Abstract
Plant invasions are a widespread and recurring phenomenon that cause significant economic and environmental damage. Invasive alien conifers are weeds that are not only costly to manage, but consistently reinvade after management efforts. Understanding how many seeds survive to germinate is a key part in understanding the weed life cycle puzzle. Here we investigated the contribution that seed predators have on reducing invasive alien conifer seed survival across both invaded and uninvaded habitats in Aotearoa New Zealand. We combined quantitative and qualitative experiments to measure seed predation across invaded and uninvaded habitats, as well as to identify which fauna are the most prolific seed predators. We utilised ex-situ empirical evidence with in-situ observations to provide realistic impacts from different seed predator species. We found that introduced mammals, particularly rodents, were the primary seed predators of invasive conifers. Seed predation pressure was highest in herbicide treated invasive alien conifer forests, indigenous beech forests, and managed pasture containing grazing livestock. Indigenous tussock areas support fewer vertebrate seed predators and as a result are particularly vulnerable to conifer invasion. The majority of seed predation occurs within the first two weeks post-dispersal. These results suggest that introduced mammal control operations, which are essential to protect endemic New Zealand species, will likely result in increasing invasive conifer populations by reducing seed predation pressure. Seed predation varies greatly between habitats, suggesting invasion and reinvasion rates are higher in ungrazed areas of lower forest density that support fewer introduced mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotic resistance, where existing communities resist the establishment of new or introduced species (Levine et al. 2004; Beaury et al. 2020), represents a major pathway for the prevention of invasive species establishment. This can be due to established native communities filling all available niche space, which prevents new species establishing, or due to negative interactions with existing species in the community (Byers and Noonburg 2003; Gioria et al. 2023). Where available niche space exists, biotic resistance can prevent or slow establishment of new species through competition for resources or via direct predation from resident animals (Levine et al. 2004; Ulloa et al. 2023). Herbivory has been shown to be equivalent to the effects of competition in resisting invasive plant encroachment (Levine et al. 2004; Santamaría et al. 2021), and similarly granivory (seed predation) is seen as a major pathway of biotic resistance (Lopez and Terborgh 2007; Pearson et al. 2011, 2012; Preukschas et al. 2014; Moyano et al. 2019b; Muschetto et al. 2022).
When confounding factors are accounted for (such as proximity to human disturbance) native species richness has been shown to negatively correlate with non-native species richness in most cases (Levine et al. 2004; Beaury et al. 2020; Halassy et al. 2023). Therefore, biotic resistance is considered to be ubiquitous across ecosystems, however, varies in magnitude and efficacy across different land uses, proximity to human-mediated disturbance, community assemblages, and ecotypes (Ackerman et al. 2017; Beaury et al. 2020). In general, proximity to human-mediated disturbance increases a community’s vulnerability to invasion and can overwhelm the effects of biotic resistance (Ackerman et al. 2017; Kröel-Dulay et al. 2019; Rojas‐Sandoval et al. 2020; Mungi et al. 2021; Gioria et al. 2023). Land use and management can influence the types and abundance of faunae found within the environment, particularly through the proportion of available woody habitat (Jenkins et al. 2013; Walker et al. 2019; Moreira-Arce et al. 2021). Stable ecosystems, resistant to environmental stress (Stotz et al. 2016), are most resistant to invasion. Certain ecosystems, such as intact forests, appear to be inherently less invasible than others through a combination of saturated niche space, high competition for nutrients and light, and by supporting a broad array of herbivores (Jenkins et al. 2013; Waddell et al. 2020; Chong et al. 2021; Moreira-Arce et al. 2021; Mungi et al. 2021).
Traditionally, biotic resistance has been viewed through the lens of native communities resisting the encroachment of non-native species (Levine et al. 2004; Beaury et al. 2020; Halassy et al. 2023), however, it is entirely reasonable to assume some non-native species will impose biotic resistance against other non-native species. Rodents, which are some of the most widespread invasive species, are generalists which have documented examples of developing preferences for either native or introduced seed based on seed size, relative abundance, or presence of secondary metabolites (Everett et al. 1978; Fischer and Türke 2016; Godó et al. 2022). Similarly, goats (among other ungulates) are generalist browsers that can develop preferences for introduced plant species (Rathfon et al. 2021). Furthermore, while it is well documented that invasive plants can facilitate one another’s establishment (Gioria et al. 2023), competition or allelopathic interactions between invasive plants could also reduce success or prevent establishment (Belote and Weltzin 2006; Wundrow et al. 2012).
The archipelago of Aotearoa New Zealand hosts more non-native plants than any other islands worldwide (Hulme 2020), and likewise hosts a number of granivorous species, both native and introduced, which could contribute to biotic resistance pressures via seed predation (Thorsen et al. 2009, 2011; Walker et al. 2019). Of these non-native plants, invasive alien conifers (colloquially known as wilding conifers) are the most successful tree weeds in Aotearoa New Zealand and the Southern Hemisphere more broadly (Nuñez et al. 2017; Policelli et al. 2023). In Aotearoa New Zealand, wilding conifers rapidly outcompete native forest regeneration, displace pasture species, and currently cover more than 1.8M ha, including indigenous-owned land (Wyatt 2018; Dickie et al. 2022; Sapere Research Group 2022). Current estimates suggest a further 7.5M ha of productive or iconic conservation land is threatened by invasion within the next 30 years (Wyatt 2018). Wilding conifers invade a variety of habitats, with different management strategies employed depending on the environment and density of invasion (NWCCP 2019). This geographical mosaic of land use and management may support different assemblages and abundances of granivorous species, suggesting a variable degree of biotic resistance across the invaded range. Understanding which environments are most at risk, and what mechanisms exist to reduce or prevent the spread of wilding conifers, is critical.
Here we sought to determine the magnitude of biotic resistance via seed predation pressure imposed across different invaded and uninvaded habitats for two of the most prolific wilding conifer species in Aotearoa New Zealand, Pinus contorta Douglas ex Loudon (Lodgepole Pine) and Pseudotsuga Menziesii (Mirb.) Franco (Douglas fir). Pinus contorta is considered the most invasive and is the only species on the list that is trade prohibited (Ledgard 2001). Ps. menziesii is also highly invasive (Ledgard 2001, 2002; Dickie et al. 2022), yet remains the second most planted timber species in NZ (MPI 2023). We sought to identify the primary seed predators and determine whether the density of available seeds would impact predator behaviour in a way that would significantly affect overall levels of predation. Similar to other studies (Chiuffo et al. 2018), we suspected that the primary seed predators in New Zealand would be invasive rodents which would present concerning implications for mammal control operations in New Zealand. We predicted high predation rates for P. contorta due to their small seed size (Moyano et al. 2019a), and that seeds positioned closer together would experience higher predation rates due to the ease of co-locating seeds.
