Abstract
While conducting a landscape genomics study of invasive tammar wallabies (Notamacropus eugenii) in Aotearoa New Zealand we discovered that parma wallabies (N. parma) are also present in the North Island. This population has gone undetected for at least 30 years (and potentially for over a century), hidden amongst the morphologically similar tammar wallabies. The fact that an invasive wallaby species could remain undetected for so long, highlights the need for greater monitoring efforts for invasive species including genomic species identification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions by non-native species are major drivers of global change and often precipitate the decline and extinction of indigenous species (Simberloff et al. 2013). For many invasions, the history of release, establishment, range expansion, and unwanted impacts are well documented; however, cryptic invasions can also occur when an invasive species either goes unnoticed, or is misidentified (Morais, Reichard 2018). Misidentifications are most common in groups such as plants and invertebrates, where taxonomic identification can be challenging, and molecular methods are often required to detect these cryptic invasions (Armstrong, Ball 2005; Morisette et al. 2021).
Few places on Earth have been as profoundly affected by invasive species as Aotearoa New Zealand (hereafter Aotearoa) (Vitousek et al. 1997). Here, indigenous species evolved without terrestrial mammals (other than bats) (Worthy, Holdaway 2002). Since European colonisation of Aotearoa began in the 1770s, a suite of 22 herbivorous mammals have established, and their cumulative effects have dramatically altered the structure and composition of many plant communities (Wardle et al. 2001).
Around 1870, Governor Sir George Grey introduced at least four species of wallaby to his free-range menagerie on Kawau Island (2070 ha) in the Hauraki Gulf (Latham, Warburton 2021). The two smallest of these, the parma wallaby (Notamacropus parma) and tammar (AKA dama) wallaby (Notamacropus eugenii) are of similar size and colour, and to an untrained eye can be mistaken for each other. Records of Grey’s liberations were not meticulous, and natural historians in the late 19th and early twentieth century assumed that all of the small wallabies on the island were tammar wallabies, unaware of the parma wallaby’s presence until 1965 (Wodzicki 1967).
A second population of tammar wallabies established on Te Ika-a-Māui (North Island) around 1912, after wallabies from Kawau Island were released at the southern end of Lake Ōkāreka, in the Bay of Plenty Region (Taylor, Cooper 1999). The tammar wallaby population in the Bay of Plenty has increased its range markedly, now occupying ~ 4000 km2 (Latham et al. 2019), causing damage to pasture, silviculture, and native plant communities (Latham et al. 2020; Latham, Warburton 2021). Consequently, in 2020 the New Zealand Ministry for Primary Industries established a national wallaby eradication programme (Tipu Mātoro) (Figs. 1 and 2).
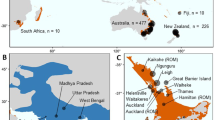
A PCA of genetic relatedness based on autosomal SNPs showing the high differentiation of parma and tammar wallabies. The three North Island parma wallabies are highlighted in black B Drawings of the two species showing morphological differences (by A.J. Veale). C Mitochondrial d-loop haplotype network showing showing the three parma wallaby haplotypes observed in New Zealand and the single tammar wallaby haplotype so far detected
We conducted a landscape genomic study of tammar wallaby across their range in Aotearoa. Our objectives were to provide baseline population genomic data to identify the sources of wallaby incursions, and to quantify the landscape connectivity of these populations. We sampled both tammar and parma wallabies on Kawau Island, along with tammar wallaby from across the mainland range. The Kawau Island parma wallaby were included to assess if there was any introgression between species, and because an eDNA sample taken from Lake Rotomā in the Bay of Plenty in November 2021, showed a possible detection of parma wallaby (Shawn Wilkinson, Wilderlabs pers. comm).
Methods
Sample collection
Tissue samples (ears) of wallabies were collected by the Bay of Plenty Regional Council (n = 173) from dead animals culled in control operations and from roadkill or found dumped bodies from hunters, with metadata including date of capture, sex, species and precise location data. Each ear was removed post-mortem, bagged separately, and stored at at − 10 °C. Further samples were also obtained from animals found or shot in culling operations outside the established populations: Taranaki (n = 2) and Wellington (n = 4). Ear tissue samples of wallabies from Kawau Island were collected from control operations in March 2019 (ears in 95% ethanol stored at − 10 °C).
