Abstract
Invasive mammalian predators are a key threat to native fauna globally. Island ecosystems that developed in isolation from mammals are particularly threatened by introduced mammalian predators. This is the case in New Zealand, where introduced mammalian predators have caused the decline of native birds, lizards, and invertebrates. In alpine areas of New Zealand, predator control targets stoats, rats, and cats as they are recognised as the key threats to native birds. Mice, which are known predators of invertebrates at lower elevations, are not actively controlled. As a result, alpine invertebrates in New Zealand represent an ideal focus for a natural experiment to understand the effects of predator control efforts and invasive mice on native invertebrates that evolved in isolation from mammals. In the Fiordland region of New Zealand, we assessed the large-bodied alpine invertebrate community at eight different sites that vary in their occurrence of mice and control of higher-order predators. We found that the recent presence of mice influenced the invertebrate community: wētā (a group of native orthopterans) were less common at sites where mice were present, and the mean body size of invertebrates collected in pitfall traps was larger at sites where mice were absent compared to sites where they were present. Control of other predators (specifically rats and mustelids) did not influence invertebrate body size, abundance, or community composition. Our findings suggest that, as in lowland environments, mice are an important predator of large-bodied invertebrates in the alpine zone and should be incorporated into future predator management programmes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Introduced mammalian predators like mice (Mus musculus), rats (Rattus spp.), stoats (Mustela erminea), and cats (Felis catus) threaten biodiversity globally (Clout 2002; McCreless et al. 2016; Doherty et al. 2015) through direct predation and competition (Medina et al. 2011; Harper and Bunbury 2015; Sánchez-Bayo and Wyckhuys 2019; Dueñas et al. 2021). The evidence for significant population declines and extinctions of native fauna caused by introduced mammalian predators is vast and extends across taxonomic groups including mammals, birds, reptiles, and invertebrates (Kats and Ferrer 2003; Gibbs 2009; Medina et al. 2011; Dueñas et al. 2021). Island ecosystems are especially at risk as many islands have lower species richness than mainland areas and fewer or no native mammalian predators (Kier et al. 2009; Medina et al. 2011). As a result, species endemic to islands can have limited behavioural and physiological defences against introduced mammalian predators (Angel et al. 2009).
New Zealand, which is made up of three large ‘mainland’ and numerous small ‘offshore’ islands, represents an ideal opportunity to explore the effects of introduced predators, including mice, on populations of native invertebrates that evolved without native mammalian predators (Parkes and Murphy 2003; Murphy et al. 2019). Early Polynesian settlers were responsible for the introduction of kiore (Rattus exulans) and kurī (domestic dogs, Canis familiaris) to New Zealand (Holdaway 1996; Clark 1997). Later, European settlers brought ship rats (R. rattus), Norway rats (R. norvegicus), and house mice (as stowaways on ships; King and Forsyth 2021), while stoats, feral cats, brushtail possums (Trichosurus vulpecula), and hedgehogs (Erinaceus europaeus) were intentionally released for reasons such as establishing a fur trade and as bio-control for other introduced mammals that had already started to become pests (King and Forsyth 2021). Many of these introduced mammalian predators have become prevalent throughout New Zealand and are key threats to the survival of native fauna (Hitchmough et al. 2016, 2021; O’Donnell et al. 2018; Robertson et al. 2021). As a result, suppressing predator populations through aerial poison applications and intense trapping programmes is a major component of New Zealand conservation action (Pryde et al. 2005; Innes et al. 2010; Reardon et al. 2012; Hoare et al. 2013). Predator control initiatives, including large-scale trapping programs and aerial 1080 poison operations, typically prioritise larger introduced predators like stoats, cats, and possums, while overlooking mice, which may play a significant role as predators for invertebrates.
There is ample evidence that invasive mice are particularly harmful to island invertebrates and are implicated in the suppression and local extinction of many invertebrate groups (St Clair 2011). In a review of the impacts of mice on islands in the Southern Ocean, (Angel et al. 2009) found evidence from comparative studies that strongly suggest mice can be highly selective predators and can influence invertebrate community composition, body size, and relative abundance. On sub-Antarctic Marion Island, where on average mice consumed 194 g (dry mass) of invertebrates per day, mice preferentially selected larger invertebrates including adult moths, moth larvae, and weevil larvae (Smith et al. 2002). Researchers have found similar trends on Guillou Island (Le Roux et al. 2002) and Macquarie Island (Copson 1986) with invertebrates making up a considerable portion of mouse diet (spiders, for example, were in 70% of mouse stomachs on Guillou Island). On Antipodes Island, where mice were abundant before their eradication, mice preferentially fed on Amphipoda, Lepidoptera and some species of Coleoptera and there were significantly fewer large macroinvertebrates compared to nearby mouse-free Archway Island (Russell 2012).
At lower elevations on mainland New Zealand, there is evidence that mice are similarly destructive to the invertebrate community. Introduced mammalian predators are key drivers of declines for invertebrate communities in forests, shrublands and grasslands (McGuinness 2001). Watts et al. (2011, 2022) have shown that mammals, and mice in particular, have significant impacts on the ground-dwelling invertebrate community within a lowland North Island forest. Watts et al. (2011) studied how the removal of thirteen mammal species (all introduced herbivores and predators except mice) from Maungatautari Sanctuary (3239 ha, 240–797 m asl) influenced the invertebrate community. The eradication resulted in an increase of adult tree wētā (Hemideina thoracica) from 0.2 to 1.2 wētā caught per pitfall trap (n = 20), while other wētā species increased from effectively 0 to 2 wētā per trap (Watts et al. 2011). In a subsequent 5-year study in a South Island grassland ecosystem (Macraes Conservation Area, eastern Otago, 400-600 m a.s.l.), Norbury et al. (2022) found that mice needed to be excluded from or extremely scarce (< 5% tracking tunnel printing rate based on the density of footprints) before invertebrate communities began to recover. Overall, our understanding of the interactions between mice and invertebrates in New Zealand is primarily confined to off-shore islands and lower elevation areas of the mainland. There is a lack of knowledge regarding whether, or to what degree, the impact of mice extends to alpine regions.
Our research represents one of the first studies to specifically explore the relationship between introduced mammalian predators, particularly mice, and the large-bodied ground-dwelling invertebrate community in the New Zealand alpine zone. On the South Island of New Zealand, common terrestrial alpine invertebrates include ground beetles, wētā (the New Zealand name for Orthoptera in the families Anostostomatidae and Rhaphidophoridae), spiders, landhoppers (terrestrial amphipods), grasshoppers, centipedes, and harvestmen (O’Donnell et al. 2017). Although mice likely influence a wide range of invertebrate groups, we focus on large-bodied invertebrates because they have been shown to be preferentially targeted in lowland studies (Watts et al. 2011, 2022). This targeting may be due to the fact that they are flightless, long-lived, relatively slow to develop, ground-dwelling, and slow-moving making them especially vulnerable to predation and potentially slower at bouncing back from predation pressure (McGuinness 2001; Stringer & Hitchmough 2012). Consequently, we also expect this group to be the first to respond to predation pressures from introduced mammalian predators in the alpine zone.
