Abstract
Cycads hold important economic and conservation value. Some species are extensively used in landscaping, while others are endangered and legally protected. The Australian cycad-attacking weevil, Siraton internatus, is notably destructive, occasionally causing infestations and invasions across various countries. This study simulated habitat suitability for S. internatus to assess its potential invasion and the impact of climate change. Habitat suitability was evaluated under current climate and four climate change scenarios over two time frames (2050 and 2090). Furthermore, we investigated the threat posed by S. internatus to cycad reserves, using Taiwanese reserves as a representative case. Our MaxEnt predictions demonstrated high accuracy, meeting multiple evaluation criteria. We explored the potential distribution of S. internatus within Australia and internationally, identifying suitable habitats in Africa, the Americas, Asia, and Europe. The case study highlighted the low habitat suitability within the two Taiwanese cycad reserves, which is projected to decrease to unsuitable levels under future climate change scenarios for this weevil species. Moreover, our results revealed that suitable habitat for S. internatus is projected to contract globally under all climate scenarios and time periods, but expansion in Chile and the southern Himalaya (e.g., Nepal). This study provides valuable insights into cycad conservation and pest invasion risks. The results support both global and local efforts to manage the invasion threats from this destructive Australian cycad-attacking weevil species. It also accentuates the urgency for continuous biosecurity inspections and prevention of exporting mature cycad caudexes from Australia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cycads (Cycadales) are ancient gymnosperms with perennial woody structures that resemble palm trees. Notably diverse and widespread during the Mesozoic, cycads have evolved with minimal morphological variation, earning them the nickname "living fossils". Despite their well-documented toxicity, cycads have played an important role in human culture and have economic value (Whiting 1963; Kobayashi et al. 1974; Whitelock 2002; Hayward and Kuwahara 2012). Cycas revoluta, for instance, is a preferred choice in horticulture, frequently found in bonsai gardens and parks (Lu 2004; Tan and Ji 2010). However, not all cycads enjoy such widespread cultivation. A majority of identified cycad species are on the IUCN Red List of Threatened Species (IUCN 2023), highlighting their conservation vulnerability. Many are also protected under the Appendices of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), and have national reserves dedicated to their protection. In Taiwan, for instance, the Coastal Range Taitung Cycas Forest Reserve (CMR) and Hongye Village Taitung Cycas Nature Reserve (HCR) are specifically designated for the protection of the endemic Cy. taitungensis (Fig. S1), underscoring the targeted efforts to conserve this unique species, alongside others like Cy. panzhihuaensis in China and Encephalarto slatifrons and E. latifrons in South Africa.
Nevertheless, despite stringent international and local legislative protections, cycads face grave challenges (IUCN 2023). The Anthropocene era has seen an escalation in human activities, such as deforestation for agricultural expansion and urban development, leading to significant losses in cycad habitats. These ancient plants also suffer from the horticultural appeal that makes rare species targets for illegal poaching and trading, further endangering their existence. Invasive pests, such as the cycad blue butterfly Chilades pandava and the scale insect Aulacaspis yasumatsui, pose additional threats, weakening the plants and making them more susceptible to diseases (Takagi 1977; Chang 1989). Climate change compounds these issues, potentially altering ecological niches and enabling more frequent biological invasions (Nogué et al. 2009; Swart et al. 2018; Naimi et al. 2022). Given these multifaceted threats, prioritizing cycad conservation is imperative. This requires strengthening legal protections, enhancing international cooperation, involving local communities, and advancing scientific research to safeguard these essential elements of global biodiversity.
