Abstract
Cities are increasingly recognised as important sites for biodiversity and essential for improving human-nature connections. However, urban areas are also hotspots for rats, which negatively impact biodiversity due to competition and depredation. Urban residents may undertake rat control on their properties, but the effectiveness of such volunteer initiatives for broader biodiversity outcomes has not been tested in urban environments. We conducted a comprehensive study inclusive of rat abundance, adult bird diversity, and bird breeding success. We monitored rat (Norway rat Rattus norvegicus; ship rat Rattus rattus) presence and modelled detection probabilities and occupancy in suburban residential backyards in Auckland, New Zealand. We also compared bird abundance and richness among backyards and recorded 140 nesting attempts from 15 bird species between September 2021 and February 2022. Despite rat control, rats were detected at some point in all backyards, though relative abundance was low, and fewer rats were detected in backyards with more frequent rat control. Higher bird abundance, and to a lesser extent richness, were associated with proximity to native vegetation patches and more frequent rat control. Overall fledging success was relatively high and predation by rats was relatively low compared to previous research. Nest survival was slightly higher for introduced bird species. Daily nest survival rate increased with nest height, proximity to native vegetation patches, and when rat detection rates decreased. Although the effect was small, frequent rat control within a backyard was associated with increased fledging success and increased bird species richness and abundance. Further research is required to explore the factors that interact with backyard rat control to improve local outcomes for birds. High levels of community participation in backyard rat control are required to improve neighbourhood-wide outcomes for birds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urban centres are often situated in biodiversity hotspots (Luck 2007), and can serve as refuges for native and endemic species (Ives et al. 2016). High-quality native vegetation patches can provide natural habitat in suburban areas, and create opportunities for city-dwellers to connect with nature and engage in conservation (Soga and Gaston 2016). On the suburban fringe, spaces set aside for biodiversity provision are usually quite small relative to the proportion of vegetation in private residential backyards (equivalent to gardens or yards in some countries). Backyards are important for wildlife connectivity and ecological processes (Goddard et al. 2010; van Heezik et al. 2013), as well as preserving biodiversity and protecting land from human development (Asaad et al. 2017).
Rats are well-adapted to residential and urban areas, due to the availability of food, water and refuges that support their populations (Feng and Himsworth 2014). Managing rats is essential for human health as rats spread zoonoses (Strand and Lundkvist 2019) and their presence impacts the mental health of urban residents (Lam et al. 2018). Globally, rats are amongst the most successful and widespread invaders (Spatz et al. 2017) and are linked to the extinction of numerous species (Doherty et al. 2016). Fewer rats can be beneficial to biodiversity, due to decreased competition for resources and depredation on species (Doherty et al. 2016), but the impact of rats on birds in urban areas is little known (Russell and Stanley 2018).
People typically privately control pests, such as rats, for self-interested reasons, such as to prevent property damage or the spread of disease, meaning any potential benefits for native biodiversity are not a primary motivation (Gerolemou et al. in press; Dunn et al. 2018). Despite this, public interest in assisting with conservation has increased (Hughey et al. 2019). Community conservation has become popular for managing invasive pest species. For example, volunteers have helped to control the American mink (Neogale vison) and grey squirrel (Sciurus carolinensis) in the UK (Mill et al. 2019) and the North American beaver (Castor canadensis) in Patagonia (Santo et al. 2015). To ensure volunteer-led pest control initiatives are achieving their aims, we need to evaluate the outcomes for native biodiversity.
Public support is needed for urban pest management (Estevez et al. 2014), as land is often divided into small parcels, meaning cooperation from multiple stakeholders is required to manage rats (Parsons et al. 2017). Pest control may not be socially acceptable (Hughey et al. 2019) due to concerns about non-target effects on pets (Crowley et al. 2017), and this limits which tools can be used in urban areas (Dearborn and Kark 2010). Promoting conservation volunteering can help with including the public in environmental and pest management (Takase et al. 2019; Taylor et al. 2022).
Little is known about the outcomes of community-led rat control in urban areas (Russell and Stanley 2018). The outcomes on rodent dynamics are also not well known, but are likely to depend on rat control activity in both the backyard itself and the wider landscape of neighbouring properties (Morgan et al. 2009). Data on the ecological outcomes of rat control remain scarce, but emerging evidence suggests some bird species strongly benefit from it, especially endemic and native species (Williams et al. 2020). Therefore, pest control is especially important on oceanic islands, where native and endemic species have not evolved natural defences against rats, putting them at risk of local and global extinction (Banks and Dickman 2007).
Aotearoa–New Zealand serves as a case study for understanding the outcomes of community-led pest control. The country has high levels of endemism, including many avian species vulnerable to predation by introduced mammalian predators (Innes et al. 2010; Walker et al. 2021). New Zealand’s endemic species have evolved while biogeographically isolated for 80 million years and without terrestrial, non-volant mammalian predators (Gibbs 2009). Consequently, they do not have behavioural defences against mammalian predators; for example, many endemic birds have lost the ability to fly or have adapted to nest on the ground (Worthy and Holdaway 2002). This increases their susceptibility to mammalian predators and their risk of extinction in comparison to native bird species that evolved more recently and which have a shared (Australasian) evolutionary history with predators.
In 2016, the New Zealand government adopted the goal to eradicate several invasive mammals from the entire country, known as ‘Predator Free 2050’ (Owens 2017). The program encourages residents to control mammalian predators by either deploying traps or toxic baits in secure bait stations in their backyards and local reserves to improve outcomes for native biodiversity (Department of Conservation 2020), with community conservation groups providing free traps and training for residents. The focal species for eradication include mustelids (e.g. stoats Mustela erminea), common brushtail possums (Trichosurus vulpecula; henceforth ‘possums’), and most notably rats (Norway (brown) rat Rattus norvegicus; ship (black) rat R. rattus). In New Zealand, ship rats are more arboreal and common than Norway rats, which facilitates them depredating nesting birds (King and Forsyth 2021). Although less arboreal, Norway rats have negatively impacted native birds globally (Doherty et al. 2016) and are the most common rat species in urban areas around the world (Capizzi et al. 2014).
Given the promotion and financial resourcing of suburban backyard rat control in New Zealand, we investigated if community-led backyard rat control improves the outcomes for birds in suburban Auckland, New Zealand’s largest city. Specifically, we asked whether rat control by householders in residential areas affects: 1) the presence of rats; 2) local bird abundance and richness; and 3) the fledging success of birds in backyards. We predicted that higher intensity rat control would be associated with lower rat presence and higher relative abundance of native and endemic birds. We expected that introduced bird species would have higher fledging success than native or endemic species, but that rat control would nevertheless improve fledging success overall. We addressed these questions by comparing nesting success and bird communities in backyards with different frequencies of householder rat control and intensities of rat control in the surrounding neighbourhood.