We used pitfall traps to compare the composition, abundance, and body size of the large-bodied invertebrate community (which we define as individuals that are greater than or equal to 1 cm in body length) across sites with and without mice and with and without predator control. Our sites consisted of four alpine areas on the South Island of New Zealand and four alpine areas on nearby offshore islands. Although predator control targeted stoats, rats, possums and cats, our study focused on the results for stoats and mice, as they are the resident introduced predators in alpine areas of Fiordland (O’Donnell et al. 2017). Given the known effects of mice on the invertebrate community at lower elevations, we expected mice to reduce invertebrate abundance and body size. Stoats are prolific hunters of invertebrates, but they have a varied diet that also includes mice (Smith et al. 2005; McAulay et al. 2020). Removing stoats from alpine zones may benefit some invertebrate groups, like wētā, which are a key part of the stoat diet (Smith et al. 2005), but it might also release predation pressure on mice or weasels (Mustela nivalis) allowing them to prey upon invertebrates (Peltzer et al. 2019; McAulay and Monks 2023). Consequently, we expected predator control not to alter large-bodied invertebrate community composition, abundance, or body size because the presence of mice would counteract any benefits received from controlling stoats.
Methods
Study area
This study occurred at eight sites above the climatic treeline in Southern New Zealand. Site altitude ranged from 700 to 1600 m above sea level (a.s.l.). Four sites were on the South Island, while the others were on smaller ‘offshore’ islands. All sites had vegetation dominated by tussock grasslands with small sections of scree, boulders, and patches of dense shrub. Tussock grasslands consisted of 50–60% mid-ribbed snow tussock (Chionochloa pallens), narrow-leafed snow tussock (C. rigida), red tussock (C. rubra), fescue tussock (Festuca novae-zelandiae), and needle snow tussock (C. acicularis). Other common plants included mountain buttercup (Ranunculus lyallii), dracophyllum (Dracophyllum rosmarinifolium), common speargrass (Aciphylla squarrosa), mirror bush (Coprosma prostrata) and species of the woody shrub Gaultheria. Scree and boulder sections were 60–80% rock-covered, ranging from small pebbles to scree to large boulders and interspersed with mosses and ferns. Shrub patches were dominated by woody shrubs no more than 1 m tall, including hebes (e.g., Veronica hectorii) and dracophyllum, as well as a variety of non-woody native plants like mountain daisies (Celmisia spp.), mountain buttercup, common speargrass, and various ferns and mosses.
Predator control regimes
The eight sites (five that are known to have mice and three that are mouse-free) were subject to a range of active predator control regimes run by government and community groups (Table 1). Sites with predator control were Homer and Gertrude Valley, Shy Lake, Secretary Island, Resolution Island and Table Hill (Table 1). Standard trapping for stoats, rats and hedgehogs in New Zealand uses small to medium-sized kill traps known as DOC150 and DOC200 traps (Warburton et al. 2008). A local conservation volunteer group runs trapping lines to control mustelids, rats, and hedgehogs at the Homer and Gertrude Valleys (Weston et al. 2018). Secretary Island is rodent-free and has an extensive 108 km trapping line to keep stoat populations at low levels (McMurtrie et al. 2011). Mice are present on Resolution Island, and there has been considerable effort to keep stoat levels low through extensive trapping (Edge et al. 2011; Murphy et al. 2016). Blaikies Hill and Table Hill are located on Stewart Island (Rakiura), which is free from mice and mustelids, but rats and cats are present. Blaikies Hill has no predator control. Predator control at Table Hill consists of trap and bait lines to control rats and feral cats (Dowding and Davis 2007). Note that Table Hill is home to a colony of New Zealand dotterel (Anarhynchus obscurus) however this is a critically endangered population (c 250 total individuals) that breed in herb-fields and exposed hilltops across the larger island of Rakiura, so we expect they have very little influence on the invertebrate community. Shy Lake is situated on the peninsula between Breaksea Sound and the Wet Jacket Arm of Dusky Sound, which received an aerial 1080 (manufactured version of fluoroacetate) predator control treatment in June 2020 (Anon. 2020). Aerial 1080 operations target possums and rats, while stoats are killed via secondary poisoning (Eason et al. 2011).
Most of the mice present sites have evidence of recent mouse activity. Mice were present but uncommon in surveys at Homer and Gertrude and Shy Lake in 2021 (tracking rate of 0.08 for both sites), while mice were very prevalent in Borland in 2020 (tracking rate of 0.82; DOC unpubl. data). Mice were fairly prevalent at Resolution Island (tracking rate of 0.36) while they were less common at Lake Roe (tracking rate of 0.10); however these surveys are not as recent, and no later data was available (2017 and 2018 respectively; DOC unpubl. data). All tracking rates are from 24-h surveys and are presented in Online Resource 1. Historically, sites with predator control have low levels of stoats and rats. Rakiura is stoat-free but there is no available trapping data for rats at Table Hill. From 21-day surveys using tracking tunnels, no rats were observed at Shy Lake, Resolution Island or Secretary Island while Homer and Gertrude had a rate of 0.05 of 35 tunnels. For stoats, no individuals were observed at Homer and Gertrude, Shy Lake, or Resolution Island. For Secretary Island, stoats occurred at low levels (an average of 13.5 stoats per hectare). Most recent tracking rates for rats and stoats and the number of traps set for each predator control site be found in Online Resource 1.
Study design
Individual sites were monitored on different dates between November 2021 and February 2022 (Austral summer) due to the large distances between sites and the costs associated with accessing them (see Table 1 for dates). We installed four pitfall grids at each of our eight field sites. Grids consisted of four rows of four pitfall traps spaced 15 m apart giving 16 pitfalls per grid (64 pitfall traps per site). Before visiting each site, we generated 16 potential grid locations by creating random GPS points within suitable alpine habitats based on aerial imagery in QGIS (QGIS Development Team 2022). We then visited each potential grid location in turn, starting at point one, and installed a grid if the area was both accessible and large enough. We made the pitfall traps from 1L clear plastic bottles with a 90 mm diameter opening. We cut off the top quarter of each bottle, inverted it to make a funnel, and trimmed the container and funnel to reduce the overall size of the trap (average trap depth around 90 mm). We derived the trap design from Hohbein and Conway (2018), who recommend using funnels to limit bycatch of non-target species (such as lizards) and suggest that traps be made of clear plastic as trap colour can influence which invertebrates are caught (Buchholz and Möller 2018). Because there was still potential to catch threatened native lizards in our traps, we filled the pitfalls with ~ 2 cm of water rather than a preserving liquid so that any lizards inadvertently caught could be released unharmed. This was possible because traps were checked daily when open.
We paired each pitfall grid with a line of five tracking tunnels (Black Trakka™ 500 × 100 × 100 mm; Gotcha Traps, Warkworth, New Zealand), spaced 50 m apart following the New Zealand Department of Conservation’s recommended distance to optimise surveying for rodents (Gillies and Williams 2013). We placed the line of tracking tunnels 50 m upslope from its paired pitfall grid running parallel to the ridgeline. If that was not possible, due to the location of the grid, the tunnel line was run downslope from the gridline. The tunnels were baited with ~ 5 g of Smooth Peanut Butter (Pams Products Ltd, New Zealand) placed on the ink in the centre of each tracking card. We left the tunnels overnight and checked them daily for three days.