Trunk-boring cycad weevils, particularly Siraton internatus (may be transferred to the genus Demyrsus Pascoe, 1872 based on the recent phylogenetic study of Hsiao et al. (2023)), pose a major threat to cycads, and prefering stressed, diseased, or dying plants (Fig. 1) (Oberprieler 1995; Tang and Oberprieler 2006). While S. internatus naturally occur in low densities and primarily feeds on Macrozamia cycads in native coastal areas from New South Wales to Queensland, it has infested various cycad genera in artificial environments, including Macrozamia, Lepidozamia, Encephalartos, Zamia, and Cycas species (Glass 1980; Kennedy 1992, 2011; Oberprieler and Caldara 2012). Its damaging potential is evident in botanical gardens and nurseries, where both seedlings and mature plants are vulnerable (Westwood 1886; Glass 1980; Kennedy 1992, 2011; Oberprieler and Caldara 2012). For example, extensive infestations of S. internatus causing the death of Encephalartos plants and a 30-year-old Zamia furfuracea (Kennedy 1992, 2011). Managing S. internatus is challenging due to its deep burrowing nature, which shields it from traditional control methods. In addition, its unintentional introduction to various global regions has been documented over the past 130 years, underscoring its invasive nature (Glass 1980; Kennedy 1992, 2011), including Belgium (Westwood 1886), Italy, Greece (Hustache 1934), USA (California) (Glass 1980; Kennedy 1992, 2011; Oberprieler and Caldara 2012; INVASIVE.ORG 2023). These historical records serve as a reminder that weevils have the potential to invade and negatively impact cycad populations worldwide, highlighting the ongoing threat they pose. However, the scarcity of detailed biological knowledge on S. internatus hampers effective control and management strategies.
The infestation of Siraton internatus and their natural habitat: a adult of S. internatus; b illustration depicting the infestation of S. internatus and the tunneling of their larvae; c preference of S. internatus for unhealthy and dying cycad plants; d natural habitat and host plants (Macrozamia communis) of S. internatus in New South Wales
This study aims to address the knowledge gap regarding the potential invasion and climate change response of S. internatus by accessing its potential distribution under present and future climate scenarios. We focused on evaluating the threat to national cycad reserves through a detailed case study (CMR and HCR in Taiwan) (Fig. S1). Our approach included compiling global occurrence of S. internatus from museum collections and literature to ensure the data accuracy. Secondly, considering the current limitations in comprehensive biological data for S. internatus, we utilized MaxEnt for modeling its habitat suitability, relying primarily on climatic variables. This approach enabled us to conduct analyses at global, Australian, and Taiwanese scales, despite the data scarcity in specific biological traits of the species. Finally, we projected future range shift under varied climate change scenarios. This study is intended to enhance our understanding of cycad conservation and weevil invasion management, equipping us with knowledge to devise effective conservation strategies.
Materials and methods
This study investigated the potential distribution of S. internatus and its impact under changing climate conditions, proposing recommendations for the conservation of cycads and management of weevil invasions. The research was conducted in three main phases: Firstly, global occurrence data for S. internatus were meticulously compiled from reputable sources, including museum collections and peer-reviewed publications, to minimize biases in our model predictions (see 'Species occurrence dataset and bioclimatic factors'). Secondly, we focused on selecting the most suitable variables and optimizing the model parameters for accurate predictions (see 'Variable selection process' and 'Model parameter settings and optimization'). Lastly, we validated the robustness of our models through various metrics and visualized potential distributions on maps to illustrate the impact of different climate scenarios on habitat suitability (see 'Model prediction and map visualization' and 'Model validation').
Species occurrence dataset and bioclimatic factors
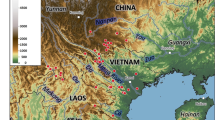
Global occurrence data for S. internatus were meticulously complied from reputable sources, including museum collections and peer-reviewed publications (Hsiao and Oberprieler 2020, 2021). To ensure the highest data accuracy, each record underwent rigorous verification, including expert taxonomic identification and cross-referencing with established distribution records to minimize biases in our model predictions. Furthermore, Quantum GIS (QGIS 2022) was employed to ensure a single distribution point within each 10-min grid cell, and to eliminate duplicate and imbalanced dataset to avoid severe spatial and environmental bias (Merow et al. 2013). Although this weevil’s primary distribution is in Australia, invasions in Europe and North America have been documented (Fig. 2a; Table S1). A comprehensive compilation of 58 S. internatus occurrences was organized and stored in a Microsoft Excel spreadsheet. The data were then saved in comma-separated value file format for further analysis and modeling using the MaxEnt software (Phillips et al. 2006). In addition, we kept all data publicly available for future validation as recommended by Liao et al. (2023).