Method
Site selection
This study was carried out in suburban backyards in Auckland, New Zealand. Around a third of the New Zealand population (1.7 M) lives in Auckland, the largest city, which has over 250 community conservation groups (Stats NZ 2021; Predator Free New Zealand 2022). We used natural boundaries (train lines, major roads, waterways) to delimit our study area to a size that made it logistically possible for data collection visits and focused on several suburbs within the eastern suburbs of Auckland (3658 ha; − 36.87, 174.83). To limit socio-economic variability and possible confounding variables among the study properties, we used the 2018 New Zealand Index of Multiple Deprivation (IMD18; a measure of relative disadvantage at the neighbourhood level comprising indicators of employment, income, crime, housing, health, education and access; Exeter et al. 2018). Most of the suburbs in this area (approx. 90%) were in the 40% least deprived compared with the country’s average. These areas typically have higher levels of conservation participation, for example, many suburbs were part of large, active community pest control groups, which facilitated finding study sites (Lukies 2020). We advertised through community group networks (D’Mello and Pannell 2019) and social media to ask people to volunteer their backyards for the study.
During an initial visit, we determined each backyard’s suitability for the study (backyard accessibility and available vegetation for bird nesting) and asked householders about their rat control activities to allow nest comparisons among backyards with different frequencies of rat control. Frequency was the operational metric at the backyard scale and referred to how often a rat control device (trap or toxic bait station) was serviced (i.e. traps checked, cleared and rebaited or toxic bait re-stocked). It was reported by householders as their existing routine and was not manipulated for the study. Rat control frequency (ordinal factor) was classified as; ‘regular’ if the backyard rat control devices were serviced weekly for the duration of the study (n = 12), ‘sporadic’ if these devices were serviced at least once but not regularly throughout the study duration (n = 11), and ‘none’ if there was no rat control occurring in that backyard (n = 9) (total sample n = 32 backyards). Householders were asked to inform us if they changed their rat control routines during the study and additionally, we asked all householders to clarify their routine every four weeks. We measured neighbourhood rat control intensity (a derived measure of both the frequency and density of rat control devices across the neighbourhood) for each property using rat control data from community conservation databases (see Analysis section). All backyards were within a kilometre of an area of significant native vegetation (henceforth ‘native vegetation patch’; as defined by the regional authority; Auckland Council 2023). Each backyard was visited weekly over 16 weeks between September 2021 and February 2022.
Procedure
Rat monitoring
To record the presence of rodents (rats and mice (Mus musculus)), we used tracking tunnels (containing an ink pad and peanut butter bait in the centre and paper to record footprints; King and Edgar 1977) and chew cards (an edible corrugated plastic card baited with peanut butter to record bite marks; Sweetapple and Nugent 2011). These monitoring devices were left in each backyard (n = 21), which excluded properties where the homeowner had dogs who interfered with the monitoring devices (n = 6), or the homeowner declined to have monitoring devices (n = 5). The devices were replaced weekly throughout the study, and any footprints/bite marks were identified to genus or species level using a reference guide (Sweetapple and Nugent; Gillies and Williams 2002). Rat species cannot be distinguished using these monitoring devices (Morgan et al. 2009). Mammalian species could also be incidentally recorded by presence of scat, trap catches by the householder, or if an animal was seen on a trail camera (Reconyx HyperFire 2, La Crosse, USA; see Nest Monitoring below) or by a researcher.
Avian community monitoring
Each backyard was surveyed once per week during the study. We used the five-minute bird count (5MBC) relative abundance method (one observer: RG), where all individual birds seen or heard within the backyard boundary (excluding birds flying overhead) were recorded once per week (on the same visit as monitoring devices were collected and replaced), one to seven hours after sunrise (Hartley 2012), depending on how many nests were found or monitored at previous backyards that day. The order of the backyards visited was randomised. We restricted the counts to within the backyard (5–15 m radius) due to the auditory and physical barriers in the suburban environment (van Heezik and Seddon 2012). Counts did not take place during heavy rain or high wind.
Nest monitoring
Bird nests were found by searching vegetation with a thermal imaging scope (Pulsar Helion XP38, Vilnius, Lithuania), observing adult birds for nesting behaviours (carrying nesting materials or food in their beaks, or returning to the same spot) and asking householders if they were aware of any nests. Once a nest was located, and the adults were away, we recorded data on the species, number of eggs or chicks, and estimated date of laying based on the size of the chicks. We did not exclude any bird species. A trail camera was installed at the nest (0.5–2 m from the nest) where possible (n = 80). Additional data collected at each nest included; geolocation, proportion of canopy cover, nest height above the ground, branch diameter at nest attachment point, tree species, tree height, and broad classification of surrounding vegetation. Nest visits were limited to 10 min to minimise the risk of abandonment. We calculated the distance to the nearest native vegetation patch in ArcGIS (ESRI 2021).
Following Fea and Hartley (2018) and van Heezik et al. (2008a), a nest concluded when no activity by adults or chicks was detected for 30 min. We used an endoscope (Teslong NTS300 with 8 mm diameter dual lens and 5 m probe, Shenzhen, China) to view the nest where required. A nest was successful if at least one chick fledged. This was determined by: 1) camera evidence of chicks fledging; 2) chicks observed fully feathered in the nest and no predator activity was detected; 3) a fledgling sighting within 10 m of the nest in the two weeks following estimated fledging date; or 4) the adults laying another clutch in the nest without evidence of predation. A nest was recorded as depredated if the nest was empty before fledging was possible and there was: 1) evidence of a predator on the camera at the nest; 2) evidence of a predator at the nest site (scat, fur, nest pulled apart); or 3) egg, chick or adult remains. Nests could also fail due to weather events or if the adults abandoned the nest for other reasons (van Heezik et al. 2008a; Fea and Hartley 2018).
The study was approved by the University of Auckland Animal Ethics Committee (22 April 2020 for three years; reference number 002243).
Analysis
Estimating the effect of rat control
All analyses were performed in R 4.0.3 (R Core Team 2020). We used single-season occupancy modelling on the monitoring data (detection by either tracking tunnel or chew card) in the 21 backyards we monitored. Using the ‘unmarked’ package (Fiske and Chandler 2011), we estimated rat presence and detection probability. We assumed that rat interactions with traps or toxic bait stations were lethal in most instances, and that property owners then service their traps and bait stations proportionally to how often they kill a rat. Rat presence was high (estimated 100% of backyards), so we tested the number of weeks without rat control in each backyard as a proxy for relative rat abundance, and therefore predation risk. In New Zealand at least, relative abundance (via indices such as tracking tunnels) and abundance have a well quantified monotonic relationship (Brown et al. 1996). To check the suitability of this proxy variable, we first used Generalised Linear Mixed Models (GLMMs) to correlate the number of rat detections and the number of weeks with rat control in each backyard (with backyard as a random effect for each week of data collection).