Methods
Seed collection and processing
Cones of P. contorta were collected from five New Zealand sites in March 2022 and 2023: Flock Hill Station (171.7370°E, 43.1511°S), Hanmer Springs (172.8947°E, 42.4654°S), Mid Dome (168.5150°E, 45.6430°S), Lake Pukaki (170.1365°E, 44.1955°S), and the Kaweka Range (176.3320°E, 39.2477°S). Cones of Ps. menziesii were collected at Mid Dome and Hanmer Springs in March 2022 and 2023 and were supplemented with seed ordered from Proseed NZ (proseed.co.nz) grown at Amberley (172.7507°E, 43.1606°S). Cones were placed into a drying oven at 30 °C for 12 h, or 65 °C for serotinous cones, and seeds were extracted. For each species, extracted seed were mixed in even proportions from each site to produce a representative batch of seed, from which a random sample of seeds was selected for this trial. Latex gloves were worn whenever handling seed to prevent any unnecessary scent transfer which could affect seed predator behaviour.
Study site
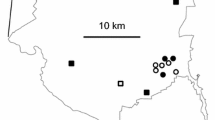
The experiment took place at Flock Hill Station (Canterbury), which is a 14,500 ha working sheep and cattle station. Flock Hill Station contains a variety of habitats, including indigenous tussock grasslands and forest, improved pasture (exotic grasses), invasive pine forest (controlled), and alpine scrub (Fig. 1). Conifers were initially planted at Flock Hill Station and surrounding areas in the mid- to late-1900s for erosion control and as part of provenance trials, with the first wilding conifers noted in 1975 (Ledgard and Paul 2008). Significant investments in controlling wilding conifers across Flock Hill station have occurred as part of the New Zealand National Wilding Conifer Control Programme (NWCCP 2019). Flock Hill Station supports a variety of potential seed predators such as native and introduced birds, invasive non-native mammals, and invertebrates (Lambie and Bennett 2021). Despite being abundant in other parts of Canterbury, Flock Hill Station does not currently host wallaby populations.
Map of Flock Hill Station and surrounding areas. Coloured points represent locations across different habitats where three experimental plots were established, each 50 m apart. Coloured camera symbols represent camera trap feeding station set ups. Inset displays map of NZ, with red point showing location of Flock Hill Station on the South Island. “World Imagery” basemap captured 2022 displayed at 1:43,000 scale (Esri et al. 2022)
The two primary conifer control methods utilised by the NWCCP are ground and aerial control (MPI 2014; NWCCP 2019). Due to the efficiencies, costs, and techniques associated with these control methods, they roughly align with low- and high-density conifer infestations respectively. Ground control commonly encompasses crews using loppers or chainsaws to cut down individual trees or seedlings and is typically reserved for scattered, or low density, infestations of conifers. Aerial control can be split into both aerial-foliar spray application (AFSA), which involves broad application of herbicide to typically dense infestations, and aerial-basal bark application (ABBA), which involves targeting scattered individual trees with herbicide.
We may expect that these control methods could have different implications regarding seed predation. Ground control reduces the amount of available woody habitat in the environment that could support seed predator species, however, supports the restoration of native scrub which could facilitate predation by native species. Aerial herbicide control typically leaves dead, standing forests of wilding conifers for 10–15 years while they naturally decay and the understory community re-establishes. The remaining dense woody habitat could provide refugia for seed predator species, and so we may expect seed predation to be high in these environments (Jenkins et al. 2013; Walker et al. 2019; Moreira-Arce et al. 2021). Depending on the surrounding environment and control measures, both ground and aerially-controlled areas could be subject to high propagule pressure from surrounding conifers.
Quantifying seed predation
This experiment was initiated in late summer, March 2023, and ran for six weeks, to coincide with the natural timing of P. contorta and Ps. menziesii seed rain. We set up 45 plots distributed across five habitat types: tussock grassland (Chionochloa spp.), improved pasture, aerially-controlled conifer forest (ACCF), ground-controlled conifer areas (GCCA), and beech (Nothofagus fusca (Hook.f.) Oerst.) forest (Fig. 1). Distinct GCCA and ACCF areas were identified using the wilding conifer information system (WCIS) database provided by the NWCCP, and subsequently ground-truthed. No areas of ABBA were selected, hence GCCA and ACCF broadly correspond to low- and high-density areas of prior wilding conifer control. Areas of improved pasture were intended to be ungrazed, however, two plots inadvertently had low density cattle grazing introduced at the 2-week mark, and the 3rd plot had high density sheep grazing introduced at the 4-week mark.
Three distinct areas of each habitat were identified (Fig. 1), separated by at least 500 m, and three plot replicates were placed in each of these areas separated by 50 m. Each plot replicate contained four groups of 25 seeds placed in a 5 × 5 grid, with each group separated by 10 m. Of these four groups, three groups were seeds of P. contorta placed at ‘low’, ‘medium’, and ‘high’ density aggregations (50 cm, 10 cm, or 2 cm between seeds respectively) and one group was seeds of Ps. menziesii at the medium (10 cm) density aggregation (Figure S1). Including density aggregations allowed us to consider how seed predator co-location behaviour affected overall seed predation. These density aggregations were selected based on the assumption that the expected seed predators (rodents, birds, invertebrates) could reasonably co-locate seeds only 2 cm apart, whilst not co-locating seeds placed 50 cm apart, potentially affecting the quantities consumed. These additional density aggregations were not repeated for Ps. menziesii due to a lack of seed. This design represented 100 seeds per replicate (75 P. contorta, 25 Ps. menziesii), and a total of 2700 seeds for each of the 5 habitat types (2025 P. contorta, 675 Ps. menziesii). The low–high density gradient would represent a density of 6.3, 156.3, and 390.6 seeds per square metre respectively when scaled up.
To accurately quantify seed predation, we adapted methods from Moyano et al. (2019a). Each seed was individually glued to a 10 cm wooden popsicle stick using non-toxic, low organic volatility, biodegradable adhesive. Sticks were pressed into the ground until only 1 cm remained aboveground (Fig. 2). Plots were checked every two weeks, and sticks that no longer contained seed were recorded and removed to be examined later for possible predator identification. At the end of the experiment all sticks were collected and accounted for. To understand whether seed predators were adversely attracted to either the sticks or adhesive, we placed out a set of 25 control sticks in each habitat arranged in the same 5 × 5 grid pattern 10 cm apart without a seed attached and subsequently checked for any stick removal or damage.
All sticks were processed into five main damage categories which we expected to correspond to different seed predators: undamaged, hollowed out seed, seed missing, seed damaged, and stick damaged (Fig. 2). The ‘stick damaged’ category was then further differentiated into damage by possums, sheep, and cattle, making a total of 7 possible damage categories. Differentiation between damage caused by possums, sheep, or cattle was possible due to dentition patterns, staining on sticks (e.g., green staining from being chewed with grass), and habitat (e.g., sheep or cattle damage were likely confined to pasture).
Identifying seed predators
Feeding stations
To determine the identity of seed predators existing in the system, and provide direct evidence of seed predation, feeding stations were set up in each habitat at Flock Hill Station and tracked with trail cameras (Moultrie M-Series Digital Game Camera). Feeding stations were present for two weeks with cameras checked weekly (Fig. 3a). Each feeding station comprised of four individual bird feeders, two for each conifer species, with an additional raised edged tray to prevent seed dispersal. For each conifer species, one feeder was placed on the ground, and another placed 1.5 m above the ground to try and facilitate predation by birds. Each feeder had an open cone affixed of the relevant conifer species, as we expected this may have encouraged interactions from birds in particular. All seed placed inside feeders were de-winged to prevent dispersal.