Map of current occurrence and potential distribution of Siraton internatus: a global occurrence data of Siraton internatus, with exotic records represented by red circles and native occurrences represented by blue circles; b potential distribution under current climate. The maps were created using QGIS version 3.22.3 (www.qgis.org) and R version 4.3.2
We sourced nineteen bioclimatic variables (1970–2000) related to temperature and precipitation, which influence small arthropods’ distribution and survival, were sourced from WorldClim (version 2.1) (Fick and Hijmans 2017). Future projection using the GCM GISS-E2-1-G (Coupled Model Inter-comparison Project Phase VI, CMIP6) under four shared socioeconomic pathway scenarios (SSP1-2.6, SSP2-4.5, SSP3-7.0, and SSP5-8.5) for 2050 and 2090 timeframes was used in our model to assess the potential impacts of climate change on habitat suitability for S. internatus. The GISS-E2-1-G climate model from NASA is renowned for its enhanced climate sensitivity, making it apt for studying future warming pattern (Kelley et al. 2020; Nazarenko et al. 2022). The four socioeconomic pathway scenarios represent contrasting levels of climate change signals, with SSP1-2.6 indicating the optimistic scenario with climate protection measures are being taken and SSP5-8.5 representing the strongest. This multi-decadal time frame allows us to track and observe trends in the distributional shift of S. internatus in response to climate change. We obtained data at a 10-min resolution for global analysis and a 30-s resolution for in-depth Australia and Taiwan analyses.
Variable selection process
We prioritized addressing multicollinearity and the ratio of sample size to features, which are critical for maintaining the integrity and predictability of our ecological models. Our initial analysis utilized 19 bioclimatic variables to comprehensively assess each variable's contribution and permutation importance, allowing us to discern their significance within the model. This selection process involved choosing the top five variables based on contribution rates and the top five based on permutation importance. This resulted in a combined list of eight unique variables due to overlap. Subsequently, these variables underwent a Variance Inflation Factor (VIF) analysis to eliminate highly multicollinear features. Following the VIF criteria (Montgomery and Peck 1992; Thompson et al. 2017), we selected three variables with VIF values below the threshold of 10. Additionally, we included bio08, which had a VIF of 13.2, slightly above our primary threshold but considered manageable. After a second round of VIF analysis, we confirmed that the four selected variables—Isothermality (bio03), Precipitation of the Driest Quarter (bio17), Mean Temperature of the Wettest Quarter (bio08), and Precipitation of the Coldest Quarter (bio19)—all exhibited low multicollinearity. These variables, detailed in Table 1, were identified as most predictive of S. internatus habitat suitability, ensuring our model was scientifically grounded and reliable.
Model parameter settings and optimization
Given the limited biological information available about S. internatus, we utilized MaxEnt for species distribution modeling as it effectively leverages presence-only data, potentially revealing informative distributions before the pest invasion (Yeh et al. 2021; Baradevanal et al. 2023; da Silva et al. 2023). We used MaxEnt version 3.4.4 (Phillips et al. 2023) in R version 4.3.2 (R Core Team 2023) with the RStudio interface (version 2023.09.01 + 494) (RStudio Team 2023) and the 'dismo' package (Hijmans et al. 2022), to predict the global and Taiwanese distribution of the weevil. Pseudo-absences were generated from presence-only data, with the model configured to select 10,000 background points, iterating until reaching 500 iterations or a convergence threshold of 0.00001. The default parameter configurations in MaxEnt can result in overly complex or overly simplified models (Warren and Seifert 2011). To identify optimal parameter settings and reduce the risk of overfitting, we employed 'ENMeval' (Kass et al. 2022), which evaluates different combinations of feature classes and regularization multipliers. We used random ten-fold cross-validation to split the datasets into training and test sets, ensuring the model's transferability across different spatial and temporal scenarios. The Akaike Information Criterion with a small sample size correction (AICc) was utilized to select the best model settings. We chose the model parameters that resulted in the lowest delta AICc value, indicating the simplest model with adequate fit (Warren and Seifert 2011). The selected features were linear and quadratic (LQ), with a regularization multiplier set at 0.5, optimizing the balance between model complexity and explanatory power.