To determine rat control intensity in the neighbouring landscape, we sourced rat control data (location of devices and number of times each trap or toxic bait station was serviced) from community conservation databases (‘CatchIT’ https://catchit.co.nz/catchit/ and ‘TrapNZ’ https://trap.nz/). To indicate what amount of rat control was the norm for a neighbourhood, we used data over a 12 month period to compensate for missing weeks of data. We used the ‘Heatmap (kernel density estimation)’ tool in Q (QGIS Development Team 2022) to create a heatmap of ‘neighbourhood rat control intensity’ across the study area between April 2021 and March 2022 inclusive. Using the distance matrix tool, we obtained a value of the neighbourhood rat control intensity (total trap and toxic bait station service records) within a 500 m radius from the centre of each 100m2 grid cell. This distance covered the likely home range of a rat (Hansen et al. 2020) and is a practical pest management unit (Carter et al. 2022). Using the raster calculator tool, we extracted the neighbourhood rat control intensity values for the specific grid cells containing each backyard rat monitoring, 5MBC, and nest. We used these neighbourhood rat control intensity values for the modelling of rat presence, bird communities and nest survival (see below).
Avian community analysis
We tested differences in bird species richness and abundance in backyards between neighbourhoods with higher (n = 16) and lower (n = 16) rat control intensities for different bird biogeographic statuses (endemic, native, and introduced bird species) with two-way Analyses of Variances (ANOVAs). We used GLMMs in the ‘lme4’ package (Bates et al. 2009) to measure the significance of environmental variables as predictors of endemic, native and introduced adult bird species 1) richness and 2) relative abundance (Table 1 for list of explanatory variables). We found the most parsimonious models (with the lowest AICc) with the dredge function in the ‘MuMin’ package (Barton 2015). Using the bird count data, we used the ‘vegan’ package (Oksanen et al. 2013) in R to test if bird community composition differed between backyards with higher and lower neighbourhood rat control intensities based on the rat control analysis (more than or less than 1 trap or toxic bait station service record in 500 m radius). We performed; 1) Nonmetric Multidimensional Scaling (NMDS; Kruskal 1964) to compare sites’ community composition, and used the ‘ggplot2’ package (Wickham 2016) to construct ellipses and display the results visually, 2) Permutational Multivariate Analysis of Variance (PERMANOVA; based on 9999 permutations and the Adonis function in ‘vegan’; using the Bray–Curtis dissimilarity index; Anderson 2001), and 3) the ‘simper’ package (Clarke 1993) in ‘vegan’ with 999 permutations to test if bird community composition differed between backyards with higher and lower neighbourhood rat control intensities.
Nest survival analysis
We used a nest survival model (Rotella 2019) to estimate daily survival. Species was included as a random effect. Nests that failed before incubation (during nest building or egg laying; n = 14) and nests with unknown data (n = 14) were excluded. Using the package ‘RMark’ (Laake 2013), we ran a nest survival analysis with the covariates in Table 1. We first checked for multicollinearity among all covariates with a correlation matrix, but none were removed (VIF < 0.8). We found the most parsimonious models (lowest AICc) using bidirectional stepwise analysis to generate the candidate models.
Results
Rat monitoring
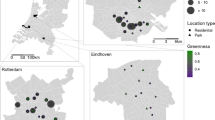
There was a positive correlation between rat detection and the number of weeks without rat control device servicing in each backyard (R2 = 0.80). The occupancy model estimated a weekly detection probability for rats of 0.362, and that rats were present at some point during the study in all (100%) of backyards. We incidentally recorded additional invasive mammals in the backyards; cats (76%, n = 16), mice (62%, n = 13), and possums (48%, n = 10). Neighbourhood rat control was more prevalent in the northeast of the study area (Fig. 1), where community rat control programs have been running for at least five years (Lukies 2020).
Heatmap indicating ‘hotspots’ (higher intensity = yellow) of rat control in urban Auckland, New Zealand. Intensity refers to the number of records of a rat control device being serviced (i.e. rat trap checked or toxic bait station restocked) between April 2021 and March 2022. The lowest intensity (dark blue) indicates 1 record within 500 m during that period. Unshaded areas (no colour) indicate no records of rat control within the surrounding 500 m radius. Data from CatchIT (https://catchit.co.nz/catchit/) and TrapNZ (https://trap.nz/). Shapes indicate residential backyard study sites (n = 32) and the frequency of rat control during the study period (white triangle = backyard with regular rat control at least once a week for the duration of the study, grey square = sporadic rat control in backyard on at least one occasion during study, black circle = no rat control during study period). Study area outline shown with dashed white line (areas outside line excluded)
Avian community
In total, 2837 individual birds and 22 species (12 introduced, seven native, three endemic) were recorded in 401 counts across the 32 backyards. The five most common species; Eurasian blackbird (Turdus merula; introduced), silvereye (Zosterops lateralis; native), house sparrow (Passer domesticus; introduced), tūī (Prosthemadera novaeseelandiae; endemic), and New Zealand fantail (Rhipidura fuliginosa; native) made up two-thirds of all records (Online Resource 1). Bird abundance was significantly higher (5.4%) in neighbourhoods with higher rat control intensities than lower rat control intensities (ANOVA Fdf = 4.65, P < 0.05, effect size = 0.0033; Fig. 2a) but species richness was not significantly different between neighbourhoods with higher and lower rat control intensities (ANOVA Fdf = 1.71, P = 0.19, effect size = 0.0012; Fig. 2b).
Bird relative abundance (a) and richness (b) for endemic, native, and introduced species in backyards in neighbourhoods with relatively higher neighbourhood rat control intensity (> 1 service record for a trap/toxic bait station in 500 m radius surrounding each 100m2 grid cell, light grey) or lower neighbourhood rat control intensity (< 1 service record for a trap/toxic bait station in 500 m radius surrounding each 100m2 grid cell, dark grey). A black dot indicates mean value
Higher native and endemic species richness and total abundance were associated with proximity to native vegetation patch, higher rat control frequency in a backyard, and higher intensity in the surrounding neighbourhood (Online Resource 2). These variables explained a proportion of variation in endemic species abundance (marginal R2 = 0.11; conditional R2 = 0.51) and richness (marginal R2 = 0.09; conditional R2 = 0.53) and native species abundance (marginal R2 = 0.10; conditional R2 = 0.35) and richness (marginal R2 = 0.12; conditional R2 = 0.28). Introduced species abundance (marginal R2 = 0.07; conditional R2 = 0.32) and richness (marginal R2 = 0.07; conditional R2 = 0. 27) were less affected by proximity to native vegetation patch and rat control intensity.