We opened the pitfall traps for three nights, except at Blaikies Hill, where traps were open for two nights because of adverse weather. At each site, we installed the four pitfall grids on the afternoon of the first day and checked all pitfall traps daily. From each trap, we identified invertebrates that were ≥ 1 cm long to the taxonomic family level, except for harvestmen which we were only able to identify to the order level. For spiders and harvestmen, we measured body size as diagonal leg span, a common measurement for this group (e.g., Seyfarth et al. 1982). For all other groups, we measured body size as the length from the front of the head to the tip of the abdomen excluding legs, antennae, and ovipositor. We kept at least three example specimens of each family that we caught and preserved these in 70% ethanol. We released additional invertebrates back into the environment after they were measured and recorded. We did not break the surface tension of the water, so many invertebrates were alive when we sampled the catch the following day (although some did drown overnight). We chose not to keep all invertebrates to limit our study’s impact on the invertebrate community. On the last day, we checked, emptied, and removed all the pitfall traps. For the entire three-day period over which pitfalls and tracking tunnels were active at each site, we recorded hourly temperature and relative humidity with two HOBO loggers (model number MX2301A, Onset Computer Corp., Pocasset, MA). We placed the loggers at ground level in shaded locations within two of the four pitfall grids selected at random.
Statistical analysis
Hill numbers, and an accumulation curve of taxonomic richness at the family level (to assess sampling effort), were generated for the invertebrate community at each site using the hillR package (Li 2018) and the random method in the Vegan package (Oksanen et al. 2020) respectively. The random method calculates a mean accumulation curve and its standard deviation based on random permutations of the data (Collwell et al. 2012). To investigate whether the presence of mice or predator control influences large-bodied invertebrate abundance, we ran a generalised linear mixed model (GLMM) with a negative binomial distribution using the package glmmTMB (Brooks et al. 2017). The model contained counts of invertebrates at all eight sites as the response variable. We combined observations of invertebrates at the pitfall grid level so there were four replicates per field site. We then included the presence of mice and predator control as categorical predictor variables in addition to the daily average maximum temperature and relative humidity as continuous predictor variables. We included the site as a random effect to account for repeated measures. We then ran a similar model with a subset of the data that only contained observations of wētā. This model contained the same structure as the model described above but with a Tweedie distribution using the package glmmTMB.
We also ran a generalised linear model (GLM) with an inverse-Gaussian distribution using the package lme4 (Bates et al. 2015) to examine the influence of mice and predator control on the body size of the largest invertebrates. To achieve this, we truncated the data to only include the largest 50% of invertebrates caught (individuals larger than 1.5 cm) to better discern whether introduced mammalian predators were removing the largest invertebrates from the community. As we were not using kill traps, there was potential to re-sample the same individual across the three-day sampling period, so we used data only from the first night of sampling. This was necessary as the smaller sample size, containing the largest 50% of invertebrates would not allow us to include a random effect. The model contained invertebrate body size as the response variable, with the presence of mice and predator control as separate categorical predictor variables and average daily minimum temperature and relative humidity as covariates.
Lastly, we used a permutational multivariate analysis of variance (PERMANOVA, 9,999 permutations; Anderson, 2001) based on a Bray–Curtis dissimilarity index to examine differences in the invertebrate community in response to the presence of mice and predator control. We also conducted a permutational analysis of multivariate dispersion (PERMDISP, Anderson, 2001, Anderson 2006) to determine if the distribution or spread of groups is significantly different. This analysis was completed using the package Vegan in R version 4.2.1 (Oksanen et al. 2020; R Core Team 2023). We then used non-metric multidimensional scaling (NMDS) with a Bray–Curtis dissimilarity index to generate minimum convex polygons (containing all points within the group) to visualise the relationships between invertebrate communities (Fig. 1).
Map of the eight field sites visited during the 2022 summer season. Black dots represent sites with active predator control, while red diamonds represent sites without predator control. Secretary Island and Stewart Island/Rakiura are mouse free while mice are present at the remaining five sites. The inset is New Zealand and the zoomed in map is Fiordland and Stewart Island. The outline of New Zealand is the NZ coastline and island Topo map generated by Land Information New Zealand accessed through the Land Resource Information System portal (LRIS; https://lris.scinfo.org.nz/ accessed Aug 2023)
Results
Overview
Over the entire study, we observed 1362 invertebrates from 8 taxonomic groups, with wolf spiders (Lycosidae), ground beetles (Carabidae), wētā (Anostostomatidae and), and landhoppers (Amphipoda) being the most common (Fig. 2). Table Hill was the most active site, with 334 invertebrate captures, while Lake Roe and Shy Lake were the least active sites, with 86 captures each (Table 2). The average nightly (9:00 pm to 6:00 am) maximum and minimum temperatures ranged from 5.5–19.8 °C and 2.3–8.6 °C respectively across the eight sites (Table 2). For invertebrates ≥ 1 cm long, average body size was 1.5 cm, and the largest invertebrate was 4.3 cm (a carabid beetle of the genus Mecodema from Borland). Among sites, Shannon diversity (q1) ranged from 1.27 to 4.19, with Secretary Island exhibiting the highest diversity. Simpson diversity (q2) varied from 1.10 to 3.70, with Secretary Island and Lake Roe showing the highest dominance. Richness (q0) ranged from 4 to 7 families, with Secretary Island having the most diversity (Table 2). The species accumulation curve (at the family level) showed little change in the number of families per sample after sample 20, suggesting that we did a fairly good job of sampling the community and that additional samples or observations would not substantially increase the number of observed families (the accumulation curve is presented in Online Resource 1). Lastly, we only recorded one mouse track across all eight field sites over the entire summer, which was recorded at Lake Roe.
Total invertebrate abundance
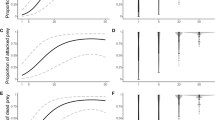
A total of 1362 observations of invertebrates across all eight field sites (i.e., a cumulative total of all invertebrates) were included in this analysis. We found the presence of mice and predator control did not significantly influence total invertebrate abundance (χ2 (1) = 0.85, P = 0.35; χ2 (1) = 0.03 P = 0.85; respectively). Invertebrate abundance was influenced by daily minimum temperature (χ2 (1) = 3 0.86, P = 0.04, model fit = 0.26). For the wētā only model, we found that the sites with mice had significantly fewer wētā compared to sites without mice (χ2 (1) = 09.98, P = 0.001; Fig. 3a). Predator control, on the other hand, did not influence wētā abundance (χ2 (1) = 0.34 P = 0.55; Fig. 3b). Wētā abundance was also significantly influenced by daily minimum temperature (χ2 (1) = 32.00 P = < 0.001) where observations of wētā increased with rising temperatures.
Invertebrate body size
To determine the effect of mouse presence and predator control on invertebrate body size, we analysed only the largest half of all invertebrates (individuals greater than 1.5 cm in body size) caught on the first night across all eight sites (n = 223/529). The presence of mice significantly influenced invertebrate body size. The invertebrates caught at sites without mice were slightly larger (mean 2.5 cm) when compared to sites with mice (mean 2.3 cm) (χ2 (1) = 7.65, P = 0.006; Fig. 4a). Conversely, invertebrate body size was not significantly influenced by the presence of predator control (χ2 (1) = 0.52, P = 0.46, Fig. 4b, model fit = 0.17). The largest invertebrates, measuring around 3.5 cm in body size at the mice absent sites were identified as being large wētā (Anostostomatidae). Wētā of similar size were not recorded at the mice present sites (Fig. 4a).