Model prediction and map visualization
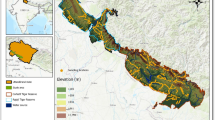
To address concerns about the impact of study area extent on model performance and predictions, we built upon the model parameters established in the previous section (Low et al. 2021; Amaro et al. 2023). Utilizing known global distribution points with 10-min resolution current climate data, we derived maximum entropy to project global potential distributions at a 10-min resolution. These projections were further refined for specific regions such as Australia and Taiwan at a higher resolution (30-s) and under various future climate scenarios to assess changes over time. Using the logistic output of MaxEnt, we estimated the probability of species presence based on environmental variables (i.e., habitat suitability) for each grid cell. This enabled the generation of potential distribution maps for different time periods and climate scenarios, illustrating the possible impacts on the species' distribution (Figs. 2b, 3, 4, 6; Figs. S5–S28). In addition, we identified potential host plants of Macrozamia cycads listed in Table S2, for which occurrence data were retrieved from the Atlas of Living Australia (ALA: http://www.ala.org.au). These data were then overlaid on the maps to visualize their correspondence with the weevil's potential distribution across Australia (Fig. S29). Specifically, for the two reserve areas of Taiwan (CMR and HCR) (Fig. S1), habitat suitability assessments were extracted for different timeframes and scenarios. Scatter plots, created using "ggplot2" (Wickham et al. 2019), helped assess the direct impacts on these reserves (Fig. 5). Dominant environmental variables were identified through a jackknifing process (Fig. S3) (Boria et al. 2014), and response curves were generated to examine the effects of individual variables on species presence (Fig. S4). All figures were optimized for clarity using Adobe Photoshop 2021 (Adobe Systems Inc.).
Habitat suitability of Siraton internatus in two Taiwanese cycad reserves under varying climate change scenarios. The graph displays changes in habitat suitability within the Coastal Mountain Range (CMR) and the High Central Range (HCR) across different timeframes (current, 2050, and 2090) and under different Shared Socioeconomic Pathways (SSP1-2.6, SSP2-4.5, SSP3-7.0, SSP5-8.5). Each point represents the average habitat suitability under a specific climate scenario, illustrating how predicted changes in climate could impact the potential distribution of Siraton internatus in these reserves. Figure was created using R version 4.3.2
Model validation
To assess the efficacy of our models, we utilized the area under the curve (AUC) of the receiver operating characteristic (ROC) curve (Fielding and Bell 1997), which measures the true positive rate against the false positive rate. AUC values span from 0 to 1, where higher values denote better predictive performance of the model (Fig. S2a) (Hernandez et al. 2006; Phillips et al. 2006; Franklin 2010; Peterson et al. 2011; Velasco et al. 2019). Additionally, we calculated the difference between the training and testing AUC values (AUC.diff) to gauge the model's generalizability (Warren and Seifert 2011). A smaller AUC.diff indicates consistent performance across both datasets, suggesting robust generalization capabilities, whereas a larger AUC.diff might indicate potential overfitting to the training data. Furthermore, we applied the continuous Boyce index (CBI) (Boyce et al. 2002; Bellard et al. 2013) to quantify the agreement of the model predictions with the actual presence data. The CBI ranges from -1 to 1, with positive values indicating that the model's predictions align well with observed distributions, and values near zero indicating performance akin to random chance. Omission rates (OR) at 0% and 10% sensitivities were also analyzed to assess the model's precision in predicting known presences of S. internatus (Pearson et al. 2006; Peterson et al. 2008; Liu et al. 2013). The model's adequacy is questioned if the default OR exceeds the predicted rate, which could signify an underprediction of known species locations (Peterson et al. 2011). The alignment of the training set OR (depicted by a blue line) with the predicted OR (black line) further served as an accuracy check, with close alignment indicating that the model reliably predicts known occurrences (Fig. S2b) (Radosavljevic and Anderson 2014).