Laboratory and bioinformatic methods
We used a DNeasy blood and tissue extraction kit on a QiaCube to extract the DNA from c. 0.5 cm2 of tissue, with an overnight digest using proteinase K according to the manufacturer’s protocols (QIAGEN, Hilden, Germany). DNA was eluted into 200 μL of Buffer AE and then stored at − 20 °C. The quality and quantity of the DNA were evaluated using a denovix DS-11 nucleic acid spectrophotometer, examining the 260/230 and 260/280 ratios to determine if there was any contamination. Any sample that did not meet the criteria for purity (260/280 = 1.7–2.1, 260/230 = 1.9–2.2) were removed.
All DNA extractions were then diluted to a uniform 50 ng/μL (with a concentration step using a SpeedVac for samples that had low concentration), with 1 μg of DNA sent for each sample for GBS sequencing. This GBS was performed at GenomNZ Animal Genomics Group (AgResearch, New Zealand). Procedures followed Dodds et al. (2015) after Elshire et al. (2011), with the following modifications. Briefly, genomic DNA was digested with PstI and MspI restriction enzymes (NEB R140L and R0106L, New England Biolabs, Ipswich, United States). We chose enzymes based on bioanalyser traces (2100 Bioanalyser, Agilent Technologies, Santa Clara, United States) showing an even digestion pattern with no evidence of repeat sequences through the region of interest. Following ligation to barcoded adapters, the uniquely barcoded individuals were pooled into two multiplexed libraries of 94 samples. Libraries post-pooling were run through PCR in multiples of four and pooled again before column clean-up, then each library was further purified and size selected (193–500 bp) using a Pippin (SAGE Science, Beverly, United States; 2% agarose, dye-free with internal standards CDF2050, Marker L CDF2010). We then sequenced each library on an Illumina HiSeq2500 using single-end reads, with 101 cycles in high-output mode (v4 chemistry).
Quality checks and adapter removal followed Dodds et al. (2015). Raw fastq files were quality checked using FastQC v. 0.10.1 (http://www.bioinformatics.babraham.ac.uk/ projects/fastqc/). Barcodes and adapters were removed using cutadapt (Martin 2011), then a random 15,000 reads were checked for contamination using BLAST + against the NT database (https://blast.ncbi.nlm.nih.gov), with the following settings: blastn -query—-task blastn—num_threads 2 -db nt -evalue 1.0e-10 -dust ‘20 64 1’ -max_target_seqs 1 -outfmt ‘7 qseqid sseqid pident evalue staxids sscinames scomnames sskingdoms stitle’. We then produced a catalogue of SNP loci (single nucleotide polymorphisms, or single base variants) following Dodds et al. (2015) and the general guidelines of (Benestan et al. 2016).
After trimming adapters with cutadapt (Martin 2011), reads were mapped and SNPs detected via a modified reference-based pipeline in bcftools (Danecek et al. 2011) using the recently completed tammar wallaby genome, which was assembled from a male obtained by lead author Dr Veale from the Waimangu thermal area in the Bay of Plenty, New Zealand (mMacEug1.pri: GCA_028372415.1). All following data filtering steps were then conducted using vcftools (Danecek et al. 2011). Filtering parameters for loci were: Minor allele count = 3, Maximum average depth = 45, Minimum depth = 5, Maximum missingness = 0.3. To retain an individual, a minimum average depth of 2 was required (measured before other filtering).
GBS analyses
We performed a Principal Coordinate Analyses (PCoA) within KGD (Dodds et al. 2015) on the genomic relatedness between individuals. Based on the KGD analyses there were clearly two highly divergent groups (which corresponded to the two species, see results), though two individuals had slight intermediate values between these groups. To further investigate this, we converted the VCF file to a Geste file to enable us to calculate the number of fixed differences between the two species (excluding the two potential hybrids). Once the alleles that were fixed differences were identified between the two species were identified we then counted the proportion of each of these categories in the two potentially hybridised individuals (as per Etherington et al., 2022). To map the distribution of samples we used the R package ggmap (Kahle & Wickham 2013).