There was limited evidence of differences in bird community composition between backyards in neighbourhoods with relatively higher or lower rat control intensities; for either native (including endemic) birds (Fig. 3a) or introduced birds (Fig. 3b). Grey warbler (Gerygone igata; endemic), shining cuckoo (Chrysococcyx lucidus; native), morepork (Ninox novaeseelandiae; native), song thrush (Turdus philomelos; introduced), common starling (Sturnus vulgaris; introduced), and greenfinch (Carduelis chloris; introduced) were recorded significantly more in neighbourhoods with a relatively higher rat control intensity (Online Resource 3). Differences among individual backyards explained the largest amount of variation in the data for native (including endemic) and introduced birds (R2 = 0.27 and 0.07, respectively; Table 2). The proportion of variation in native (including endemic) and introduced bird communities explained by rat control intensity in the neighbourhood was low (R2 = 0.02 for native and endemic species and 0.00 for introduced species). Distance to native vegetation patch and rat control frequency were significant for native and endemic bird community composition, but the proportion of data they each explained was low (R2 = 0.02).
Nonmetric Multidimensional Scaling (NMDS) ordinations of a native (inc. endemic) and b introduced bird community composition in backyards in areas with relatively higher rat control intensity (> 1 trap/toxic bait station per 100m2 in surrounding area, light grey) or lower rat control intensity (< 1 trap/toxic bait station per 100m2 in surrounding area, dark grey). Neighbourhood rat control intensity = in surrounding 500 m radius. Stress = 0.188 for native and endemic birds, 0.203 for introduced birds
Nest survival
We found 140 nests of 15 species (eight introduced, four native, three endemic) across 29 backyards (Online Resource 4, Fig. 4). The most common species found nesting were Eurasian blackbird, New Zealand fantail and silvereye. At least one chick fledged in 60.7% of nests (55.7% native and endemic species, 65.7% introduced species), while 29.3% of nests (28.6% native and endemic species, 30.0% introduced species) failed and the outcome was indeterminable in 10.0% of nests (5.7% native and endemic, 14.3% introduced species). Failures where the cause of failure was known (n = 34) were due to predation by mammalian predators 44.2% of the time (rats = 3, cats = 3, possums = 2, unknown = 7), one nest (common starling) was depredated by the common myna (Acridotheres tristis; introduced), two nests were abandoned, two were damaged by bad weather and seven failed for an unknown reason (Online Resource 4). We were unable to determine the nest outcome for 14 nests and another 14 failed before incubation (during nest building or egg laying). The most common trees that birds nested in were phoenix palms (Phoenix canariensis; n = 11), Pittosporum spp. (n = 11), cabbage trees (Cordyline australis; n = 11), privet (Ligustrum spp.; n = 10), Camellia spp. (n = 9), Coprosma spp. (n = 9), and nikau (Rhopalostylis sapida; n = 6). Nests above 5 m (the mean value) were 13.5% more likely to fledge at least one chick compared to nests below that height.
Nest (n = 140) outcome of birds in Auckland backyards (n = 29) with and without rat control. Backyards with regular rat control (n = 12), backyards with sporadic rat control (n = 10), backyards without any rat control (n = 7). ‘Active’ = period from nest building to fledging. Fledge includes nests where there was evidence of at least one chick fledging. Fail includes predation (n = 16), abandonment (n = 12), weather (n = 6), unknown cause (n = 7). * Native to New Zealand; ** Endemic to New Zealand
The multi-model assessment for modelling daily nest survival included 35 models (Table 3 shows the top 9 models). Branch diameter, distance to native vegetation patch and nest height were variables consistently included in the most parsimonious models predicting nest success, with branch diameter included in all the top ten models. Daily nest survival rate was higher for nests in backyards with more frequent rat control, nests on a larger branch diameter, and nests further from the ground. Survival decreased when nests were further from native vegetation patches, rat detection was higher, and neighbourhood rat control intensity was higher.
Discussion
Rat control and rat abundance
The more frequently rat control devices were serviced, the less likely rats were detected, suggesting that regular and consistent backyard rat control can suppress rats. Regardless of rat control, rats were detected at some point in every backyard we monitored (except one backyard with sporadic monitoring due to construction work). The occupancy modelling showed that the monitoring tools had a high probability of detection when rats were present over multiple weeks. This indicates at the localised scale of the backyard rat control does not permanently eliminate rats. Rats in lower density populations are likely to roam more widely (Innes et al. 2011) and, with inconsistent control, would still be periodically present across neighbourhoods and backyards (Taylor et al. 2020). This could explain why we found that increases in neighbourhood rat control intensity decreased fledging success, although the effect was minimal, as rats subject to inconsistent control may roam further, and therefore encounter more nests. Ship rats may have been less susceptible to predator control as they are more arboreal, and traps and toxic bait stations are typically at ground level (Nance et al. 2023). Although a single instance of monitoring alone may mistakenly suggest rats are absent (Blackwell et al. 2002): regular ongoing monitoring is required to confirm prolonged rat absence or detect rats that have immigrated or are transient (Ruffell et al. 2015). To discourage rats, householders can reduce potential food sources, such as containing garbage and compost and removing fallen fruit from their backyards (van Heezik et al. 2008a).
There were some limitations to using community-sourced rat control data in the analysis. Each type of trap (A24, T-rex, etc.) had to be treated equally for the analysis, even though different traps may have different success rates. Further research would be required to investigate differences among trap types. The data were also collected by multiple people, which can lead to discrepancies in the way the data are recorded. The dataset may contain missing data, especially where a trap was checked, nothing was found but no data were recorded. Despite the limitations, community-sourced data are often high quality, especially when participants follow a protocol (Kosmala et al. 2016), such as the ones provided by both TrapNZ and CatchIT (Fewster 2023; TrapNZ 2023). Community-sourced data provide information about neighbourhoods that would otherwise go unrecorded (Dickinson et al. 2012) and, in our study, it allowed us to look at rat control across a much larger area than we would have been able to study otherwise. Despite its limitations, using community-sourced rat control data is a useful tool for pest management, and should be encouraged, especially when supported by skilled partners (Peters et al. 2016).
Avian communities
Backyard rat control was associated with higher native and endemic bird species richness and abundance, although the biological effect size was small. Previous studies also found that rat suppression, as opposed to eradication, only has a limited effect on increasing bird species richness and abundance (Ruffell and Didham 2017). This is because most remaining urban bird species are more adaptable than species absent from urban areas, having adapted to increased habitat disturbances (Blair 1996). Introduced species had the smallest response to rat control (compared to endemic and native birds) in our study, as they are more tolerant of mammalian predators than New Zealand native and endemic species (Starling-Windhof et al. 2011; Walker et al. 2021). Bird species highly vulnerable to predation by invasive predators have already disappeared from urban areas, and would therefore require higher intensities of rat control to return (Walker et al. 2021).
How people manage their backyards can affect the bird communities present. In our analysis and other studies (Galbraith et al. 2015; McNaughton et al. 2021), individual backyard differences had a large effect on bird community composition. We could not control for all potential explanatory factors, such as backyard size, supplementary bird feeding, and vegetation composition, which were heterogenous across the study area, and can affect bird communities (van Heezik et al. 2013). As native vegetation complexity is important for birds in urban areas (Heggie-Gracie et al. 2020), encouraging people to plant a variety of native plants in their backyards could support more native and endemic bird species.