Results
Model performance in predicting Siraton internatus potential distribution
Our MaxEnt model underwent rigorous validation, displaying exemplary performance across a range of metrics, confirming its suitability for accurately predicting the potential distribution of S. internatus. Achieving a Delta AICc of 0 and an AICc of 969.9185, the model demonstrated an optimal balance between complexity and fit. This was further evidenced by the incorporation of 10 coefficients, reflecting a sophisticated approach to modeling ecological patterns. The model excelled in validation metrics, evidenced by a high average validation AUC of 0.979265, with an AUC difference of just 0.0120902 between the training and validation sets. This small difference underscores the model’s robust generalization capabilities across datasets. Additionally, the 10% training omission rate averaged at 0.145, indicating the model's precision in capturing known species locations within the training data. The continuous Boyce Index values of 0.908 for training and 0.6468 for validation affirm the model's alignment with actual species distributions, suggesting that the predictions are not only accurate but also ecologically meaningful. Visual evaluations (Fig. S2) through omission rate graphs and the ROC curve further affirmed the model's reliability. The omission graph closely matched predicted with observed omissions, highlighting the model’s fidelity in representing the species' actual presence across its potential range. Moreover, the ROC curve displayed an impressive training AUC of 0.985, significantly surpassing the threshold for random predictions, which illustrates the model's exceptional predictive power (Franklin 2010; Peterson et al. 2011; Velasco et al. 2019).
Predicting the potential distribution for Siraton internatus under the current climate
Our predictions showed that S. internatus’s potential global distribution (Fig. 2b) encompasses areas specifically highlighted in Australia (Fig. 3) and Taiwan (Fig. 4). Green zones represented high habitat suitability, red indicated moderate habitat suitability, and white represented low suitability. The weevil’s potential global distribution (Fig. 2b) highlights high-risk coastal areas, including regions beyond its native Australian habitat like Argentina, Brazil, Chile, France, Mexico, Nepal, New Caledonia, New Zealand, Philippines, Portugal, South Africa, Spain, Taiwan, the USA, and Vietnam. Figure 3 showed that the highly suitable habitats of S. internatus include the coastal regions of northern Queensland to Victoria, southwestern Western Australia and Tasmania. When considering the distribution of potential host plants in the genus Macrozamia, overlaid with the weevil's potential distribution, there is a noticeable concentration of suitable habitats along the eastern coast of Australia (Fig. S29). This overlap suggests areas where Macrozamia populations could be at risk due to weevil invasion, providing critical information for conservation efforts. Focusing on Taiwan (Fig. 4), our analysis revealed that the high mountain areas exhibit high habitat suitability for S. internatus. Contrastingly, the northern and western regions of Taiwan are predominantly characterized by large areas of low suitability, marked as white areas on the map. Notably, when factoring in Taiwanese cycad reserves (Fig. 5), the Coastal Range Taitung Cycas Forest Reserve (CMR) and Taitung Hongye Village Taitung Cycas Nature Reserve (HCR) both show low habitat suitability for S. internatus (0.126988 and 0.2773997). The contribution rate of the environmental variables and their permutation importance according to the jackknife test are presented in Table 1 and Fig. S3. Response curves for four selected variables of S. internatus are shown in Fig. S4, which all showing unimodal relationships, which indicate the relationship with the habitat suitability and variable values.
Projection of future potential distribution for weevil under the future climate
Projections of S. internatus’s potential distribution under varying SSP climate scenarios are depicted in Fig. 6 and Figs. S5–S28, considering the four climate change scenarios over two-time frames, including the least severe (SSP1-2.6) and most severe (SSP5-8.5) climate change scenarios. Our global analysis revealed that suitable habitats for the species are subject to noticeable variability in response to climate change. Although a general trend of contraction is evident, particularly in regions such as North America and Europe, unique variations are observed in specific locales over the next seven decades. Our projections identify a distinct southward shift in suitable habitats within Australia, reflecting a change from traditional ranges towards new potential habitats further south. Conversely, in Taiwan, particularly within the CMR and HCR regions (Figs. 4, 5), the suitability for S. internatus starts from a low baseline and is further exacerbated by climate change, with projections indicating a significant decline to below 0.1 by 2090 under the harsher climate scenarios, signaling a worrying trend towards greater unsuitability. Additionally, distinct expansion of suitable habitats for S. internatus was identified along a specific elongated region in the southern Himalayas, notably encompassing areas within Nepal and Bhutan. This linear area of increased habitat suitability stands in stark contrast to the global trend of habitat contraction and indicates potential new refuges for the species in these high-altitude regions despite broader climate-induced pressures.