Genetic (mitochondrial) species ID methods
The following novel PCR primers were designed to amplify c. 800 bp of the mitochondrial D-loop across wallaby species in NZ:
Macropus_Dloop1F: TGAAGAGTCCAAGTAATTTAACAAAA.
Macropus_Dloop1R: AGCAACCAGTAGCCAGTCAG.
PCRs consisted of 1 × FastStart Taq DNA Polymerase PCR Buffer with MgCl2 (Roche), 0.2 mM dNTP Mix (Roche), 1 U of Fast-Start Taq DNA Polymerase (Roche), 0.2 µM of each primer (Macropus_Dloop1F and Macropus_Dloop1R, 0.4 µg/µl of bovine serum albumin (BSA), 2 µl of genomic DNA extraction, and 16 µl of PCR grade water for a total volume of 25 µl. Thermocycling conditions were an initial denaturation step of 95 °C for 4 min; then 38 cycles of denaturation at 95 °C for 45 s, annealing at 55 °C for 45 s, and extension at 72 °C for 1 min; and a final extension step at 72 °C for 10 min. Samples were sequenced using the BigDye® Terminator v3.1.
Results
As predicted, our genomic data accurately identified the two highly divergent species on Kawau Island (parma and tammar wallabies). Of the 28,935 autosomal SNPs retained after filtering, 16,796 (58%) were fixed differences between these two species. Unexpectedly, however, our results revealed three parma wallabies in the Bay of Plenty, along with the eighty tammar wallabies genotyped from the region. Once this surprising result was obtained, the culled wallabies brought to the Kaharoa School hunting competition (September 17th 2023) were assessed morphologically by a Bay of Plenty Regional Council wallaby specialist, with 17/183 identified as parma wallabies (Dale Williams pers comm); this species identification was confirmed using mitochondrial markers (GenBank accession # PQ217621 - PQ217624).
Neither the parma or tammar wallabies sampled from Kawau Island, nor the three parma wallaby sequenced in the Bay of Plenty showed any trace of hybridisation. However, two predominantly tammar individuals sampled from the Bay of Plenty showed possible minor traces of historic introgression, with < 2% likely parma ancestry, based on the proportion of parma-specific SNPs present.
Discussion
The important and surprising result obtained is that parma wallabies are present in the Bay of Plenty, and this population has gone undetected for a considerable period. There are two possible scenarios for the origins of parma wallabies in the Bay of Plenty. First, they may have been liberated at the same time as tammar wallabies around 1912. The presence of parma wallabies on Kawau Island was only discovered in 1965 and those that liberated the tammar wallabies will not have known the difference (Wodzicki 1967). Alternatively, it is possible that parma wallabies destined for export to zoos may have been illegally moved from Kawau Island to holding pens near Rotorua prior to the 1990s, with some of these individuals then escaping, or being released (Dale Williams pers comm). Whatever their origins, these parma wallabies have gone unidentified for decades, if not over a century. With only seven parma wallabies sampled for GBS (4 from Kawau Island, 3 from the Bay of Plenty) we cannot accurately assess the relative genetic diversity of these populations or attempt to model the timing of the introduction to the Bay of Plenty, nor the number of founders. With further sampling we may be able to ascertain these parameters which will help us understand the population’s history.
Differences in the ecology and behaviour of tammar and parma wallabies may help explain how parma wallabies have gone unnoticed for such an extended period. Tammar wallabies in their native Australia are grazers, often feeding in large aggregations, preferring open grassland, scrub, and dry sclerophyll forest, while parma wallabies are both grazers and browsers, are generally solitary, and in Australia they prefer wet sclerophyll forest with dense understories, and rainforest (Lentle et al. 2003; Read, Fox 1991; Van Dyck, Strahan 2008). In Aotearoa, if parma wallaby have maintained their preference for more densely forested habitats, they may be less likely to be culled in control operations compared with tammar wallabies. This potential habitat preference difference, and their more solitary nature, coupled with their similar physical appearance, may explain their previous anonymity.