As in previous New Zealand studies, proximity to a native vegetation patch had a positive effect on species richness and abundance, especially for native and endemic species (Deconchat et al. 2009; Heggie-Gracie et al. 2020; van Heezik et al. 2008b). Native and endemic species depend more on native resources for survival than introduced species, with native vegetation patches providing resources such as food, singing perches, nesting sites, and acting as stepping-stones for movement across the landscape (Fernández-Juricic and Jokimäki 2001; Donnelly and Marzluff 2004; Noe et al. 2022). Therefore, urban conservation management has generally focused their efforts on these areas (Savard et al. 2000), which likely has a halo effect on the surrounding area (Glen et al. 2013). Besides having higher abundances of native and endemic birds, native vegetation patches and backyards with complex vegetation likely harbour more pest mammals (Miller et al. 2022). Higher abundances of pest mammals may make people more likely to undertake pest control, and this could in part explain why areas with pest control have higher abundances of native and endemic birds.
Our results may not be applicable to areas of lower socio-economic status, which may not have the same natural environment infrastructure to support native birdlife. In Auckland, the districts with the lowest tree and native vegetation cover are often the most deprived neighbourhoods (Exeter et al. 2018; Auckland Regional Council 2019) and may have less avian diversity as a result. However, people in deprived areas of Auckland are also the least likely to be engaged in backyard pest control due to lack of time and resources alongside other priorities. It is not appropriate to seek generalisation when there is an inherent bias in the communities undertaking pest control.
Rat control and nest survival
Nests were more likely to fledge at least one chick if they were in a backyard with more frequent servicing of rat control devices, or where rat abundance was low. Nest failure rate due to predation was lower than comparable urban studies in New Zealand (39.8–52.4%; Fea and Hartley 2018; Morgan et al. 2011; van Heezik et al. 2008a). This could be because predation risk is lower in backyards compared to forest fragments (the focus of most urban research), nest predators have more resource availability, domestic cats alter rat behaviour, or mammalian predator abundance is lower in Auckland than other cities, perhaps due to coordinated efforts to control rats, which reduces their density (Gillies and Clout 2003; Brown et al. 2015). The relationship between fledging success and pest control is complex, with many other factors contributing to the outcomes for native and endemic species. Although we found a small positive effect between fledging success and pest control, further research is warranted, as our study found that differences between backyards, such as distance to a native vegetation patch, were also significant. Fledgling survival can be highly variable between seasons, so ideally any study on nest survival would take place over several years to reflect this. Due to the COVID-19 pandemic, we were unable to conduct our study over more than one nesting season. Further research is needed that compares suburbs with differing levels of neighbourhood rat control.
Overall fledging success was high in our study compared to other published data. International studies found nest survival is typically higher for birds in urban than rural areas (Kentish et al. 1995; Vincze et al. 2017). Most urban bird species are already tolerant of predation by rats, and predation is therefore not a major selective pressure (MacGregor-Fors et al. 2022). Fledging success and predation rates in our study were similar for native, endemic, and introduced species.
Eradication is costly and often unmaintainable if reinvasion by pests is high (Doherty and Ritchie 2017). A better strategy might be to rely on damage thresholds; the relationship between a pest species’ abundance and a specific impact (Yokomizo et al. 2009). Also known as density-impact functions, damage thresholds are thresholds above which damage to a specific biodiversity variable (e.g. nest mortality) is no longer acceptable and pest managers must reduce pests to bring damage (e.g. nest mortality) down to an acceptable level, or a maximum allowable pest density to achieve a specific biodiversity outcome (e.g. % increase in bird breeding success) (Norbury et al. 2015). To calculate damage thresholds, a model explores the relationship between a ‘damage,’ such as mortality of nests of a species, and the density of a predator (Yokomizo et al. 2009). This indicates the density at which a predator population significantly impacts nest mortality. Community conservation could aim to reduce pest numbers to tolerable levels of predation, thus allowing native and endemic species populations to recover.
Like other New Zealand studies, we found that increased nest height had a positive effect on survival (van Heezik et al. 2008a; Fea and Hartley 2018). Higher nests are less detectable (through sight or smell) and accessible to terrestrial predators. These studies also found additional variables affected fledging success, for example, New Zealand fantail fledging success was also associated with lower nest visibility (van Heezik et al. 2008a) and a smaller branch diameter which inhibits predators from reaching nests (Fea and Hartley 2018). In contrast, we found that an increase in branch diameter was associated with increased nest survival across all species. We attribute this to low rates of nest predation by mammalian predators, meaning any advantage smaller branches offer in reducing access for predators was low. Instead, larger branches may have offered other advantages, such as more stability for nests in harsh weather conditions or reduced visibility to avian predators (Attisano et al. 2020). These results indicate that rat control is only one factor affecting fledging success. Therefore, how people manage their backyards, including the trees and shrubs within, can make a difference to outcomes for urban birds.
Management implications
More frequent rat control in a backyard had better outcomes for birds. Therefore, servicing rat control devices (traps and toxic bait stations) weekly would be more effective than the pulsing often recommended by conservation managers, where devices are only serviced once per season (Auckland Council 2020). Although pulsing may be beneficial in sustaining volunteer commitment by having breaks from servicing equipment, not servicing frequently enough may reduce the effectiveness of the control. In urban areas, suppression might be a more realistic goal for pest management than eradication, given the current levels of participation and technologies available. The narrative around pest control programs might be better served by focusing on the social benefits for individuals and communities (Gerolemou et al. 2022, in press). Community conservation could also focus on biodiversity outcome targets, such as an increase in the abundance of native and endemic species to help maintain volunteers’ motivation. Further research is required to identify the maximum interval between servicing.
Our findings support the prioritisation of rat control in suburban native vegetation patches and surrounding buffer zones extending into residential areas. Connecting these areas by planting native vegetation corridors, for example, in volunteers’ backyards, would provide positive benefits for biodiversity (Goddard et al. 2010). Pest control will be required in these areas to ensure native wildlife can thrive. However, care must be taken not to ignore suburbs with low socio-economic status that may not have native vegetation patches but would still benefit from connecting with birds.
As cats are a popular pet globally (23% of households; GFK 2016) and we did record instances of cat predation on fledglings in our study. Although cats also eat rodents, van Heezik et al. (2010) showed that cats have a significant impact on New Zealand’s urban bird populations regardless of their consumption of rodents, and that both rats and cats require management. Engaging with cat owners about responsible pet ownership is likely to be one of the most acceptable and effective management strategies for urban cats (Ovenden et al. 2024).
Conclusion
Rat control can suppress rats and is slightly associated with higher fledging success. It is also associated with higher local relative abundance of native and endemic birds. Rat control intensity in the surrounding neighbourhood had a low impact on local outcomes for birds, suggesting that higher levels of participation in backyard rat control would be required for improvements at the neighbourhood level. Increases in rat control did not dramatically alter bird community composition. Other factors, such as habitat availability, should be considered, and require further research (Noe et al. 2022). If communities want to encourage extant native and endemic birds to return to urban areas, additional actions, such as planting appropriate vegetation, will be required alongside rat control.