Left: Feeding station setup in beech forest with trail cameras recording seed predator interactions. Feeding stations contained four feeders, two for Pinus contorta and two for Pseudotsuga menziesii, and with one of each conifer species at ground level and 1.5 m aboveground. Where necessary, white corflute was used behind feeders to prevent accidental triggers. Right: Examples of visitations from potential seed predators at feeding stations. Clockwise from top-left: common brushtail possum (Trichosurus vulpecula), hedgehog (Erinaceus europaeus), tomtit (Petroica macrocephala), rat (Rattus spp.), mouse (Mus musculus), and European greenfinch (Chloris chloris)
Trail cameras were set with a 10-s delay between triggers (all maximum sensitivity settings) and therefore would often trigger by the same animal multiple times during a single visit. Visits were considered unique if 5-min had passed between triggers, or if it was evident that the captured subject was different than the one prior. Each unique visit was recorded, even if no interaction with our feeding stations occurred, to provide evidence of seed predators in the system. We recorded direct interactions with our feeding stations, including the height of feeder and conifer seed species interacted with. Visitations to only one conifer seed species were common, and therefore we considered predators that visited both conifer species as 2 unique visits (1 for each conifer species). Feeding events were considered as images or videos that demonstrated seed being ingested, beyond a reasonable doubt, with the caveat that the images could be obscured if the predators were facing away from the camera.
Ex-situ experiment
In order to determine the identification of predators interacting with sticks, we established a qualitative experiment under controlled conditions at Willowbank Wildlife Park, Christchurch, and at green spaces of our office site in Christchurch city centre. At Willowbank Wildlife Park, sticks were placed into two different brushtail possum (Trichosurus vulpecula Kerr) enclosures, as well as the wallaby (Notamacropus eugenii Desmarest) enclosure. At the office site, rats, mice, and introduced birds are present. This experiment was established to reliably determine the type of damage to sticks or seeds attributable to different seed predators and receive reliable examples of dentition patterns for comparison with the in-situ samples. Trail cameras were positioned to record animal interactions with the sticks at each location (set at maximum sensitivity) and checked daily. Where seed were damaged or missing, the corresponding sticks were removed. All sticks were removed and examined after three days, and seed predators were identified to species level where possible from the trail camera footage.
Analysis
We assessed overall seed predation as the proportion of seed eaten out of available seed (n = 25) in each aggregation (sub-plot) at the end of the experiment. Cumulative proportion of seed eaten was used as the dependent variable in a one-inflated beta regression model with a logit link function. Habitat, seed species, their interaction, and density aggregation were included as independent variables, with plot ID included as a random effect. The accuracy of the one-inflated beta regression model was assessed through standard diagnostic tools, specifically: residual plots, worm plots, and normalized randomized quantile residual plots (Stasinopoulos and Rigby 2008). One-inflated beta regression models were run using the gamlss package (Rigby and Stasinopoulos 2005). Confidence intervals (95%) were calculated using the broom.mixed package (Bolker and Robinson 2022).
To analyse whether the proportion of seed predated varied between timepoints, which would help us understand if seed predators returned to the food source after finding it the first time, we calculated relative treatment effects for an F1-LD-F1 design (Noguchi et al. 2012). This analysis involves several tests and computes both Wald-type statistics (WTS) and ANOVA-type statistics (ATS) among others to approximate the effect that time has on predicting seed predation using the nparLD package (Noguchi et al. 2012). Where WTS and ATS disagreed, we reverted to ATS as the more appropriate measure of significance (Noguchi et al. 2012).
We assessed whether the likelihood of seed predation occurring at bait stations varied across different habitats, as recorded by camera traps, using a binomial regression model with a logit link function. Occurrence of feeding was included as a binary dependent variable, predicted by conifer seed species, height of feeding station, their interaction, and habitat included as independent variables. Identity of predator species was not included as an independent variable due to being heavily dominated by a single species. Significance of fixed effects were assessed using pairwise tests from the emmeans package (Lenth 2023), and significance was considered where Tukey-adjusted p-values were less than 0.05. The performance package was used to test for multicollinearity amongst fixed effects (Lüdecke et al. 2021).
We assessed whether seed predation varied between different damage categories (as a proxy for seed predator identity) using a Kruskal–Wallis test. A post-hoc Dunn test using the Holm p-adjustment method was used to determine which damage categories were most common (Ogle et al. 2023). All analyses were completed using R version 4.3.1 (R Core Team 2023).
Results
Proportion of seed predated
The average percentages of seed eaten in each habitat by the end of the experiment for P. contorta and Ps. menziesii respectively were highest in Beech Forest (92.8%; 95.9%), followed by ACCF (91.7%; 82.7%), Pasture (90.7%; 72.4%), GCCA (70.0%; 69.8%), and finally Tussock (52.1%; 31.6%; Fig. 4, 5). For all habitats the majority of seed predation occurred in the first two weeks, by the first measurement timepoint (Fig. 4). Only two control sticks total had evidence of tampering—both of which were present in ACCF and both gnawed by a lone hedgehog during a single visit captured by our trail camera. As no other control sticks had evidence of tampering, we considered that neither sticks nor glue were adversely attracting seed predator attention.
Cumulative percentage of seed predation over time in each habitat. Timepoints were 2 weeks apart, with the experiment lasting 6 weeks total. Error bars show ± standard error. Figure titles refer to the species of, and distance between, seeds—with distances relating to low (50 cm), medium (10 cm), and high (2 cm) densities of seeds which could affect co-location behaviour
The zero-inflated mixed effects model predicting overall seed predation had substantial explanatory power (Pseudo R2 = 0.37). Overall seed predation was significantly lower in tussock habitats (β = − 1.03, 95% CI [− 1.67, − 0.385], p < 0.003). There was no significant overall difference observed between P. contorta and Ps. menziesii seed predation (β = 0.574, 95% CI [− 0.631, 1.78], p = 0.353), however, significant interactions showed Ps. menziesii had lower seed predation rates in pasture and tussock habitats (Pasture: β = − 1.69, 95% CI [− 3.16, − 0.212], p = 0.027; Tussock: β = − 1.82, 95% CI [− 3.17, − 0.470], p = 0.010). The close (2 cm) aggregation had significantly lower predation than either far (50 cm) or standard (10 cm) aggregations (β = − 0.720, 95% CI [− 1.24, − 0.202], p = 0.007; Figure S2; Table S1).
Seed predation varied significantly between timepoints (WTS p < 0.001; ATS p < 0.001) and habitats (WTS p < 0.001; ATS p < 0.001; Modified ATS p = 0.001; Figure S3; Table S2). The habitat effect was primarily driven by the lower seed predation experienced in tussock areas for P. contorta, and lower seed predation in tussock, and pasture habitats for Ps. menziesii. Differences over time primarily related to increases in seed predation over successive timepoints seen in both tussock and pasture habitats (Figure S3). The interaction between habitat and timepoint was considered non-significant (ATS p = 0.100).