Parma wallabies may have greater negative effects on native biodiversity, as the breadth of their diet is far higher than that of the tammar wallabies (Vujcich 1979). Given we now know these species are sympatric, all previous research on wallabies in the Bay of Plenty requires re-evaluation. For instance, one study found that wallabies from forested habitats had shorter skulls and were more likely to browse shrubs than those in the pasture (Williamson 1986). These observations may relate to differences in wallaby species compositions in each habitat, rather than intraspecific variation. Similarly, studies looking at niche modelling and their likely future spread (Latham et al. 2019) will be confounded if the dispersal rates of the two species differ substantially.
In Australia, tammar and parma wallabies are allopatric, and they diverged approximately 3 million years ago (Nilsson et al. 2018). These species will occasionally hybridize in captivity, but their offspring are generally sterile (Close, Lowry 1989). Our results showing only occasional potential minor introgression reaffirms their general incompatibility (Taylor et al. 1999). With such a small number of parma individuals sequenced, and with only two possible slightly introgressed individuals in the dataset, further sequencing is required to confirm if admixture is truly present in these individuals (ruling out cross-contamination, barcode-jumping or sequencing errors). All seven parma wallabies identified in the GBS appear to have pure parma ancestry both on Kawau Island, and in the Bay of Plenty.
For the management of this newly discovered population, parma wallabies are able to be identified morphologically, and species confirmation can be confirmed using mitochondrial markers when necessary. Genomic sequencing in this instance while critical in the initial detection of the species appears unnecessary for ongoing species identification. Targeting culling and accurate species identification will be important to determine the geographic distribution, population density, and dispersal characteristics of parma wallabies in the Bay of Plenty. As their ecology and behaviour is likely to differ from tammar wallabies specific research is also required on the efficacy of monitoring and control techniques for this species to inform future eradication planning.
The fact that a parma wallaby invasion can go undetected for so long, in a country that invests considerably in invasive mammal control and research, highlights the need for greater monitoring of invasive species, particularly using genetic techniques. If wallabies can go unnoticed, how many invasive insects are being missed? For mammals, a similar situation has been recorded for the Asian house rat Rattus tanezumi, with this species (or their hybrids with R. rattus) being discovered in both California and South Africa only after genetic testing (Bastos et al. 2011; Conroy et al. 2013). Sometimes we see what we expect to see, and if we do not suspect that something is present, we do not look. This delay in detection may mean the difference between a rapid decisive incursion response and having to manage a sizeable outbreak.
Data availability
All mitochondrial sequences uploaded to GenBank (GenBank accession # PQ217621 - PQ217624), and all metadata and processed GBS data is available on Dryad, https://doi.org/10.5061/dryad.80gb5mkzz.
References
Armstrong K, Ball S (2005) DNA barcodes for biosecurity: invasive species identification. Philos Trans R Soc B: Biol Sci 360:1813–1823
Bastos AD, Nair D, Taylor PJ et al (2011) Genetic monitoring detects an overlooked cryptic species and reveals the diversity and distribution of three invasive Rattus congeners in south Africa. BMC Genet 12:26
Benestan LM, Ferchaud A-L, Hohenlohe PA et al (2016) Conservation genomics of natural and managed populations: building a conceptual and practical framework. Mol Ecol 25:2967–2977
Close R, Lowry P (1989) Hybrids in marsupial research. Aust J Zool 37:259–267
Conroy CJ, Rowe KC, Rowe KMC et al (2013) Cryptic genetic diversity in Rattus of the San Francisco Bay region. California Biol Invasions 15:741–758
Danecek P, Auton A, Abecasis G et al (2011) The variant call format and VCFtools. Bioinformatics 27:2156–2158
Dodds KG, McEwan JC, Brauning R et al (2015) Construction of relatedness matrices using genotyping-by-sequencing data. BMC Genom 16:1047
Van Dyck S, Strahan R (2008) The mammals of Australia. (No Title)
