for Scientific Research (NWO), Grant No. 474-03-385.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Noordijk, J., Musters, C.J.M., van Dijk, J. et al. Invertebrates in field margins: taxonomic group diversity and functional group abundance in relation to age. Biodivers Conserv 19, 3255–3268 (2010). https://doi.org/10.1007/s10531-010-9890-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-010-9890-1