Discussion
Distribution of Siraton internatus in Australia, with discussion on its ecology
Our model predicted the potential distribution of S. internatus based on current climatic variables and occurrence data. These predictions align with known occurrence records (Fig. 2 and Table S1), barring Victoria, northern Queensland, Tasmania and southwestern Western Australia. In Victoria, northern Queensland and Tasmania, natural populations of Macrozamia are absent. Additionally, southwestern Western Australia is inhabited by another species of Siraton (S. roei (Boheman,)). A notable report by Hsiao and Oberprieler (2020) reported a suspicious record of S. internatus near Cooktown in northern Queensland (Fig. 3), outside its recognized range. Yet, our models do highlight northern Queensland areas like Koombooloomba National Park and Girringun National Park as highly suitable for this weevil. The presence of several botanical gardens in northern Queensland raises the possibility of accidental introductions via cycad bonsais. As Hsiao and Oberprieler (2020) pointed out, certain specimens from the Australian National Botanical Garden in Canberra, where native cycads are absent, suggest inadvertent introductions. The same could apply to northern Queensland, although no subsequent specimens have been discovered. Hsiao and Oberprieler (2020) mapped seven potential Macrozamia cycad hosts (Table S2) for S. internatus, with M. polymorpha subsequently confirmed as a host in Hsiao and Oberprieler (2021). Our predictions showed a considerable overlap between these cycads and S. internatus’s potential distribution, supporting earlier findings. Furthermore, we identified 19 new potential Macrozamia hosts from eastern Australia for S. internatus (Table S2) based on its potential distribution. This indicates that S. internatus can potentially infest most Macrozamia species in their natural habitats.
Australia's domestic botanical or home gardens are notably susceptible to cycad-attacking weevils. The threat is magnified in gardens within the native cycad and weevil range, such as the Cairns Botanic Gardens, Brisbane Botanic Gardens, City Botanic Gardens in Queensland, the North Coast Regional Botanic Garden, Orange Botanic Gardens, and Royal Botanic Garden Sydney in New South Wales, and the Australian National Botanic Gardens in the Australian Capital Territory. These botanical gardens, due to their diverse cycad collections, can be prime weevil habitats. For example, Kennedy (1992) reported the loss of several Encephalartos plants and a mature Zamia furfuracea due to a large infestation of S. internatus. Small-scale occurrence of S. internatus have also been reported at the Australian National Botanical Garden in the Australian Capital Territory (Hsiao and Oberprieler 2020). Additionally, Maria Walford-Huggins, who established an impressive collection of endemic and exotic cycads in Julatten, northern Queensland in the 1980s (Birkbeck and Birkbeck 2014), recorded an infestation of Demyrsus digmon Hsiao and Oberprieler 2020 on Encephalartos cycads (Hsiao and Oberprieler 2020). Our results indicated that both infestation events by S. internatus occurred within the potential distribution range of the weevil. Furthermore, our future projection covered four different climatic scenarios over two future time frames, highlights the ongoing vulnerability of artificial facilities in eastern Australia to S. internatus (Figs. 6, S5–S28). However, we previously considered that the response curves of the model (Table 1 and Fig. S5) can provide insufficient biological data of the weevil, but Smith and Sonto (2020) caution against over-interpreting the importance of specific climatic variables based solely on model accuracy. Overall, our findings offer crucial insights for cycad conservation and managing S. internatus infestations, beneficial for botanical gardens, the horticultural industry, and cycad enthusiasts.