Generalised linear model results of the influence of a mouse presence and b predator control (focusing on stoats and rats) on invertebrate body size. We truncated the data to the largest 50% of invertebrates (individuals larger than 1.5 cm) and only included individuals caught on the first night. Figures represent the mean and 95% confidence intervals for each factor
Invertebrate community composition
There was no difference in dispersion between sites with and without mice, nor between sites with and without predator control (PERMDISP; Table 3). We found a small but significant difference in community composition between sites with and without mice (R2 = 0.17, P < 0.001; Table 3; Fig. 5). Wētā were less common in sites where mice were present (Fig. 5). Predator control had no significant influence on community composition (R2 = 0.03, P = 0.36; Table 3; Fig. 6).
Non-metric multidimensional scaling (NMDS) ordination of large-bodied terrestrial invertebrate groups as measured at sites with and without mice (k = 3). Red points represent sites where mice are absent and blue points represent sites where mice are present. The red polygon is an ellipsoid hull that encompasses all sites where mice are absent, while the blue polygon encompasses all sites where mice are present. Taxonomic group labels are centered around sites that they are the most associated in the 2D space
Non-metric multidimensional scaling (NMDS) ordination of large-bodied terrestrial invertebrate groups as measured at sites with (blue points) and without (orange points) predator control (k = 3). The orange polygon is an ellipsoid hull that encompasses all sites that have no active predator control, while the blue polygon encompasses all sites with predator control. Taxonomic group labels are centered around sites that they are the most associated in the 2D space
Discussion
Our study provides new evidence suggesting that mice are influencing the large-bodied invertebrate community in alpine areas of the southern South Island of New Zealand. We found that wētā were less common in sites where mice were present. The largest 50% of invertebrates on the first sampling day were also slightly larger at mouse-free sites than at sites with a history of mice. We did not find the presence of mice to influence invertebrate abundance, but we did find that wētā were more abundant at sites that were mice-free. Lastly, the presence of predator control targeting stoats, rats and or cats had no significant effect on invertebrate community composition, abundance, or body size.
At lower elevations, there is strong international evidence that mice influence invertebrate abundance, body size and community composition (Marris 2000; Angel et al. 2009; St Clair 2011; Houghton et al. 2019). For example, comparisons of invertebrates on sub-Antarctic Marion Island with those on nearby mouse-free Prince Island suggest the presence of mice influenced invertebrate community composition, causing a decrease in the body size of medium to large-bodied invertebrates (Crafford and Scholtz 1987; Crafford 1990; Chown and Smith 1993). Similarly, in the North Island of New Zealand, the invertebrate community within a mammal-proof fence where mice are actively controlled (within Zealandia Ecosanctuary) was found to be significantly different from the community outside the fence where mice are not controlled, and some beetle species were up to 20 times more prevalent within the mouse-free enclosure (Vergara et al. 2021). Watts et al. (2022) removed mice from a 24-ha area within the fenced Maungatautari sanctuary and allowed mice to remain in an adjacent, independently fenced, 17-ha area. After surveying the invertebrate community in each enclosure, the treatments were swapped. Watts et al. found invertebrate abundance and body size increased substantially after removing mice, then decreased again after mice were allowed to build up once more.
The lack of an effect of the presence of mice on the invertebrate community in our study could be a result of the low number of mice that were active during our sampling period. Although mice are known to be consistently absent from our mouse-free sites, mouse abundance can vary greatly at sites where they are present as mouse populations boom and bust with food availability (often associated with Chionochloa or beech mast seeding in the alpine zone). For instance, Wilson & Lee (2010) investigated the impact of tussock mast seeding during the summer of 2005/06 on mouse populations. They discovered that mouse density surged from 4 individuals per hectare in autumn to 39 individuals per hectare by spring of 2006, before declining to 8 individuals per hectare the following autumn (May 2007). By the subsequent spring (November 2007), no mice were captured across 768 trap-nights. This research highlights the boom-bust relationship that mice have with seed masts, where populations boom during the mast when food is available, and then crash as the inflated population strips the environment of resources. The last seed mast event on the South Island was in 2019 (Nichols et al. 2021; O’Malley et al. 2022). As a result, our study took place two years after the mast when mice had become relatively scarce, and the invertebrate community may have had time to recover from the intense predation pressure associated an inflated mouse population driven by the tussock mast. To test our hypothesis that mice are driving the differences that we observed in the invertebrate community, we would need to repeat this study before, during and immediately after a mast year to observe the influence of mice on the invertebrate community when predation pressure is at its peak.
Though there is little information on mouse diet in alpine areas of New Zealand, Wilson and Lee (2010) found mouse stomach contents to be dominated by arthropods and to switch to seeds during a mass seed event. Studies at lower elevations and offshore islands show that mice consume a range of plant, vertebrate and invertebrate species, but that invertebrates often make up much of their diet (Copson 1986). For example, in a study of the stomach contents of 102 mice near Dunedin Otago, 86% of stomachs contained plant material, and 90% contained invertebrates, where Lepidoptera, Coleoptera and Araneae were commonly consumed (Miller and Webb 2001). On Antipodes Island, mouse stomachs were found to contain plant matter, bird remains, and invertebrates including Lepidoptera larvae, Acarina, adult Coleoptera and Araneae (Russell et al. 2020). There is also evidence of mice influencing native lizards in New Zealand, where skink populations were found to have a negative relationship with mouse abundance in the Eglinton Valley, Fiordland National Park (Monks et al. 2024).
Although we detected likely effects of mouse pressure on the alpine invertebrate fauna, there are alternative explanations for why invertebrate body size may differ between sites with and without mice. For example, there are likely to be natural geographic differences in the species present at the different sites, even within taxonomic groups, and these species may differ in size. Indeed, we caught multiple species of Hemiandrus wētā, a genus whose body size can vary greatly. Hemiandrus focalis was species of wētā common to our field sites that are classified as a medium to large wētā (maximum body size of 24 mm, head width 6.8–9.6 mm, used as a proxy for body size; Salmon 1950), while H. maculifrons is classified as a small wētā (head width of 3.6–4.9 mm; Smith et al. 2013). In our study, the maximum body size for wētā in sites with and without mice was similar (3.0 and 3.3 cm, respectively), as was the body size of spiders (2.2 and 2.3 cm, respectively). This suggests that similarly sized wētā and other species of invertebrates were present at all sites, but larger individuals were less common at sites where mice were present.
Temporal effects could also influence our body-size data from different sites as our sampling occurred over several months. Adults of different species of holometabolous groups, like beetles, can emerge at different points in the season (Harry et al. 2011), and juveniles of hemimetabolous groups, like wētā and spiders, get larger over time as individuals mature and eventually become adults. We sampled our eight sites at different times across the austral summer, therefore it would have been possible to sample all the ‘mice present’ sites when there were more early-instar hemimetabolous invertebrates present. Similarly, mice-present sites could have been sampled before large adult beetles emerged or after they reproduced and died. However, we surveyed mice free and mice present sites fairly evenly throughout the summer, visiting representatives of both site categories in early summer (Nov-Dec) and mid-summer (January). Additionally, large Hemiandrus species, including H. fiordensis (which was common at our field sites), may take up to 3 years to reach sexual maturity (Johns 2001; Van Wyngaarden 1975), and at sites we have visited repeatedly (unpublished data) we observed both juveniles and adults to be present in all summer months, rather than distinct periods with only adults or immatures. Thus, it is likely that a range of differently sized wētā and other invertebrates inhabit our sites throughout the summer. Differences in body sizes observed between sites are unlikely to be driven primarily by the time at which we sampled and the correlation we observed with predator presence is likely to be a real effect.