Data availability
The datasets generated and analysed during the study are available in the Figshare repository, https://figshare.com/s/aaaee7550142071ac9b8
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Asaad I, Lundquist CJ, Erdmann MV, Costello MJ (2017) Ecological criteria to identify areas for biodiversity conservation. Biol Conserv 213:309–316. https://doi.org/10.1016/j.biocon.2016.10.007
Attisano A, Groß LB, Sato NJ et al (2020) Impact of brood parasitism and predation on nest survival of the fan-tailed gerygone in New Caledonia. J Avian Biol 51:1–13. https://doi.org/10.1111/jav.02476
Auckland Regional Council (2019) Auckland’s Urban Ngahere (Forest) Strategy. Auckland, New Zealand
Banks P, Dickman C (2007) Alien predation and the effects of multiple levels of prey naiveté. Trends Ecol Evol 22:229–230
Barton K (2015) Package ‘mumin.’ p.439. https://cran.hafro.is/web/packages/MuMIn/MuMIn.pdf
Bates D, Maechler M, Bolker B, et al (2009) Package ‘lme4’ http://lme4.r-forge.r-project.org
Blackwell GL, Potter MA, McLennan JA (2002) Rodent density indices from tracking tunnels, snap-traps and Fenn traps: Do they tell the same story? N Z J Ecol 26:43–51
Blair RB (1996) Land use and avian species diversity along an urban gradient. Ecol Appl 6:506–519. https://doi.org/10.2307/2269387
Brown K, Elliott G, Innes J, Kemp J (2015) Ship rat, stoat and possum control on mainland New Zealand: an overview of techniques, successes and challenges. Wellington, New Zealand: New Zealand Department of Conservation 36. http://www.doc.govt.nz/Documents/conservation/threats-and-impacts/animal-pests/ship-rat-stoatpossum-control.pdf
Brown K, Elliott G, Innes J, Kemp J (2015) Ship rat, stoat and possum control on mainland New Zealand: an overview of techniques, successes and challenges. New Zeal Dep Conserv 36
Capizzi D, Bertolino S, Mortelliti A (2014) Rating the rat: global patterns and research priorities in impacts and management of rodent pests. Mamm Rev 44:148–162. https://doi.org/10.1111/mam.12019
Carter ZT, Hanson JO, Perry GLW, Russell JC (2022) Incorporating management action suitability in conservation plans. J Appl Ecol. https://doi.org/10.1111/1365-2664.14258
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Auckland Council (2020) Pest animal control guidelines for the Auckland region. Auckland, New Zealand
Auckland Council (2023) Auckland Unitary Plan. Auckland, New Zealand
Crowley SL, Hinchliffe S, McDonald RA (2017) Conflict in invasive species management. Front Ecol Environ 15:133–141. https://doi.org/10.1002/fee.1471
D’Mello K, Pannell J (2019) Community restoration database Auckland. figshare. Dataset. Available at: https://doi.org/10.6084/m9.figshare.7796840
Dearborn DC, Kark S (2010) Motivations for conserving urban biodiversity. Conserv Biol 24:432–440. https://doi.org/10.1111/j.1523-1739.2009.01328.x
Deconchat M, Brockerhoff EG, Barbaro L (2009) Effects of surrounding landscape composition on the conservation value of native and exotic habitats for native forest birds. For Ecol Manage 258:196–204. https://doi.org/10.1016/j.foreco.2009.08.003
Department of Conservation (2020) Towards a Predator Free New Zealand, Department of Conservation. Wellington, New Zealand
Dickinson JL, Shirk J, Bonter D et al (2012) The current state of citizen science as a tool for ecological research and public engagement. Front Ecol Environ 10:291–297. https://doi.org/10.1890/110236
Doherty TS, Ritchie EG (2017) Stop jumping the gun: a call for evidence-based invasive predator management. Conserv Lett 10:15–22. https://doi.org/10.1111/conl.12251
Doherty TS, Glen AS, Nimmo DG et al (2016) Invasive predators and global biodiversity loss. Proc Natl Acad Sci U S A 113:11261–11265. https://doi.org/10.1073/pnas.1602480113
Donnelly R, Marzluff JM (2004) Importance of reserve size and landscape context to urban bird conservation. Conserv Biol 18:733–745. https://doi.org/10.1111/j.1523-1739.2004.00032.x
Dunn M, Marzano M, Forster J, Gill RMA (2018) Public attitudes towards “pest” management: perceptions on squirrel management strategies in the UK. Biol Conserv 222:52–63. https://doi.org/10.1016/j.biocon.2018.03.020
ESRI (2021) ArcGIS Desktop. Redlands, CA, USA: ESRI
Estevez RA, Anderson CB, Pizarro JC, Burgman MA (2014) Clarifying values, risk perceptions, and attitudes to resolve or avoid social conflicts in invasive species management. Conserv Biol 29:19–30. https://doi.org/10.1111/cobi.12359
Exeter DJ, Lee ACL, Zhao J, et al (2018) 2018 New Zealand Index of Multiple Deprivation. Auckland, New Zealand: University of Auckland. Available at: https://imdmap.auckland.ac.nz/download/
Fea N, Hartley S (2018) The balancing act of nest survival: survival of a small endemic bird in the face of ship rat predation and other risk factors. Avian Conserv Ecol 13:11. https://doi.org/10.5751/ACE-01284-130211
Feng AYT, Himsworth CG (2014) The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosyst 17:149–162. https://doi.org/10.1007/s11252-013-0305-4
Fernández-Juricic E, Jokimäki J (2001) A habitat island approach to conserving birds in urban landscapes: case studies from southern and northern Europe. Biodivers Conserv 10:2023–2043
Fewster R (2023) Entering Trap Data. Auckland, New Zealand. Available at: https://catchit.co.nz/tutorials/CatchITUser-Guide-Traplines.pdf
Fiske IJ, Chandler RB (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23. https://doi.org/10.18637/jss.v043.i10
Galbraith JA, Beggs JR, Jones DN, Stanley MC (2015) Supplementary feeding restructures urban bird communities. Proc Natl Acad Sci U S A 112:E2648–E2657. https://doi.org/10.1073/pnas.1501489112
Gerolemou RV, Russell JC, Stanley MC (2022) Social capital in the context of volunteer conservation initiatives. Conserv Sci Pract. https://doi.org/10.1111/csp2.12765
Gerolemou R V, Russell JC, Stanley MC (in press) Community-led vertebrate pest management in urban areas barriers and motivations. Ecol Soc
GFK (2016) Global pet ownership and feeding trends. https://www.gfk.com/insights/mans-best-friend-global-pet-ownership-and-feeding-trends. Accessed 1 Feb 2024
Gibbs GW (2009) The end of an 80-million year experiment: A review of evidence describing the impact of introduced rodents on New Zealand’s “mammal-free” invertebrate fauna. Biol Invasions 11:1587–1593. https://doi.org/10.1007/s10530-008-9408-x