Global invasion of Siraton internatus and its potential impacts to cultivated and wild cycads
While S. internatus originates from Australia, the weevil's suitable habitats under the current climatic conditions stretch beyond its native confines. Areas of pronounced risk include locales spanning Argentina, Brazil, Chile, France, Mexico, Nepal, New Caledonia, New Zealand, Philippines, Portugal, South Africa, Spain, Taiwan, USA, and Vietnam (Fig. 2). Notably, many of these regions harbor native cycad species: Mexico boasts 26 species, New Caledonia one, Philippines two, South Africa 24, the USA three, Taiwan one, and Vietnam seven (detailed in Table S3). This overlap underscores the invasive threat S. internatus poses to cycad conservation. This threat isn’t limited to wild cycads. Cultivated cycad plants, vital for ex-situ conservation of endangered cycad species, are equally vulnerable, especially when situated in or near these high-risk zone (Westwood 1886; Glass 1980; Kennedy 1992, 2011; Oberprieler and Caldara 2012). Historical data evidences the weevil’s capacity to inflict significant damage in botanical or domestic gardens and nurseries. A multitude of these risk areas are proximate to botanical gardens, intensifying concerns about accidental biological invasions. Examples include the La Plata Botanical Garden and E.N.Orfila Botanical Garden in Argentina, the Botanical Gardens of Porto Alegre and Jardim Botânico De Caxias Do Sul in Brazil, the Nacional de Viña del Mar Botanical Garden and Parque Paraiso Escalante in Chile, the Arquebuse Botanical Garden and Botanical Garden of Lyon in France, the Rock Garden of Liwang and Banke Botanical Garden in Nepal, the Parc Zoologique et Forestier Michel Corbasson and de Félix et Janine BOLE Botanical Garden in New Caledonia, the Timaru Botanic Gardens and Christchurch Botanic Gardens in New Zealand, the Baguio Botanical Garden in Philippines, the Tropical Botanical Garden of Lisboa and Jardim Botânico do Porto in Portugal, the Makana Botanical Gardens and Durban Botanic Gardens in South Africa, the Jardín Botánico Atlántico de Gijón and Barakaldo Botanical Garden in Spain, the Botanical Garden of National Museum of Natural Science in Taiwan, the San Diego Botanic Garden and South Coast Botanic Garden in California and McKee Botanical Gardens in Florida in USA, and the Phong Nha Botanic Garden in Vietnam. Furthermore, botanical gardens, pivotal for biosecurity, can inadvertently act as gateways for pest and pathogen invasions (Wondafrash et al. 2021). Thus, they might inadvertently catalyze the establishment of weevil populations in surrounding environments.
However, it's noteworthy that only a few isolated areas are marked as high risk. This suggests a low likelihood of S. internatus evolving into a dominant global cycad pest akin to the cycad blue butterfly or cycad scale insect. Interestingly, despite multiple invasions spanning over a century, S. internatus hasn’t entrenched itself in foreign territories, causing localized damages rather than widespread establishment (Westwood 1886; Glass 1980; Kennedy 1992, 2011; Oberprieler and Caldara 2012). This observation casts doubt on the accuracy of the pest threat ranking system proposed by Tang and Oberprieler (2006), which might have overestimated the threat posed by Australian cycad-boring weevils.
The evaluation of invasive threat of Siraton internatus to Taiwan’s endemic cycads
Taiwan, an East Asia island celebrated for its rich biodiversity despite its modest size, is home to the singular endemic cycad species, Cycas taitungensis (Shao and Chung 2022). Restricted to two petite populations, this species is safeguarded within two nature reserves: Coastal Range Taitung Cycas Forest Reserve (CMR) and the Taitung Hongye Village Taitung Cycas Nature Reserve (HCR) (Fig. S1). Beyond the challenges of habitat destruction, illegal harvesting, and horticultural trade, Cy. taitungensis grapples with invasive threats like the cycad blue butterfly and the cycad aulacaspis scale insect (Chang 1989; Chao and Lai 2005). These challenges have led to its classification as endangered (EN) on the latest IUCN Red List (Bösenberg 2022). To gauge the potential fallout of the Australian cycad-attacking weevils on this indigenous species, we embarked on a focused assessment of the weevil's habitat compatibility within these Taiwanese nature reserves. Overlaying the confines of these reserves onto a map delineating the weevil's suitable habitats in Taiwan (as seen in Fig. 5), it’s evident that both CMR and HCR have low habitat suitability for S. internatus establishment, which is beneficial for the survival of Cy. taitungensis. However, occasional infestation events of S. internatus may still occur in some local botanic gardens, as observed previously (Westwood 1886; Glass 1980; Kennedy 1992, 2011; Oberprieler and Caldara 2012). Furthermore, the growing trend of cultivating cycad bonsai for decorative purposes in Taiwan heightens the risk of unintentionally ushering in the Australian cycad weevil. Although there’s no documented instance of Cy. taitungensis serving as a host for S. internatus, recent studies spotlighting species delimitation draw parallels between Cy. taitungensis and Cy. revoluta (Chang et al. 2022). Given that the latter has been a known host for S. internatus, the potential vulnerability of Cy. taitungensis becomes palpable. This research unveils a fresh, looming menace to the conservation endeavors for Cy. taitungensis. Coupled with the onslaught from existing invasive pests, its limited population, isolated habitats, and unlawful trade, the prospect of Cy. taitungensis's survival dims considerably should S. internatus gain a foothold.