Our community composition results and wētā only model highlight that mice may be particularly threatening to alpine wētā. Other studies have shown that wētā are consumed by mice at lower elevations, and they are a common part of stoat diet in alpine areas of the South Island (Smith et al. 2005, 2010). For example, McAulay et al. (2020) found that stoat diet had a greater reliance on invertebrates, especially wētā, during the summer when invertebrates were more readily available in the environment. This is concerning as many alpine wētā are long-lived and develop slowly compared to other invertebrates. As noted above, H. fiordensis found in our study can take up to three years to reach sexual maturity, and there is evidence that larger species in other alpine genera (Hemideina, Deinacrida) can live even longer; for example the mountain stone wētā (Hemideina maori) can take three to four years to reach sexual maturity and may live for one to four breeding seasons as an adult (Jamieson et al. 2000; Leisnham et al. 2003). While ground wētā have fairly high fecundity (clutch size of 20–60 eggs; Gwynne 2004), stoat diet analyses demonstrate that an individual can consume upwards of seven wētā in a single night (Purdey et al. 2004; Smith et al. 2008). Given how long it takes for wētā to fully develop into adults, this suggests wētā recruitment could struggle to keep up with predation pressure from introduced mammalian predators. Unfortunately, we don’t understand the level of pressure they can tolerate or whether they can fully recover from intense periods of predation pressure such as what occurs following masting events.
Predator control and mesopredator release
Predator control did not appear to influence community composition, abundance, or body size for the large-bodied alpine invertebrate community. Current predator control strategies in New Zealand focus on protecting avifauna through large-scale poisoning and trapping programmes (Elliott and Kemp 2016; Innes et al. 2019; Leathwick and Byrom 2023; Nichols et al. 2021; Reardon et al 2012). Work in the alpine zone is an extension of large-scale predator control or species-specific localised control for threatened species, like rock wren (Xenicus gilviventris) and focuses on larger predators like stoats (Little et al. 2017; Weston et al. 2018). Trapping and aerial 1080 poisoning are effective tools to control stoat populations (Dilks et al. 2020; Nichols et al. 2021), which likely benefits the invertebrate community. However, mouse kills from pest control tools such as 1080 are variable, and mouse populations bounce back relatively quickly after predator control operations, making large-scale operations less effective for mice (van Heezik et al. 2020). As a result, fauna susceptible to mouse predation may remain vulnerable and continue to face high predation pressure even when they reside in areas with active predator control. It is likely that invertebrates in our predator control sites still face predation pressure from mice (if not in the study season, then in recent previous seasons), which could explain why we saw no difference between locations with predator control and those without.
Controlling for specific predators, instead of the full suite of introduced mammalian predators, can limit conservation outcomes, or even generate negative outcomes for native species. In New Zealand, suppression of cats and stoats resulted in a boom of rodent populations when there was enough food in the system to support them, which increased predation pressure on native fauna (Norbury et al. 2013; Tompkins et al. 2013; Whitau et al. 2023). Similarly, on Little Barrier Island, lethal control of cats increased the number of predatory rats, causing a decline in the breeding success of the Cook’s petrel (Pterodroma cookii). Globally there are multiple examples of mesopredator release as an unintended consequence of predator control that has led to adverse conservation outcomes for species and habitat (Barton and Roth 2008; Ritchie and Johnson 2009; Colman et al. 2014; Marlow et al. 2015). While we lack sufficient data to confirm whether meso-predator release is occurring in alpine areas of New Zealand, it is important to note that excluding mice and other potential predators from predator control efforts could potentially hinder conservation outcomes. As a result, predator control efforts should be more comprehensive and be cognisant of the trophic interactions between predators (Doherty et al. 2015).
To make predator control more comprehensive and support large-bodied invertebrates in the New Zealand alpine zone, our findings suggest that adaptive management strategies including mouse control are warranted given the mounting evidence that mice are key predators for many endemic species. While it is difficult to monitor mouse populations when they are at low densities (we only detected one mouse print from tracking tunnels across the five sites where mice are known to occur), mice can still influence the invertebrate community when they are scarce in the environment (Norbury et al. 2022). On smaller scales, mice have been successfully eradicated from multiple offshore islands through intensive poisoning programmes using anticoagulant rodenticides (Griffiths et al. 2015; Horn et al. 2019; Martin and Richardson 2019). In these cases, bait programmes were meticulously planned to ensure all rodents had a high chance of being exposed to the bait and to limit exposure to non-target wildlife and potable water supplies (Martin and Richardson 2019). As the size of the target area for control increases, so do the logistical challenges, costs, and risk of failure (Livingstone et al. 2022). Consequently, intensive aerial eradication efforts are currently impractical for the scale of poisoning required to eradicate mice from large mainland areas. Intensive baiting in smaller locations with natural barriers to mouse movement may be a way to support populations of individual threatened invertebrate species or localised invertebrate communities in the alpine zone. However, these efforts require constant upkeep, and mice often re-invade quickly (Gillies et al. 2003; Elliott and Kemp 2016); therefore, investment in new tools that are effective against mice across large areas is essential if we hope to support native species at greater scales.
Conclusion
The interactions between introduced predators and native species are complex. Our study makes initial contributions to understanding the relationship between large-bodied invertebrates and the suite of introduced predators in New Zealand’s alpine areas. Our results suggest that mice influence large-bodied invertebrate body size and community composition, particularly the presence of wētā, and we were able to detect these differences in a year when mouse abundance was low. Further work comparing periods of high and low mouse abundance is required to better understand this dynamic. Predator control efforts, primarily targeting stoats, were not found to influence the invertebrate community despite stoats being known predators of invertebrates. These results suggest that predator control needs to be extended to include mice to benefit large-bodied invertebrates in alpine areas of New Zealand. Our findings also support trends in other studies where controlling for a single predator instead of the full suite of predators can limit conservation outcomes for native fauna. As a result, we need to ensure that our predator control programmes are more comprehensive and account for the interactions between predator species to maximise conservation outcomes.
Competing interests
The authors have no competing interests to disclose.
Data availability
The data for this paper are not publicly available, but will be made available on request.