Elshire RJ, Glaubitz JC, Sun Q et al (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6:e19379
Etherington GJ, Ciezarek A, Shaw R, Michaux J, Croose E, Haerty W, Di Palma F (2022) Extensive genome introgression between domestic ferret and European polecat during population recovery in Great Britain. J Hered 113(5):500–515
Kahle DJ, Wickham H (2013) ggmap: spatial visualization with ggplot2. R J 5(1):144
Latham ADM, Warburton B (2021) Family Macropodidae. In: King CM, Forsyth DM (eds) The handbook of New Zealand mammals, 3rd edn. CSIRO, Melbourne, Australia, pp 1–27
Latham ADM, Latham MC, Warburton B (2019) Current and predicted future distributions of wallabies in mainland New Zealand. N z J Zool 46:31–47
Latham ADM, Latham MC, Norbury GL et al (2020) A review of the damage caused by invasive wild mammalian herbivores to primary production in New Zealand. N z J Zool 47:20–52
Lentle R, Haslett S, Hume I et al (2003) Foraging behaviour in tammar (Macropus eugenii) and parma (Macropus parma) wallabies. Aust J Zool 51:297–305
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17:10–12
Morais P, Reichard M (2018) Cryptic invasions: a review. Sci Total Environ 613–614:1438–1448
Morisette J, Burgiel S, Brantley K et al (2021) Strategic considerations for invasive species managers in the utilization of environmental DNA (eDNA): steps for incorporating this powerful surveillance tool. Management of Biological Invasions: International Journal of Applied Research on Biological Invasions 12:747
Nilsson MA, Zheng Y, Kumar V et al (2018) Speciation generates mosaic genomes in kangaroos. Genome Biol Evol 10:33–44
Read D, Fox B (1991) Assessing the habitat of the parma wallaby, Macropus parma (Marsupialia: Macropodidae). Wildl Res 18:469–477
Simberloff D, Martin J-L, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Taylor A, Cooper D (1999) Microsatellites identify introduced New Zealand tammar wallabies (Macropus eugenii) as an ‘extinct’taxon Animal Conservation forum. Cambridge University Press, Cambridge, pp 41–49
Taylor AC, Sunnucks P, Cooper D (1999) Retention of reproductive barriers and ecological differences between two introduced sympatric Macropus spp. in New Zealand. Anim Conserv 2:195–202
Vitousek PM, D’antonio CM, Loope LL et al (1997) Introduced species: a significant component of human-caused global change. N Z J Ecol 21:1–16
Vujcich VC (1979) Feeding ecology of the parma, Macropus parma (Waterhouse) and tammar Macropus eugenii (Desmarest) wallabies on Kawau Island. University of Auckland, Biology
Wardle DA, Barker GM, Yeates GW et al (2001) Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecol Monogr 71:587–614
Williamson GM (1986) The ecology of the dama wallaby (Macropus eugenii, Desmarest) in forests at Rotorua, with special reference to diet: a thesis submitted in partial fulfilment of the requirements for the degree of Master of Science, Massey University. Massey University, Palmerston North
Wodzicki K, Flux JE (1967) Re-discovery of the white-throated wallaby, Macropus parma Waterhouse 1846, on Kawau Island, New Zealand. Australian J Sci 29:429–430
Worthy TH, Holdaway RN (2002) The lost world of the moa: prehistoric life of New Zealand. Canterbury University Press, Christchurch
Acknowledgements
Many thanks to Carl Weaver, Dale Williams, Greg Corbett, Davor Bejakovic and the team at Bay of Plenty Regional Council for their work archiving the wallaby ear samples. Thanks to Travis Ashcroft for organising the study. Funding was provided by the Tipu Matoro National Wallaby Eradication Programme under Ministry for Primary Industries contract C0033146.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding was provided by the Tipu Matoro National Wallaby Eradication Programme under Ministry for Primary Industries contract C0033146. The research was supported by the Strategic Science Investment Fund (SSIF) for Manaaki Whenua Landcare Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest.
Ethical approval
No ethics approval was sought as all animal samples were obtained from animals culled in pest control operations.
Human and animal rights
All animal samples used in this study were obtained from culled animals from pest control operations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Veale, A.J., McEwan, J.C., Brauning, R. et al. Genomics detects cryptic wallaby invasion. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03429-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03429-9