Identification of seed predators
In total, 824 images and videos containing seed predators (hits) were captured by camera traps on Flock Hill Station, representing 152 unique visits. Hits were unevenly distributed across habitats, with no seed predators recorded in GCCA (Figure S5). By far the most common recorded species were rodents (mice and rats; 126 unique visits), followed by birds (13 unique visits), possums (8 unique visits), and hedgehogs (5 unique visits; Figs. 3, S4). At the office site rodents were the most commonly recorded species, and only five total feeding events were recorded by bird species—all of which were introduced species. No other species interactions with seed were recorded at the office site.
The binomial logistic model predicting whether feeding would occur at bait stations had substantial explanatory power (R2Tjur's = 0.68). There was a significant positive effect of feeding occurring in ACCF (β = 4.12, 95% CI [2.38, 7.07], p < 0.001). The number of feeding events was significantly greater in ACCF compared to any other habitat, despite a similar number of visitations occurring in beech forest (42% and 48% respectively; Figure S5). Roughly half of all visitations resulted in feeding in ACCF and Tussock, compared to only ~ 20% of visitations resulting in feeding in beech forest. There was a significant positive interaction between possum damage and Ps. menziesii seed (β = 4.12, 95% CI [2.38, 7.07], p < 0.001). No other effects were significant (Table S3).
Hedgehogs, deer, and pigs are also present at Flock Hill Station, however, of these only hedgehogs were observed on trail cameras, and none of these species (including hedgehogs) were suspected of predating any seeds from this experiment. Wallabies did not appear to show any preference for eating conifer seed, however, did tamper with sticks placed into their enclosure.
Seed predator preferences
The Kruskal–Wallis rank sum test testing the difference in ranks between number of seeds damaged by each damage category suggests that the effect is statistically significant, and large for both conifer species (P. contorta: Kruskal–Wallis χ2 = 452.64, p < 0.001; ε2 (rank) = 0.56, 95% CI [0.53, 1.00]; Ps. menziesii: Kruskal–Wallis χ2 = 113.37, p < 0.001; ε2 (rank) = 0.42, 95% CI [0.34, 1.00]). Missing seeds were the most common “damage” type (P. contorta: x̄ = 16.20 ± 0.50 SE; Ps. menziesii: x̄ = 11.78 ± 1.32 SE; Figure S6), followed by possum damage (P. contorta: x̄ = 1.16 ± 0.13 SE; Ps. menziesii: x̄ = 1.64 ± 0.46 SE). Pairwise comparisons between ranked proportions of damage types were broadly consistent between conifer species (Table S4) with only the Damaged Seed–Hollow Seed, Sheep–Cattle, Possum–Hollow Seed, and Possum–Damaged Seed comparisons considered non-significant.
Evidence from ex-situ experiments provided and indication of how damage categories related to seed predator identity. Rodents were responsible for instances of “missing” or “damaged” seeds (Fig. 2c, d). While occasionally rodents caused minor markings on sticks, we found no evidence of rodents causing major damage to sticks as per Fig. 2e. Major stick damage was attributed to possums, cattle, or sheep, which were less careful in removing seeds from sticks. Hollow seeds were attributed to invertebrates in the field due to the size of holes in the seed coat and, although we had no direct ex-situ comparison, appeared in a spatially idiosyncratic pattern which would suggest little co-location of seeds by the seed predator.
Discussion
By combining qualitative and quantitative experiments we found that wilding conifer seed predation levels significantly vary between habitats in Aotearoa New Zealand. High levels of seed predation were observed in similarly structured beech forest and aerially-controlled conifer forests which likely support greater seed predator populations than other habitats. Unsurprisingly, rodent species were the most commonly observed seed predator species but contrary to anecdotal evidence from experts in NZ neither native nor introduced birds significantly contributed to seed predation in any habitat. Our experiment included similar, and additional, environments to Moyano et al. (2019a) and found further support that that smaller seeds (P. contorta = ~ 4 mg) are more commonly predated on than larger seeds (Ps. menziesii = ~ 12.5 mg; Veech et al. 2000). By comparing damage from the wild with that of captive animals, and with known damage confirmed by trail cameras, we are confident we were able to accurately identify seed predators into broad morphological categories.
The highest rates of seed predation occurred in beech forest and ACCF, which were also the habitats that had the most feeding station visitations (Fig. 5, S4). This would imply that these forest habitats have higher seed predator abundances, however, despite similarities in visitations we recorded more than twice as many feeding events at feeding stations in ACCF compared to beech forest. This may suggest that food sources are scarcer in ACCF, and seed predators are less choosy, or that seed predators have become more accustomed to wilding conifer seed as a food source which would naturally be more common in ACCF. We know that woody, forested habitats typically support a greater abundance of vertebrates (Jenkins et al. 2013; Walker et al. 2019; Moreira-Arce et al. 2021), so it is unsurprising that seed predation by vertebrates would be highest in these environments. Compounding this effect, forests are typically moist, shaded, and have smothering leaf- or needle-litter which would reduce seed germination (Mazia et al. 2001; Jensen and Gutekunst 2003), resulting in these habitats being less susceptible to invasion or re-invasion.
Tussock grassland and native scrub areas had the lowest levels of seed predation suggesting they could be the most vulnerable to wilding conifer invasion. These findings support Dylewski et al. (2020) who found that small seeded species are less likely to be predated by small mammals in grasslands than in forests. We did however find lower levels of seed predation in unimproved grasslands than reported in Moyano et al. (2019a), despite our experiment continuing for longer. Aside from differences in predator abundance (and diversity) and seed palatability, this may be explained by the smaller scale presented by Moyano et al. (2019a) with all seeds placed within 2 m2 providing easier predation by co-location. However, we note that overall seed predation in our experiment was most variable between plots in GCCA compared to other habitats, which is likely a reflection of the variability of habitats that were classified as GCCA; habitats were classified as GCCA if wilding conifers had been controlled by ground crews within 5 years and were dominated by unimproved introduced grasses. These control measures had varying levels of re-invasion, resulting in GCCA plots with high variation in the availability of both dead and alive woody-habitat. While more similar to one another than to other habitats, the variation among GCCA plots could have had an impact vertebrate seed predator abundance.
Grazed pasture (of either sheep or cattle) represents another habitat where seed predation is high (Fig. 4, 5), however, this environment differs from both beech forest and ACCF. Rather than intentionally targeting these seeds, we expect that grazing livestock will unintentionally ingest seed passively while grazing (Janzen 1984; Delibes et al. 2019). Interestingly, this passive seed predation can still represent a significant mechanism for biotic resistance. The inadvertent introduction of cattle and sheep into pasture plots at the 2- and 4-week marks correspond with marked increases in seed predation (Fig. 4, Figure S6). However, the rate of seed predation will of course vary according to stocking density. In addition, sheep in particular will impact seedlings with browsing damage (Ledgard and Norton 2008; Zamora Nasca et al. 2018), which is likely to be the larger mechanism preventing successful invasions in pasture.