References
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253
Angel A, Wanless RM, Cooper J (2009) Review of impacts of the introduced house mouse on islands in the Southern Ocean: are mice equivalent to rats? Biol Invasions 11:1743–1754
Anon (2020) Operational report for Norway rat, possum, and ship rat control in the Wet Jacket Peninsula. Department of Conservation, Te Anau
Barton BT, Roth JD (2008) Implications of intraguild predation for sea turtle nest protection. Biol Conserv 141:2139–2145. https://doi.org/10.1016/j.biocon.2008.06.013
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bertoia A, Murray T, Robertson BC, Monks JM (2023) Pitfall trapping outperforms other methods for surveying ground-dwelling large-bodied alpine invertebrates. J Insect Conserv 27:679–692
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/RJ-2017-066
Buchholz S, Möller M (2018) Assessing spider diversity in grasslands—Does pitfall trap color matter? J Arachnol 46:376–379. https://doi.org/10.1636/JoA-S-16-062.1
Chown SL, Smith VR (1993) Climate change and the short-term impact of feral house mice at the sub-Antarctic Prince Edward Islands. Oecologia 96:508–516. https://doi.org/10.1007/BF00320508
Clark GR (1997) Maori subsistence change: zooarchaeological evidence from the prehistoric dog of New Zealand. Asian Perspect 36:200–219
Clout MN (2002) Biodiversity loss caused by invasive alien vertebrates. Eur J Wildl Res 48:51–58. https://doi.org/10.1007/BF02192392
Colman NJ, Gordon CE, Crowther MS, Letnic M (2014) Lethal control of an apex predator has unintended cascading effects on forest mammal assemblages. Proc R Soc B Biol Sci 281:20133094. https://doi.org/10.1098/rspb.2013.3094
Colwell RK, Chao A, Gotelli NJ et al (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5:3–21
Copson GR (1986) The Diet of the Introduced Rodents Mus-Musculus L and Rattus-Rattus L on Sub-Antarctic Macquarie Island. Wildl Res 13:441–445. https://doi.org/10.1071/wr9860441
Crafford JE (1990) The role of feral house mice in ecosystem functioning on Marion Island. In: Antarctic ecosystems. Springer, pp 359–364
Crafford JE, Scholtz CH (1987) Quantitative differences between the insect faunas of sub-Antarctic Marion and Prince Edward Islands: a result of human intervention? Biol Conserv 40:255–262
Davies D, Dilley B, Bond A et al (2015) Trends and tactics of mouse predation on Tristan Albatross Diomedea dabbenena chicks at Gough Island. South Atlantic Ocean. Avian Conserv Ecol 10:1
Dilks P, Sjoberg T, Murphy EC (2020) Effectiveness of aerial 1080 for control of mammal pests in the Blue Mountains, New Zealand. N Z J Ecol 44:1–7
Doherty TS, Dickman CR, Nimmo DG, Ritchie EG (2015) Multiple threats, or multiplying the threats? Interactions between invasive predators and other ecological disturbances. Biol Conserv 190:60–68. https://doi.org/10.1016/j.biocon.2015.05.013
Dowding JE, Davis AM (2007) New Zealand dotterel (Charadrius obscurus) recovery plan, 2004–14. Department of Conservation, Wellington Report 58
Dueñas M-A, Hemming DJ, Roberts A, Diaz-Soltero H (2021) The threat of invasive species to IUCN-listed critically endangered species: a systematic review. Glob Ecol Conserv 26:e01476. https://doi.org/10.1016/j.gecco.2021.e01476
Eason C, Miller A, Ogilvie S, Fairweather A (2011) An updated review of the toxicology and ecotoxicology of sodium fluoroacetate (1080) in relation to its use as a pest control tool in New Zealand. N Z J Ecol 1:1–20
Edge KA, Crouchley D, McMurtrie P et al (2011) Eradicating stoats (Mustela erminea) and red deer (Cervus elaphus) off islands in Fiordland. Isl Invasives Erad Manag 1:166–171
Elliott G, Kemp J (2016) Large-scale pest control in New Zealand beech forests. Ecol Manag Restor 17:200–209
Foster NJ, Maloney RF, Recio MR et al (2021a) European hedgehogs rear young and enter hibernation in New Zealand’s alpine zones. N Z J Ecol 45:1–6
Foster NJ, Maloney RF, Seddon PJ et al (2021b) Altitudinal distribution of the entire invasive small mammal guild in the eastern dryland zone of New Zealand’s Southern Alps. Biol Invasions 23:1837–1857. https://doi.org/10.1007/s10530-021-02474-y
Gibbs GW (1998) Why are some weta (Orthoptera: Stenopelmatidae) vulnerable yet others are common? J Insect Conserv 2:161–166. https://doi.org/10.1023/A:1009660200402
Gibbs GW (2009) The end of an 80-million year experiment: a review of evidence describing the impact of introduced rodents on New Zealand’s ‘mammal-free’ invertebrate fauna. Biol Invasions 11:1587–1593. https://doi.org/10.1007/s10530-008-9408-x
Gillies C, Williams D (2013) DOC tracking tunnel guide v2.5.2: using tracking tunnels to monitor rodents and mustelids. Department of Conservation, Hamilton
Gillies CA, Leach MR, Coad NB et al (2003) Six years of intensive pest mammal control at Trounson Kauri Park, a Department of Conservation “mainland island”, June 1996—July 2002. N Z J Zool 30:399–420. https://doi.org/10.1080/03014223.2003.9518349
Griffiths R, Buchanan F, Broome K et al (2015) Successful eradication of invasive vertebrates on Rangitoto and Motutapu Islands, New Zealand. Biol Invasions 17:1355–1369
Gwynne DT (2004) Reproductive behavior of ground weta (Orthoptera: Anostostomatidae): drumming behavior, nuptial feeding, post-copulatory guarding and maternal care. J Kans Entomol Soc 77:414–428. https://doi.org/10.2317/E-34.1
Harper GA, Bunbury N (2015) Invasive rats on tropical islands: their population biology and impacts on native species. Glob Ecol Conserv 3:607–627. https://doi.org/10.1016/j.gecco.2015.02.010
Harry I, Drees C, Höfer H, Assmann T (2011) When to sample in an inaccessible landscape: a case study with carabids from the Allgäu (northern Alps) (Coleoptera, Carabidae). ZooKeys 1:255–271. https://doi.org/10.3897/zookeys.100.1531
Hawes TC (2015) Canalization of freeze tolerance in an alpine grasshopper. Cryobiology 71:356–359
Hitchmough RA, Adams L, Reardon J, Monks J (2016) Current challenges and future directions in lizard conservation in New Zealand. J R Soc N Z 46:29–39. https://doi.org/10.1080/03036758.2015.1108923
Hitchmough RA, Barr B, Knox C, et al (2021) Conservation status of New Zealand reptiles, 2021. Department of Conservation, Wellington Report 35
Hoare JM, Monks A, O’Donnell CFJ (2013) Do population indicators work? Investigating correlated responses of bird populations in relation to predator management. Ecol Indic 25:23–34. https://doi.org/10.1016/j.ecolind.2012.09.007
Hohbein RR, Conway CJ (2018) Pitfall traps: a review of methods for estimating arthropod abundance. Wildl Soc Bull 42:597–606
Holdaway RN (1996) Arrival of rats in New Zealand. Nature 384:225–226. https://doi.org/10.1038/384225b0
Horn S, Greene T, Elliott G (2019) Eradication of mice from Antipodes Island, New Zealand. Isl Invasives Scaling Meet Chall 131:136
Houghton M, Terauds A, Merritt D et al (2019) The impacts of non-native species on the invertebrates of Southern Ocean Islands. J Insect Conserv 23:435–452. https://doi.org/10.1007/s10841-019-00147-9