Gillies CA, Williams D (2002) A short guide for identifying footprints on tracking tunnel papers. Dep. Conserv. 1–26. http://www.rimutakatrust.org.nz/downloads/Footprints_OLDDM-630181.doc
Gillies CA, Clout M (2003) The prey of domestic cats (Felis catus) in two suburbs of Auckland City, New Zealand. J Zool 259:309–315. https://doi.org/10.1017/S095283690200328X
Glen AS, Pech RP, Byrom AE (2013) Connectivity and invasive species management: towards an integrated landscape approach. Biol Invasions 15:2127–2138. https://doi.org/10.1007/s10530-013-0439-6
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25:90–98. https://doi.org/10.1016/j.tree.2009.07.016
Hansen N, Hughes NK, Byrom AE, Banks PB (2020) Population recovery of alien black rats rattus rattus: a test of reinvasion theory. Austral Ecol 45:291–304. https://doi.org/10.1111/aec.12855
Hartley LJ (2012) Five-minute bird counts in New Zealand. N Z J Ecol 36:1
Heggie-Gracie SD, Krull CR, Stanley MC (2020) Urban divide: predictors of bird communities in forest fragments and the surrounding urban matrix. Emu 120:333–342. https://doi.org/10.1080/01584197.2020.1857650
Hughey KFD, Kerr GN, Cullen R (2019) Public perceptions of New Zealand’s environment: 2019. Christchurch, New Zealand
Innes J, Kelly D, Overton JMC, Gillies C (2010) Predation and other factors currently limiting New Zealand forest birds. N Z J Ecol 34:86–114
Innes J, Watts C, Fitzgerald NL, et al (2011) Behaviour of invader ship rats experimentally released behind a pest-proof fence, Maungatautari, New Zealand. In: Veitch CR, Clout MN, Towns DR (eds) Island Invasives: Eradication and Management. IYCN, Gland, Switzerland, pp 437–440
Ives CD, Lentini PE, Threlfall CG et al (2016) Cities are hotspots for threatened species. Glob Ecol Biogeogr 25:117–126. https://doi.org/10.1111/geb.12404
Kentish BJ, Dann P, Lowe KW (1995) Breeding biology of the common blackbird turdus merula in Australia. Emu 95:233–244. https://doi.org/10.1071/MU9950233
King CM, Edgar RL (1977) Techniques for trapping and tracking stoats (Mustela erminea); a review, and a new system. New Zeal J Zool 4:193–212. https://doi.org/10.1080/03014223.1977.9517953
King CM, Forsyth DM (2021) The Handbook of New Zealand Mammals, 3rd edn. Otago University Press, Dunedin, New Zealand
Kosmala M, Wiggins A, Swanson A, et al (2016) Assessing data quality in citizen science Assessing data quality in citizen science. Front Ecol Environ 14:551–560. https://doi.org/10.1002/fee.l436
Kruskal JB (1964) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29:115–129
Laake JL (2013) RMark: An R Interface for Analysis of Capture-Recapture Data with MARK
Lam R, Byers KA, Himsworth CG (2018) Beyond zoonosis: the mental health impacts of rat exposure on inner-city residents. J Environ Health 81:8–13
Luck GW (2007) A review of the relationships between human population density and biodiversity. Biol Rev Camb Philos Soc 82:607–645
Lukies K (2020) Ecological Corridors. Auckland, New Zealand: East Bays Songbird Project 1-32. https://drive.google.com/file/d/1MlLAUS8_7rrSLaktaD4OQY3WH5v4LuFa/view?pli=1
MacGregor-Fors I, García-Arroyo M, Quesada J (2022) Keys to the city: an integrative conceptual framework on avian urban filtering. J Urban Ecol 8:1–5. https://doi.org/10.1093/jue/juac026
McNaughton EJ, Beggs JR, Gaston KJ et al (2021) Retrofitting streetlights with LEDs has limited impacts on urban wildlife. Biol Conserv 254:108944. https://doi.org/10.1016/j.biocon.2020.108944
Mill AC, Crowley SL, Lambin X et al (2019) The challenges of long-term invasive mammal management lessons from the UK. Mamm Rev 50:136–146. https://doi.org/10.1111/mam.12186
Miller KF, Wilson DJ, Hartley S et al (2022) Invasive urban mammalian predators: distribution and multi-scale habitat selection. Biology (basel) 11:1527
Morgan DKJ, Waas JR, Innes J (2009) An inventory of mammalian pests in a New Zealand city. New Zeal J Zool 36:23–33. https://doi.org/10.1080/03014220909510136
Morgan DKJ, Waasa JR, Innes J, Fitzgerald N (2011) Identification of nest predators using continuous time-lapse recording in a New Zealand city. New Zeal J Zool 38:343–347. https://doi.org/10.1080/03014223.2011.607835
Nance AH, Wilson M, Burns PA et al (2023) Arboreal activity of invasive rodents: conservation implications for the control of an island pest. Pacific Conserv Biol. https://doi.org/10.1071/pc23011
Noe EE, Innes J, Barnes AD et al (2022) Habitat provision is a major driver of native bird communities in restored urban forests. J Anim Ecol 91:1444–1457. https://doi.org/10.1111/1365-2656.13700
Norbury GL, Pech RP, Byrom AE, Innes J (2015) Density-impact functions for terrestrial vertebrate pests and indigenous biota: guidelines for conservation managers. Biol Conserv 191:409–420. https://doi.org/10.1016/j.biocon.2015.07.031
Oksanen J, Blanchet FG, Kindt R et al (2013) Package ‘vegan.’ Community Ecol Packag Version 2:1–295
Ovenden K, Bassett I, Sumner CL (2024) ‘I want you to want me’: how owners value cats’ choices has implications for cat containment. People Nat 00:1–14. https://doi.org/10.1002/pan3.10580
Owens B (2017) The big cull-can new Zealand pull off an audacious plan to get rid of invasive predators by 2050? Nature 541:148–150
Parsons MH, Banks PB, Deutsch MA et al (2017) Trends in urban rat ecology: a framework to define the prevailing knowledge gaps and incentives for academia, pest management professionals (PMPs) and public health agencies to participate. J Urban Ecol 3:1–8. https://doi.org/10.1093/jue/jux005
Peters MA, Hamilton D, Eames C et al (2016) The current state of community-based environmental monitoring in New Zealand. N Z J Ecol 40:279–288. https://doi.org/10.20417/nzjecol.40.37
Predator Free New Zealand (2022) Find a group. https://predatorfreenz.org/big-picture/national-map/find-a-group/. Accessed 17 Aug 2022
QGIS Development Team (2022) QGIS. Grüt, Böschacherstrasse, Switzerland: QGIS Development Team. https://version.qgis.org/en/site/forusers/download.html
R Core Team (2020) RStudio: Integrated Development for R. Vienna, Austria: R Core Team. https://www.r-project.org/
Rotella J (2019) Chapter 17: nest survival models. Progr MARK a Gentle Introd 17:1–17
Ruffell J, Didham R (2017) Conserving biodiversity in New Zealand’s lowland landscapes: does forest cover or pest control have a greater effect on native birds? N Z J Ecol 41:23–33. https://doi.org/10.20417/nzjecol.41.12