Predation of P. contorta seeds was greater than that of Ps. menziesii (Fig. 5), reinforcing the finding from Moyano et al. (2019a) that smaller seeds are preferentially predated. They suggest this is due to: a) the opportunity for seed predators with smaller gape sizes to ingest smaller seeds more easily, and b) a lower threshold to break through seed defences of smaller seeds. It may be that P. contorta seed are simply more palatable than those of Ps. menziesii, as noted by Lobo et al. (2009) when comparing seed predation between P. contorta, Abies lasiocarpa, and Picea glauca. Scent causing volatile compounds differ between these genera (Backlund et al. 2014; Mitić et al. 2021) and Ps. menziesii (Record et al. 1976) which could affect their attractiveness to seed predators. Given that Ps. menziesii seeds are less likely to be predated, their seedlings are shade tolerant (Ledgard 2002; Ledgard et al. 2005), and that larger-seeded species are more likely to be cached (Vander Wall 2003, 2008) we may expect Ps. menziesii to be a successful late-successional species that replaces P. contorta after control measures. This would broadly agree with McAlpine et al. (2016) who found that ACCF is more likely to be succeeded by non-target species than by the original invasive pine species (P. contorta in our case). While McAlpine et al. (2016) view this in the context of native species re-establishment, Ps. menziesii would likely outcompete any native re-establishment in areas where propagule pressure is high enough. However, the successional ability of Ps. menziesii will likely be limited by its preference for wetter environments when competing with P. contorta drier areas (Miller and Ecroyd 1986; Miller and Knowles 1994a), or due to the much earlier coning and aggressive spreading nature of P. contorta which could overwhelm any losses from seed predation (Moyano et al. 2019b).
As rodents were the most commonly recorded seed predators (Figure S6), levels of seed predation are likely to fluctuate according to predictable spatial and temporal patterns of rodent populations (Wilson et al. 2007; Walker et al. 2019). Rodents are widespread across Aotearoa (Brown et al. 1996; Ruscoe et al. 2001), suggesting that levels of seed predation are likely to be high across NZ. However, Walker et al. (2019) demonstrated that mouse and rat populations are irruptive in cooler climates, indicating that areas higher in elevation or further south may have intermittently lower seed predation pressure and therefore lower biotic resistance. Rodent populations are also known to experience periods of rapid growth and decline in accordance with the increased food availability from masting (synchronised high seed production) years (Choquenot and Ruscoe 2000). The New Zealand flora is particularly rich in species that exhibit masting behaviour (Schauber et al. 2002), and consequently interannual fluctuation in wilding conifer seed predation pressure is likely in New Zealand.
Our results indicate that large-scale rodent removal programs could have unintended consequences on wilding pine communities by relieving seed predation pressures (Peltzer et al. 2019). However, realistically the total removal of rodents, mice in particular (Burns et al. 2011), is unattainable in the near future, and thus is not likely to be of great concern. Even under the most optimistic scenarios where the NZ predator free 2050 strategy succeeds (DOC 2020); mice are not included in the predator free 2050 strategy and therefore a large seed predation pressure will still remain for wilding conifers, or even increase as mesopredator numbers reduce (Wilson et al. 2018). Hypothetically, pest-free areas or islands could be vulnerable to exotic conifer invasion in the absence of rodents, however, rationally these areas would either struggle to completely remove rodents or would likely be remote areas far from conifer source populations.
Birds were not observed to be significant seed predators of either P. contorta or Ps. menziesii. Anecdotal evidence suggests that flocks of exotic birds (finch species in particular; N. Ledgard, personal communication, October 19, 2023) will readily feed on Pinus nigra seeds during cone opening, however, this is likely due to the time of year when cone opening occurs. Pinus nigra cones open in spring (Miller and Knowles 1986), whereas P. contorta and Ps. menziesii cones open in late summer-autumn (Miller and Ecroyd 1987; Miller and Knowles 1994b). If birds are eating P. nigra seed, from either open cones or the ground, this may reflect the need for additional food sources in spring, whereas birds can be more selective with their food sources later in summer. This finding is consistent with Chiuffo et al. (2018) who also recorded no predation of P. contorta, Ps. menziesii, or P. ponderosa seeds by birds in South America. While we followed best practise guidelines in camera trap usage to maximise our hit rate (Randler and Kalb 2018; Palencia et al. 2022), some studies have noted discrepancies between trail camera trigger rates between mammals and birds (Ortmann and Johnson 2021). Given these data were only assessed qualitatively (the presence or absence of feeding) as opposed to quantitively, any discrepancies as a result of camera trap efficacy are unlikely to affect our results. It is also however possible that our feeding stations were avoided by birds (or other taxa) through neophobia. Rates of neophobia are recorded as higher in birds than other taxa, however reduces over time (Stryjek and Modlinska 2016; Crane and Ferrari 2017). While we intentionally left our feeding stations out for 2 weeks in hopes of familiarising seed predators and reducing the risk of missing risk-averse individuals, we note that our limited bird records were all captured in the first week suggesting neophobia was not a large influence.
Interestingly, possums were also identified as seed predators on the ground, whereas they previously have only been reported as occasionally chewing cones or ringbarking trees (Miller and Ecroyd 1987). Although this effect is likely small compared to that of rodents, we have shown that possums could also act as agents of biotic resistance. While wallabies were not suspected to be a significant seed predator, their inclusion was necessary to determine whether our results are generalisable across New Zealand, as they are not currently present at Flock Hill but are present in many other conifer invaded areas. Our results suggested wallabies showed little interest in wilding conifer seed, and any seed predation would likely be unintentional whilst grazing. However, similar to lagomorphs, wallabies would be more likely to impact wilding conifer populations through browsing of seedlings and saplings (Ledgard and Norton 2008; McAlpine et al. 2016; Latham et al. 2020).
The only native species that we suspect to be noteworthy seed predators, although far less effective than non-native species, are invertebrates. While we did not endeavour to identify specific invertebrate seed predators, iNaturalist records (www.inaturalist.org. Accessed October 2023) demonstrate there are large populations of native and exotic granivorous species in New Zealand, including but not limited to grasshoppers (Brachaspis spp., Paprides spp., Phaulacridium spp., Conocephalus spp.), crickets (Teleogryllus spp.) and wētā (Pleioplectron spp., Hemiandrus spp.; Griffin et al. 2011). There are known introduced seed parasitoids for Ps. menziessii (Lee et al. 2021), however, we recorded no instances either in the field or in our remaining seed stock of the Douglas fir seed chalcid (Megastigmus spermotrophus Wachtl).
The effect of density aggregation on seed predation appears counterintuitive at first, as seeds placed close together (2 cm apart) were generally predated less than those placed further apart (50 cm and 10 cm). However, upon further investigation it is evident that the close aggregation trended towards either low or high predation—an “all or nothing” approach (Figure S2). Seed predation of the close aggregation was extremely high in habitats with greater seed predation pressures (beech forest, ACCF, and pasture), and quite low in tussock and GCCA. This pattern likely reflects the relative abundance of granivorous vertebrates supported by each habitat. With fewer granivorous mammals, some replicates of the 2 cm aggregate were likely missed by the predominant seed predators (rodents). Rodents are known to primarily follow established trails (Jamon 1994; Bennett and Buckley 1996), and thus, where fewer rodents and fewer trails exist, our positioned conifer seeds were less likely to intersect with a rodent trail. The 2 cm aggregate represented an area of 0.064 m2, compared to 0.16 m2 and 4 m2 for the 10 cm and 50 cm aggregates respectively, and thus more easily missed by chance.