Innes J, Kelly D, Overton JM, Gillies C (2010) Predation and other factors currently limiting New Zealand forest birds. N Z J Ecol 34:86
Innes J, Fitzgerald N, Binny R et al (2019) New Zealand ecosanctuaries: types, attributes and outcomes. J R Soc N Z 49:370–393
Jamieson IG, Forbes MR, McKnight EB (2000) Mark-recapture study of mountain stone weta Hemideina maori (Orthoptera: Anostostomatidae) on rock tor “islands.” N Z J Ecol 24:209–214
Kats LB, Ferrer RP (2003) Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers Distrib 9:99–110. https://doi.org/10.1046/j.1472-4642.2003.00013
Johns P (2001) Distribution and conservation status of ground weta, Hemiandrus species (Orthoptera: Anostostomatidae. Department of Conservation, Wellington New Zealand
Kier G, Kreft H, Lee TM et al (2009) A global assessment of endemism and species richness across island and mainland regions. Proc Natl Acad Sci 106:9322–9327. https://doi.org/10.1073/pnas.0810306106
King CM, Forsyth DM (eds) (2021) The handbook of New Zealand mammals. CSIRO Publishing, Collingwood
Koot EM, Morgan-Richards M, Trewick SA (2022) Climate change and alpine-adapted insects: modelling environmental envelopes of a grasshopper radiation. R Soc Open Sci 9:211596
Leathwick JR, Byrom AE (2023) The rise and rise of predator control: a panacea, or a distraction from conservation goals? N Z J Ecol 47:3515
Leisnham PT, Cameron C, Jamieson IG (2003) Life cycle, survival rates and longevity of an alpine weta Hemideina maori (Orthoptera: Anostostomatidae) determined using mark-recapture analysis. N Z J Ecol 27:191–200
Le Roux V, Chapuis J-L, Frenot Y, Vernon P (2002) Diet of the house mouse (Mus musculus) on Guillou Island, Kerguelen archipelago, Subantarctic. Polar Biol 25:49–57
Li D (2018) hillR: taxonomic, functional, and phylogenetic diversity and similarity through Hill Numbers. J Open Source Softw 3:1041
Little L, King C, O’Donnell CF (2017) Behaviour of stoats (Mustela erminea) raiding the nests of rock wrens (Xenicus gilviventris) in alpine New Zealand. Notornis 64:124–135
Livingstone J, Horn SR, Broome KG, Sagar RL (2022) How low can you sow? House mouse eradication on Motuareronui/Adele Island. N Z J Ecol 46:3498
Marlow NJ, Thomas ND, Williams AA et al (2015) Cats (Felis catus) are more abundant and are the dominant predator of woylies (Bettongia penicillata) after sustained fox (Vulpes vulpes) control. Aust J Zool 63:18–27
Marris JWM (2000) The beetle (Coleoptera) fauna of the Antipodes Islands, with comments on the impact of mice; and an annotated checklist of the insect and arachnid fauna. J R Soc N Z 30:169–195
Martin AR, Richardson MG (2019) Rodent eradication scaled up: clearing rats and mice from South Georgia. Oryx 53:27–35
McAulay J, Monks J (2023) Interspecific variation in predation patterns of stoats and weasels in an alpine conservation programme. N Z J Ecol 47:3520
McAulay J, Seddon PJ, Wilson DJ, Monks JM (2020) Stable isotope analysis reveals variable diets of stoats (Mustela erminea) in the alpine zone of New Zealand. N Z J Ecol 44:1–13
McCreless EE, Huff DD, Croll DA et al (2016) Past and estimated future impact of invasive alien mammals on insular threatened vertebrate populations. Nat Commun 7:12488. https://doi.org/10.1038/ncomms12488
McGuinness CA (2001) The conservation requirements of New Zealand’s nationally threatened invertebrates. Department of Conservation, Wellington Report 20
McMurtrie P, Edge KA, Crouchley D et al (2011) Eradication of stoats (Mustela erminea) from Secretary Island, New Zealand. Isl Invasives Erad Manag 1:455–460
Medina FM, Bonnaud E, Vidal E et al (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Change Biol 17:3503–3510. https://doi.org/10.1111/j.1365-2486.2011.02464.x
Miller AP, Webb PI (2001) Diet of house mice (Mus musculus L.) on coastal sand dunes, Otago, New Zealand. N Z J Zool 28:49–55. https://doi.org/10.1080/03014223.2001.9518256
Monks JM, Besson AA, O’Donnell CF (2024) Landscape scale control of selected mammalian predators fails to protect lizards. Biol Invas 26:107–118
Morgan-Richards M, Gibbs GW (2001) A phylogenetic analysis of New Zealand giant and tree weta (Orthoptera: Anostostomatidae: Deinacrida and Hemideina) using morphological and genetic characters. Invertebr Syst 15:1–12. https://doi.org/10.1071/it99022
Murphy EC, Gillies C, Maddigan F et al (2016) Flexibility of diet of stoats on Fiordland islands, New Zealand. N Z J Ecol 40:114–120
Murphy EC, Russell JC, Broome KG et al (2019) Conserving New Zealand’s native fauna: a review of tools being developed for the Predator Free 2050 programme. J Ornithol 160:883–892. https://doi.org/10.1007/s10336-019-01643-0
Newman DG (1994) Effects of a mouse, Mus musculus, eradication programme and habitat change on lizard populations of Mana Island, New Zealand, with special reference to McGregor’s skink, Cyclodina macgregori. N Z J Zool 21:443–456. https://doi.org/10.1080/03014223.1994.9518015
Nichols M, Nathan H, Mulgan N (2021) Dual aerial 1080 baiting operation removes predators at a large spatial scale. N Z J Ecol 45:1–10
Norbury G, Byrom A, Pech R et al (2013) Invasive mammals and habitat modification interact to generate unforeseen outcomes for indigenous fauna. Ecol Appl 23:1707–1721
Norbury G, Wilson DJ, Clarke D et al (2022) Density-impact functions for invasive house mouse (Mus musculus) effects on indigenous lizards and invertebrates. Biol Invas 1:1–15
O’Donnell CFJ, Weston KA, Monks JM (2017) Impacts of introduced mammalian predators on New Zealand’s alpine fauna. N Z J Ecol 41:1–22
O’Donnell C, Borkin K, Christie J, et al (2018) Conservation status of New Zealand bats. Department of Conservation, Wellington
Oksanen J, Blanchet FG, Friendly M, et al (2020) vegan: community ecology package
O’Malley TDR, Stanley MC, Russell JC (2022) Assessing two different aerial toxin treatments for the management of invasive rats. Animals 12:309. https://doi.org/10.3390/ani12030309
Parkes J, Murphy E (2003) Management of introduced mammals in New Zealand. N Z J Zool 30:335–359. https://doi.org/10.1080/03014223.2003.9518346
Peltzer DA, Bellingham PJ, Dickie IA et al (2019) Scale and complexity implications of making New Zealand predator-free by 2050. J R Soc N Z 49:412–439
Pryde MA, O’Donnell CF, Barker RJ (2005) Factors influencing survival and long-term population viability of New Zealand long-tailed bats (Chalinolobus tuberculatus): implications for conservation. Biol Conserv 126:175–185
Purdey DC, King CM, Lawrence B (2004) Age structure, dispersion and diet of a population of stoats (Mustela erminea) in southern Fiordland during the decline phase of the beech mast cycle. N Z J Zool 31:205–225. https://doi.org/10.1080/03014223.2004.9518373