Ruffell J, Innes J, Bishop C et al (2015) Using pest monitoring data to inform the location and intensity of invasive-species control in New Zealand. Biol Conserv 191:640–649. https://doi.org/10.1016/j.biocon.2015.08.022
Russell JC, Stanley MC (2018) An overview of introduced predator management in inhabited landscapes. Pacific Conserv Biol 24:371–378. https://doi.org/10.1071/PC18013An
Santo AR, Sorice MG, Donlan CJ et al (2015) A human-centered approach to designing invasive species eradication programs on human-inhabited islands. Glob Environ Chang 35:289–298
Savard JPL, Clergeau P, Mennechez G (2000) Biodiversity concepts and urban ecosystems. Landsc Urban Plan 48:131–142
Soga M, Gaston KJ (2016) Extinction of experience: the loss of human-nature interactions. Front Ecol Environ 14:94–101. https://doi.org/10.1002/fee.1225
Spatz DR, Zilliacus KM, Holmes ND et al (2017) Globally threatened vertebrates on islands with invasive species. Sci Adv 3:e1603080
Starling-Windhof A, Massaro M, Briskie JV (2011) Differential effects of exotic predator-control on nest success of native and introduced birds in New Zealand. Biol Invasions 13:1021–1028. https://doi.org/10.1007/s10530-010-9886-5
Stats NZ (2021) Subnational population estimates (RC, SA2), by age and sex, at 30 June 1996–2021. https://nzdotstat.stats.govt.nz/wbos/Index.aspx?DataSetCode=TABLECODE7979. Accessed 30 Mar 2022
Strand TM, Lundkvist Å (2019) Rat-borne diseases at the horizon. a systematic review on infectious agents carried by rats in Europe 1995–2016. Infect Ecol Epidemiol 9:1–9. https://doi.org/10.1080/20008686.2018.1553461
Sweetapple P, Nugent G (2011) Chew-track-cards: a multiple-species small mammal detection device. N Z J Ecol 35:153–162
Sweetapple P, Nugent G (n.d.) Chewcards: A guide to the interpredtation of animal tooth impressions. Lincoln, New Zealand: Manaaki Whenua Landcare Res 2–26
Takase Y, Hadi AA, Furuya K (2019) The relationship between volunteer motivations and variation in frequency of participation in conservation activities. Environ Manage 63:32–45. https://doi.org/10.1007/s00267-018-1106-6
Taylor CN, Russell JC, Russell KJ (2020) A strategic social impact assessment for Predator-Free Rakiura, New Zealand, with a human-ecological approach. Socio-Ecol Pract Res 2:161–174. https://doi.org/10.1007/s42532-020-00049-0
Taylor L, Maller CJ, Soanes K et al (2022) Enablers and challenges when engaging local communities for urban biodiversity conservation in Australian cities. Sustain Sci 17:779–792. https://doi.org/10.1007/s11625-021-01012-y
TrapNZ (2023) Trap.NZ Comprehensive User Guide. https://help.trap.nz/books/trapnz-comprehensive-user-guide. Accessed 25 Jul 2023
van Heezik Y, Seddon PJ (2012) Accounting for detectability when estimating avian abundance in an urban area. N Z J Ecol 36(3):1
van Heezik Y, Ludwig K, Whitwell S, McLean IG (2008a) Nest survival of birds in an urban environment in New Zealand. N Z J Ecol 32:155–165
van Heezik Y, Smyth A, Mathieu R (2008b) Diversity of native and exotic birds across an urban gradient in a New Zealand city. Landsc Urban Plan 87:223–232. https://doi.org/10.1016/j.landurbplan.2008.06.004
van Heezik Y, Smyth A, Adams A, Gordon J (2010) Do domestic cats impose an unsustainable harvest on urban bird populations? Biol Conserv 143:121–130. https://doi.org/10.1016/j.biocon.2009.09.013
van Heezik Y, Freeman C, Porter S, Dickinson KJM (2013) Garden size, householder knowledge, and socio-economic status influence plant and bird diversity at the scale of individual gardens. Ecosystems 16:1442–1454. https://doi.org/10.1007/s10021-013-9694-8
Vincze E, Seress G, Lagisz M et al (2017) Does urbanization affect predation of bird nests? a meta-analysis. Front Ecol Evol. https://doi.org/10.3389/fevo.2017.00029
Walker S, Monks A, Innes JG (2021) Life history traits explain vulnerability of endemic forest birds and predict recovery after predator suppression. N Z J Ecol 45:1–13. https://doi.org/10.20417/NZJECOL.45.25
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag
Williams DR, Child MF, Dicks LV et al (2020) Bird Conservation. In: Sutherland WJ, Dicks LV, Petrovan SO, Smith RK (eds) What Works in Conservation 2020. Open Book Publishers, Cambridge, UK, pp 137–281
Worthy T, Holdaway R (2002) The lost world of the moa: prehistoric life of New Zealand. Canterbury University Press, Christchurch, New Zealand
Yokomizo H, Possingham HP, Thomas MB, Buckley YM (2009) Managing the impact of invasive species: the value of knowing the density-impact curve. Ecol Appl 19:376–386. https://doi.org/10.1890/08-0442.1
Acknowledgements
We are grateful to all the householders who volunteered their properties for our research. We thank Zach Carter, James Fazzolari, Jarden Howard, and Shub Sharma for their help with conducting the fieldwork. We thank Imogen Bassett, Brett Butland, Sandra Jack, and Mary Stewart for their guidance on pest management in Auckland. We are grateful to the people and organisations who helped to find volunteer study properties, namely the Eastern Bays Songbird Project, Margaret Voyce, and Spencer Cooper. We thank the two anonymous reviewers and the editors for their helpful suggestions to this manuscript. We thank CatchIT and TrapNZ for allowing us to use their data in our analysis. This research was funded by Auckland Council’s Natural Environment Targeted Rate.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by Auckland Council’s Natural Environment Targeted Rate.
Author information
Authors and Affiliations
Contributions
All authors designed the study; Rosie Gerolemou collected the data; Rosie Gerolemou and James Russell analysed the data; all authors contributed to writing and editing the text and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
All authors receive funding from Auckland Council. James Russell was a science advisor to Zero Invasive Predators.
Ethics approval
The study was approved by the University of Auckland Animal Ethics Committee (22 April 2020 for three years; reference number 002243).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gerolemou, R.V., Russell, J.C. & Stanley, M.C. Outcomes of community-led urban rat control on avifauna. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03401-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03401-7