Under natural conditions seed would land on the top layer of undergrowth and over time would be agitated and sink further into the understory layers. This may affect the level of seed predation over time, making them more or less susceptible to predation by different seed predators that are active in different understory layers. This uncertainty is not accounted for in the current methodology, as seeds were kept stationary in the understory, at the surface of the litter layer. This could lead to minor variation between our results and true estimates of seed predation; however, this uncertainty is unavoidable to ensure that we could keep track of individual seeds over time. We would expect any variation in our results to be minor, in particular because the majority of seed predation occurred by the first timepoint (within 2 weeks), and therefore the majority of seed is likely predated while on the litter surface or accessible upper undergrowth layers (Record et al. 1976). We are confident our results are as representative as possible while still retaining accurate observations over time.
Conclusion
Here we provided an example of how biotic resistance can be facilitated by other invasive species. We demonstrated seed predation pressures for wilding conifers are high when introduced granivorous species, or grazing livestock, are present. Levels of wilding conifer seed predation will likely fluctuate with spatiotemporal variation in rodent populations. Most seed are consumed within 2 weeks of release from the cone. Native tussock and scrub provide the lowest levels of granivory-based biotic resistance, indicating a high vulnerability to invasion and re-invasion, with forest biomes the most resilient. Few, if any, native species provide significant seed predation pressure on wilding conifers, which gives worrying implications for unintended consequences of invasive mammal control operations on wilding conifer populations.
Data availability
Upon acceptance, all data used in this manuscript will be made available at: https://github.com/TomC-93/VLR
Code availability
While no novel code was developed in this manuscript, the code used is available upon reasonable request.
References
Ackerman JD, Tremblay RL, Rojas-Sandoval J, Hernández-Figueroa E (2017) Biotic resistance in the tropics: patterns of seed plant invasions within an island. Biol Invasions 19:315–328
Backlund I, Arshadi M, Hunt AJ, McElroy CR, Attard TM, Bergsten U (2014) Extractive profiles of different lodgepole pine (Pinus contorta) fractions grown under a direct seeding-based silvicultural regime. Ind Crops Prod 58:220–229
Beaury EM, Finn JT, Corbin JD, Barr V, Bradley BA (2020) Biotic resistance to invasion is ubiquitous across ecosystems of the United States. Ecol Lett 23:476–482
Belote RT, Weltzin JF (2006) Interactions between two co-dominant, invasive plants in the understory of a temperate deciduous forest. Biol Invasions 8:1629–1641
Bennett GGJ, Buckley LL (1996) Use of foraging trails by Norway rats. Anim Behav 51:765–771
Bolker B, Robinson D (2022) broom.mixed: Tidying Methods for Mixed Models. Version R package version 0.2.9.4, CRAN.
Brown K, Moller H, Innes J, Alterio N (1996) Calibration of tunnel tracking rates to estimate relative abundance of ship rats (Rattus rattus) and mice (Mus musculus) in a New Zealand forest. N Z J Ecol 20:271–275
Burns B, Innes J, Day T (2011) The use and potential of pest-proof fencing for ecosystem restoration and fauna conservation in New Zealand. Fenc Conserv Restrict Evolut Potential Riposte Threat Process. https://doi.org/10.1007/978-1-4614-0902-1_5
Byers JE, Noonburg EG (2003) Scale dependent effects of biotic resistance to biological invasion. Ecology 84:1428–1433
Chiuffo MC, Moyano J, Rodriguez-Cabal MA, Nuñez MA (2018) Seed predation of non-native species along a precipitation gradient. Plant Ecol 219:1307–1314
Chong KY, Corlett RT, Nuñez MA, Chiu JH, Courchamp F, Dawson W, Kuebbing S, Liebhold AM, Padmanaba M, Souza L (2021) Are terrestrial biological invasions different in the tropics? Annu Rev Ecol Evol Syst 52:291–314
Choquenot D, Ruscoe WA (2000) Mouse population eruptions in New Zealand forests: the role of population density and seedfall. J Anim Ecol 69:1058–1070
Crane AL, Ferrari MC (2017) Patterns of predator neophobia: a meta-analytic review. Proc R Soc B Biol Sci 284:20170583
Delibes M, Castañeda I, Fedriani J (2019) Spitting seeds from the cud: a review of an endozoochory exclusive to ruminants. Front Ecol Evol 7:265
Dickie IA, Sprague R, Green J, Peltzer DA, Orwin K, Sapsford S (2022) Applying ecological research to improve long-term outcomes of wilding conifer management. N Z J Ecol 46:1–16
DOC (2020) Towards a Predator Free New Zealand: Predator Free 2050 Strategy. Wellington. https://www.doc.govt.nz/globalassets/documents/conservation/threats-and-impacts/pf2050/pf2050-towards-predator-freedom-strategy.pdf
Dylewski Ł, Ortega YK, Bogdziewicz M, Pearson DE (2020) Seed size predicts global effects of small mammal seed predation on plant recruitment. Ecol Lett 23:1024–1033
Esri, Maxar, Earthstar Geographics, Community GU (2022) World Imagery [Basemap]. 1:43,700., Esri
Everett RL, Meeuwig RO, Stevens R (1978) Deer mouse preference for seed of commonly planted species, indigenous weed seed, and sacrifice foods. Rangel Ecol Manag J Range Manag Arch 31:70–73
Fischer C, Türke M (2016) Seed preferences by rodents in the agri-environment and implications for biological weed control. Ecol Evol 6:5796–5807
Gioria M, Hulme PE, Richardson DM, Pyšek P (2023) Why are invasive plants successful? Annu Rev Plant Biol 74:635–670
Godó L, Valkó O, Borza S, Deák B (2022) A global review on the role of small rodents and lagomorphs (clade Glires) in seed dispersal and plant establishment. Glob Ecol Conserv 33:e01982
Griffin MJ, Morgan-Richards M, Trewick SA (2011) Is the tree weta Hemideina crassidens an obligate herbivore? N Z J Nat Sci 6:11–19
Halassy M, Batáry P, Csecserits A, Török K, Valkó O (2023) Meta-analysis identifies native priority as a mechanism that supports the restoration of invasion-resistant plant communities. Commun Biol 6:1100
Hulme PE (2020) Plant invasions in New Zealand: global lessons in prevention, eradication and control. Biol Invasions 22:1539–1562
Jamon M (1994) An analysis of trail-following behaviour in the wood mouse, Apodemus sylvaticus. Anim Behav 47:1127–1134
Janzen DH (1984) Dispersal of small seeds by big herbivores: foliage is the fruit. Am Nat 123:338–353
Jenkins CN, Pimm SL, Joppa LN (2013) Global patterns of terrestrial vertebrate diversity and conservation. Proc Natl Acad Sci 110:E2602–E2610