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ramløv H, Wharton DA, Wilson PW (1996) Recrystallization in a freezing tolerant Antarctic nematode, Panagrolaimus davidi, and an alpine weta, Hemideina maori (Orthoptera; Stenopelmatidae). Cryobiology 33:607–613
Rawlence TE (2019) The efficacy of aerial 1080 poison applied on a landscape scale to control alpine predators and the reproductive response of rock wren (Xenicus gilviventris). University of Otago, Masters
Reardon JT, Whitmore N, Holmes KM et al (2012) Predator control allows critically endangered lizards to recover on mainland New Zealand. N Z J Ecol 1:141–150
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998. https://doi.org/10.1111/j.1461-0248.2009.01347.x
Robertson H, Baird K, Elliott G, et al (2021) Conservation status of birds in Aotearoa New Zealand, 2021. Department of Conservation, Wellington Report 36
Russell JC (2012) Spatio-temporal patterns of introduced mice and invertebrates on Antipodes Island. Polar Biol 35:1187–1195
Russell JC, Peace JE, Houghton MJ et al (2020) Systematic prey preference by introduced mice exhausts the ecosystem on Antipodes Island. Biol Invasions 22:1265–1278. https://doi.org/10.1007/s10530-019-02194-4
Salmon JT (1950) A revision of the New Zealand wetas Anostostominae (Orthoptera: Stenopelmatidae) Dominion Museum Records in Entomology. Wellington 1:121–177
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27. https://doi.org/10.1016/j.biocon.2019.01.020
Seyfarth E-A, Hergenröder R, Ebbes H, Barth FG (1982) Idiothetic orientation of a wandering spider: Compensation of detours and estimates of goal distance. Behav Ecol Sociobiol 11:139–148. https://doi.org/10.1007/BF00300103
Smith BT, Morgan-Richards M, Trewick SA (2013) New Zealand ground wētā (Anostostomatidae: Hemiandrus): descriptions of two species with notes on their biology. New Zealand Journal of Zoology 40:314–329
Smith D, Jamieson I, Peach R (2005) Importance of ground weta (Hemiandrus spp.) in stoat (Mustela erminea) diet in small montane valleys and alpine grasslands. N Z J Ecol 1:207–214
Smith DHV, Moller H, Wilson DJ et al (2010) Prey switching by stoats (Mustela erminea): a supplemental food experiment. Wildl Res 37:604–611. https://doi.org/10.1071/WR10088
Smith DHV, Wilson DJ, Moller H et al (2008) Stoat density, diet and survival compared between alpine grassland and beech forest habitats. N Z J Ecol 32:166–176
Smith DHV, Avenant N, Chown S (2002) The diet and impact of house mice on a sub-Antarctic island. Polar Biol 25:703–715. https://doi.org/10.1007/s00300-002-0405-8
St Clair JJH (2011) The impacts of invasive rodents on island invertebrates. Biol Conserv 144:68–81. https://doi.org/10.1016/j.biocon.2010.10.006
Stringer I, Hitchmough RA (2012) Assessing the conservation status of New Zealand’s native terrestrial invertebrates. New Zealand Entomologist 35:77–84. https://doi.org/10.1080/00779962.2012.686309
Tompkins DM, Byrom AE, Pech RP (2013) Predicted responses of invasive mammal communities to climate-related changes in mast frequency in forest ecosystems. Ecol Appl 23:1075–1085. https://doi.org/10.1890/12-0915.1
Towns DR, Wardle DA, Mulder CP et al (2009) Predation of seabirds by invasive rats: multiple indirect consequences for invertebrate communities. Oikos 118:420–430
van Heezik Y, Ray SM, Jamieson IG et al (2020) Impacts of aerial 1080 predator control on nest success and adult survival of South Island robins. N Z J Ecol 44:1–11
Van Wyngaarden F (1975) The ecology of the Tekapo ground wētā (Hemiandrus new sp., Orthoptera: Anostostomatidae) and recommendations for the conservation of a threatened close relative. Thesis, University of Canterbury
Vergara OE, Nelson N, Hartley S (2021) Effects of mammal exclusion on invertebrate communities in New Zealand. Austral Ecol 46:776–791
Warburton B, Poutu N, Peters D, Waddington P (2008) Traps for killing stoats (Mustela erminea): improving welfare performance. Anim Welf 17:111–116. https://doi.org/10.1017/S0962728600027615
Watts C, Armstrong D, Innes J, Thornburrow D (2011) Dramatic increases in weta (Orthoptera) following mammal eradication on Maungatautari – evidence from pitfalls and tracking tunnels. N Z J Ecol 35:261–272
Watts C, Innes J, Wilson DJ et al (2022) Do mice matter? Impacts of house mice alone on invertebrates, seedlings and fungi at Sanctuary Mountain Maungatautari. N Z J Ecol 46:1–15
Weston KA, O’Donnell CF, van dam-Bates P, Monks JM (2018) Control of invasive predators improves breeding success of an endangered alpine passerine. Ibis 160:892–899
Whitau K, Kelly D, Galloway TN et al (2023) Effects of altitude, seedfall and control operations on rat abundance in South Island Nothofagus forests 1998–2016. N Z J Ecol 47:3502
Wilson DJ, Lee WG (2010) Primary and secondary resource pulses in an alpine ecosystem: snow tussock grass (Chionochloa spp.) flowering and house mouse (Mus musculus) populations in New Zealand. Wildl Res 37:89–103
Worland MR, Wharton DA, Byars SG (2004) Intracellular freezing and survival in the freeze tolerant alpine cockroach Celatoblatta quinquemaculata. J Insect Physiol 50:225–232. https://doi.org/10.1016/j.jinsphys.2003.12.001
Acknowledgements
Thank you to the Department of Zoology, the University of Otago, and the New Zealand Department of Conservation (DOC) for logistical support. We would like to highlight the staff at DOC Te Anau and Rakiura for all they did to help us get to some of our more remote field sites. Additionally, we would like to thank the DOC Alpine Research Programme for funding this research and supporting alpine invertebrate conservation. We would like to give a special thank you to Tōrea Scott-Fyfe and Tessa Mackenzie for all the help and support in the field. Our research was a part of the DOC Alpine Research Programme and followed consultation with the Ngāi Tahu Research Consultation Committee of the University of Otago.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by the University of Otago and the Department of Conservation alpine research programme.
Author information
Authors and Affiliations
Contributions
Aaron Bertoia: Conceptualization (equal), Methodology (equal), Formal Analysis (lead), Investigation (lead), Visualisation (lead), Preparation (lead), Validation (equal), Writing – Original Draft (lead), Writing – Review & Editing (equal). Tara Murray: Conceptualisation (equal), Methodology (equal), Writing – Review & Editing (equal), Supervision (supporting). Bruce Robertson: Writing – Review & Editing (equal), Methodology (supporting), Supervision (supporting). Jo Monks: Conceptualization (equal), Methodology (equal), Resources (lead), Supervision (lead), Validation (equal), Writing – Review & Editing (equal), Funding Acquisition (lead).
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bertoia, A., Murray, T.J., Robertson, B.C. et al. Introduced mice influence the large-bodied alpine invertebrate community. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03370-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03370-x