Jensen K, Gutekunst K (2003) Effects of litter on establishment of grassland plant species: the role of seed size and successional status. Basic Appl Ecol 4:579–587
Kröel-Dulay G, Csecserits A, Szitár K, Molnár E, Szabó R, Ónodi G, Botta-Dukát Z (2019) The potential of common ragweed for further spread: invasibility of different habitats and the role of disturbances and propagule pressure. Biol Invasions 21:137–149
Lambie J, Bennett M (2021) Flockhill Biodiversity Management Plan. Flockhill. https://www.flockhillnz.com/media/lizette-folder/biodiversity-plan/biodiversity-plan-lizette-design.pdf
Latham ADM, Latham MC, Norbury GL, Forsyth DM, Warburton B (2020) A review of the damage caused by invasive wild mammalian herbivores to primary production in New Zealand. N Z J Zool 47:20–52
Ledgard N (2001) The spread of lodgepole pine (Pinus contorta, Dougl.) in New Zealand. Forest Ecol Manag 141:43–57
Ledgard N (2002) The spread of Douglas-fir into native forests. N Z J for 47:36–38
Ledgard N, Norton D (2008) The impact of browsing on wilding conifers in the South Island high country. N Z J for 52:29
Ledgard N, Paul T (2008) Vegetation successions over 30 years of high country grassland invasion by Pinus contorta. N Z Plant Prot 61:98–104
Ledgard N, Knowles L, De La Mare P (2005) Douglas-fir-the current New Zealand scene. N Z J for 50:13–16
Lee S, Fowler SV, Lange C, Smith LA, Evans AM (2021) Unexpected parasitism of Douglas-fir seed chalcid limits biocontrol options for invasive Douglas-fir in New Zealand. N Z Plant Prot 74:70–77
Lenth R (2023) Emmeans: Estimated Marginal Means, aka Least-Squares Means. Version 1.8.9. CRAN, R package
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Lobo N, Duong M, Millar J (2009) Conifer-seed preferences of small mammals. Can J Zool 87:773–780
Lopez L, Terborgh J (2007) Seed predation and seedling herbivory as factors in tree recruitment failure on predator-free forested islands. J Trop Ecol 23:129–137
Lüdecke D, Ben-Shachar M, Patil I, Waggoner P, Makowski D (2021) Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw 6:3139
Mazia NC, Chaneton EJ, Ghersa CM, León RJ (2001) Limits to tree species invasion in pampean grassland and forest plant communities. Oecologia 128:594–602
McAlpine KG, Howell CJ, Wotton DM (2016) Effects of tree control method, seed addition, and introduced mammal exclusion on seedling establishment in an invasive Pinus contorta forest. N Z J Ecol 40:302–309
Miller J, Ecroyd C (1986) Introduced forest trees in New Zealand: Recognition, Role and Seed Source. 2. Pinus contorta Loud. Contorta pine. Rotorua.
Miller JT, Knowles FB (1994a) Introduced forest trees in New Zealand: recognition, role, and seed source. 14. Douglas-fir. Pseudotsuga menziesii (Mirbel) Franco, Rotorua, 38 p
Miller JT, Ecroyd CE (1987) Introduced forest trees in New Zealand: recognition, role, and seed source. 2. Pinus contorta Loudon-contorta pine. N Z for Res Inst for Res Bull 124:12
Miller JT, Knowles FB (1986) Introduced forest trees in New Zealand: recognition, role, and seed source. 1. Pinus nigra Arn.—European Black Pine. N Z for Res Inst for Res Bull 124:10
Miller JT, Knowles FB (1994b) Introduced forest trees in New Zealand: recognition, role, and seed source. 14. Douglas-fir. Pseudotsuga menziesii (Mirbel) Franco. N Z for Res Inst for Res Bull 124:38
Mitić ZS, Stojanović-Radić Z, Cvetković VJ, Jovanović SČ, Dimitrijević M, Ickovski JD, Jovanović N, Mihajilov-Krstev T, Stojanović GS (2021) Pseudotsuga menziesii (Pinaceae): volatile profiles, antimicrobial activity and toxicological evaluation of its essential oil. Chem Biodivers 18:e2100424
Moreira-Arce D, Vergara PM, Fierro A, Pincheira E, Crespin SJ, Alaniz A, Carvajal MA (2021) Standing dead trees as indicators of vertebrate diversity: bringing continuity to the ecological role of senescent trees in austral temperate forests. Ecol Ind 129:107878
Moyano J, Chiuffo M, Nuñez M, Rodriguez-Cabal M (2019a) Seed predation does not explain pine invasion success. Oecologia 189:981–991
Moyano J, Chiuffo MC, Policelli N, Nuñez MA, Rodriguez Cabal MA (2019b) The interplay between propagule pressure seed predation and ectomycorrhizal fungi in plant invasion. NeoBiota. https://doi.org/10.3897/neobiota.42.30978
MPI (2014) New Zealand Wilding Conifer Management Strategy 2015–2030. Wellington. https://www.mpi.govt.nz/dmsdocument/51013-The-wilding-conifer-management-strategy-20152030
MPI (2023) National exotic forest desription. Industries MfP, Wellington. https://www.mpi.govt.nz/dmsdocument/55996-2022-NEFD-Report
Mungi NA, Qureshi Q, Jhala YV (2021) Role of species richness and human impacts in resisting invasive species in tropical forests. J Ecol 109:3308–3321
Muschetto E, Chaneton EJ, Mazía N, Tripodi MA, Busch M (2022) Biotic resistance in a stochastic world: Do rodents act as a filter to alien tree invasion in pampean old fields? Ecol Res 37:568–581
Noguchi K, Gel Y, Brunner E, Konietschke F (2012) nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw 50:1–23
Nuñez MA, Chiuffo MC, Torres A, Paul T, Dimarco RD, Raal P, Policelli N, Moyano J, García RA, Van Wilgen BW (2017) Ecology and management of invasive Pinaceae around the world: progress and challenges. Biol Invasions 19:3099–3120
NWCCP (2019) National Wilding Conifer Control Programme Annual Report 2017–18. Wellington. https://www.wildingpines.nz/assets/Documents/National-Wilding-Conifer-Control-Programme-Annual-Report-2017-18.pdf
Ogle D, Doll J, Wheeler A, Dinno A (2023) FSA: simple fisheries stock assessment methods. Version R package version 0.9.5, CRAN
Ortmann C, Johnson S (2021) How reliable are motion-triggered camera traps for detecting small mammals and birds in ecological studies? J Zool 313:202–207
Palencia P, Vicente J, Soriguer RC, Acevedo P (2022) Towards a best-practices guide for camera trapping: assessing differences among camera trap models and settings under field conditions. J Zool 316:197–208
Pearson DE, Callaway RM, Maron JL (2011) Biotic resistance via granivory: establishment by invasive, naturalized, and native asters reflects generalist preference. Ecology 92:1748–1757
Pearson DE, Potter T, Maron JL (2012) Biotic resistance: exclusion of native rodent consumers releases populations of a weak invader. J Ecol 100:1383–1390
Peltzer DA, Bellingham PJ, Dickie IA, Houliston G, Hulme PE, Lyver POB, McGlone M, Richardson SJ, Wood J (2019) Scale and complexity implications of making New Zealand predator-free by 2050. J R Soc N Z 49:412–439
Policelli N, Hoeksema JD, Moyano J, Vilgalys R, Vivelo S, Bhatnagar JM (2023) Global pine tree invasions are linked to invasive root symbionts. New Phytol 237:16–21
Preukschas J, Zeiter M, Fischer M, Stampfli A (2014) Biotic resistance to plant invasion in grassland: Does seed predation increase with resident plant diversity? Basic Appl Ecol 15:133–141
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Randler C, Kalb N (2018) Distance and size matters: a comparison of six wildlife camera traps and their usefulness for wild birds. Ecol Evol 8:7151–7163
Rathfon RA, Greenler SM, Jenkins MA (2021) Effects of prescribed grazing by goats on non-native invasive shrubs and native plant species in a mixed-hardwood forest. Restor Ecol 29:e13361
Record CR, Marsh RE, Howard WE, Stern DJ (1976) Olfactory responses of deer mice to Douglas-fir seed volatiles
Rigby RA, Stasinopoulos DM (2005) Generalized additive models for location, scale and shape, (with discussion). Appl Stat 54:507–554
Rojas-Sandoval J, Ackerman JD, Tremblay RL (2020) Island biogeography of native and alien plant species: contrasting drivers of diversity across the Lesser Antilles. Divers Distrib 26:1539–1550
Ruscoe WA, Goldsmith R, Choquenot D (2001) A comparison of population estimates and abundance indices for house mice inhabiting beech forests in New Zealand. Wildl Res 28:173–178
Santamaría J, Tomas F, Ballesteros E, Ruiz JM, Bernardeau-Esteller J, Terrados J, Cebrian E (2021) The role of competition and herbivory in biotic resistance against invaders: a synergistic effect. Ecology 102:e03440
Sapere Research Group (2022) Benefits and costs of additional investment in wilding conifer control. Wellington. https://www.mpi.govt.nz/dmsdocument/58519-2022-Benefits-and-costs-of-additional-investment-in-wilding-conifer-control
Schauber EM, Kelly D, Turchin P, Simon C, Lee WG, Allen RB, Payton IJ, Wilson PR, Cowan PE, Brockie R (2002) Masting by eighteen New Zealand plant species: the role of temperature as a synchronizing cue. Ecology 83:1214–1225
Stasinopoulos DM, Rigby RA (2008) Generalized additive models for location scale and shape (GAMLSS) in R. J Stat Softw 23:1–46
Stotz GC, Pec GJ, Cahill JF (2016) Is biotic resistance to invaders dependent upon local environmental conditions or primary productivity? A meta-analysis. Basic Appl Ecol 17:377–387
Stryjek R, Modlinska K (2016) Neophobia in wild rats is elicited by using bait stations but not bait trays. Int J Pest Manag 62:158–164
Thorsen MJ, Dickinson KJ, Seddon PJ (2009) Seed dispersal systems in the New Zealand flora. Perspect Plant Ecol Evolut Syst 11:285–309
Thorsen MJ, Seddon PJ, Dickinson KJ (2011) Faunal influences on New Zealand seed dispersal characteristics. Evol Ecol 25:1397–1426
Ulloa J, Fuentes-Lillo E, Fuentes-Ramírez A, Pauchard A, García RA (2023) Native bamboo increases biotic resistance to Pinus contorta invasion in temperate forest ecosystems. Biol Invasions 25:3905
Vander Wall SB (2003) Effects of seed size of wind-dispersed pines (Pinus) on secondary seed dispersal and the caching behavior of rodents. Oikos 100:25–34
Vander Wall SB (2008) On the relative contributions of wind versus animals to seed dispersal of four Sierra Nevada pines. Ecology 89:1837–1849
Veech JA, Charlet DA, Jenkins SH (2000) Interspecific variation in seed mass and the co-existence of conifer species: a null model test. Evol Ecol Res 2:353–363
Waddell EH, Chapman DS, Hill JK, Hughes M, Bin Sailim A, Tangah J, Banin LF (2020) Trait filtering during exotic plant invasion of tropical rainforest remnants along a disturbance gradient. Funct Ecol 34:2584–2597
Walker S, Kemp JR, Elliott GP, Mosen CC, Innes JG (2019) Spatial patterns and drivers of invasive rodent dynamics in New Zealand forests. Biol Invasions 21:1627–1642
Wilson DJ, Ef W, Canham CD, Ruscoe WA (2007) Neighbourhood analyses of tree seed predation by introduced rodents in a New Zealand temperate rainforest. Ecography 30:105–119
Wilson DJ, Innes JG, Fitzgerald NB, Bartlam S, Watts C, Smale MC (2018) Population dynamics of house mice without mammalian predators and competitors. N Z J Ecol 42:192–203
Wundrow EJ, Carrillo J, Gabler CA, Horn KC, Siemann E (2012) Facilitation and competition among invasive plants: a field experiment with alligatorweed and water hyacinth. PLoS ONE 7:e48444
Wyatt S (2018) Benefits and costs of the wilding pine management programme phase 2. Wellington. https://www.wildingpines.nz/assets/Documents/MPI-Long-Term-Management-Wilding-Conifers-Cost-Benefit-Analysis.pdf
Zamora Nasca LB, Relva MA, Nuñez MA (2018) Ungulates can control tree invasions: experimental evidence from nonnative conifers and sheep herbivory. Biol Invasions 20:583–591
Acknowledgements
We would like to acknowledge Richard and Anna Hill, who allowed us access onto Flock Hill Station, without whom this work would not have been possible. We are also grateful to Willowbank Wildlife Park for allowing us to run a sister experiment on their site. In particular, Shaun Horan and all of the keepers, who kindly donated their time to helping us set this up. In addition, we are grateful to the possums, Tiny and Dobbie, as well as all of the wallabies at Willowbank Wildlife Park for acting as our guinea pigs. We would like to acknowledge Dave Henley, Pedro Capelino, Georgia Dickson, and Mariann Eagle who all helped with either fieldwork, seed preparation, or both. And finally, we would also like to express our gratitude to the two anonymous reviewers whose comments greatly improved this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding for this work was provided by MBIE under Science investment contract C04X2102 (Endeavour programme Vive la résistance).
Author information
Authors and Affiliations
Contributions
Thomas Carlin, Thomas Paul, Jan Dudenhoeffer, Carol Rolando, and Matthew Scott contributed to the study conception and design. Funding was secured by Thomas Paul and Carol Rolando. Material preparation and data collection were performed by Thomas Carlin, Jan Dudenhoeffer, Matthew Scott, Max Novoselov, Ryan Vorster, and Casey Springford. Data analyses and the initial draft were completed by Thomas Carlin. All authors provided meaningful and detailed revisions throughout the experiment and on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carlin, T.F., Paul, T.S.H., Dudenhoeffer, J.H. et al. The enemy of my enemy… Exotic mammals present biotic resistance against invasive alien conifers. Biol Invasions 26, 2647–2662 (2024). https://doi.org/10.1007/s10530-024-03336-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-024-03336-z