Impact of climate change on the distribution of Siraton internatus, with suggestions to future cycad conservation and pest management
Climate change is reshaping global ecosystems, leading to significant alterations in species distribution patterns (Nogué et al. 2009; Naimi et al. 2022), which in turn challenges S. internatus habitats worldwide. Our ecological niche modeling indicates a global trend of habitat contraction for S. internatus, with notable exceptions highlighting the complex nature of these shifts. Specifically, we observe expansion of suitable habitats in regions such as southern Chile and the southern Himalayas, including Nepal and Bhutan (Fig. 6, Figs. S5–S12). These areas represent unexpected refuges where S. internatus could potentially thrive amidst global contractions. Conversely, in Australia (Figs. S13–20), the weevil's native habitats, there's a discernible southward shift in suitable habitats, reflecting climate-driven migration within its endemic range. This adaptation suggests that while traditional habitats may become less viable, new areas could emerge as suitable, necessitating updates to current management and conservation strategies to account for these shifts. Moreover, while our modeling efforts provide a critical foundation for understanding these distributional changes, following insights from Smith and Santos (2020), we acknowledge the importance of increasing sample size in future modeling efforts. Enhanced sample sizes are crucial for a more reliable inference of variable importance and ensuring more accurate and robust predictions of habitat suitability and invasion risks. This becomes especially significant as we continue to adapt our conservation strategies in response to shifting ecological niches and emerging threats.
The contrasting patterns of habitat contraction globally, with expansions in specific locales such as Chile and the southern Himalayas, and shifts within Australia, underline the need for nuanced conservation approaches. These should be tailored to local conditions and include both preventive measures in newly suitable areas and adaptive strategies in regions experiencing habitat shifts. Enhanced monitoring and biosecurity are crucial, particularly in regions facing new risks from S. internatus expansion, to prevent its spread to ecosystems previously unaffected by this pest. Therefore, our findings underscore the imperative for a multifaceted approach to conservation in the face of climate change, balancing the need to address habitat contractions globally with the necessity to manage emerging threats and opportunities in specific regions.
Conclusion and future works
This study has investigated the distribution and potential invasion risks of the trunk-boring cycad weevils, S. internatus, with a focus on both its native territory in Australia and its potential impacts globally, particularly in Taiwan. Modeling habitat suitability under current and future climate scenarios revealed a nuanced response to climate change, with global habitat contractions anticipated, alongside localized expansions and shifts, such as in southern Chile and the southern Himalayas. Given these insights, we continue to recommend stringent quarantine measures on cycad imports and advocate for the regulation of cycad caudex exports from Australia to prevent further spread of S. internatus. To refine future predictions, extensive faunistic surveys of S. internatus should be prioritized. Furthermore, attention should be given to the other three known Australian cycad-attacking weevils: S. roei, Demyrsus meleoides Pascoe, and D. digmon. The invasion of D. meleoides into regions like Italy, South Africa, and the UK, coupled with its affinity for African Encephalartos cycads, underscores its economic significance (Covassi 1974; Oberprieler 1995; INVASIVE.ORG 2023). Employing alternative models (e.g., physiologically based demographic models, (Ponti et al. 2021)), can enhance our understanding of the potential invasion when more data become available. Future research should explore the potential spatial overlap and interaction between S. internatus and various cycad species. This includes a detailed investigation into the weevil's impact on both known and potential cycad hosts, enhancing our understanding of its ecological dynamics and informing targeted conservation efforts. As a pioneering effort in this niche field, we aim to lay the groundwork for advanced cycad conservation strategies and the management of invasive pests like S. internatus. This study underscores the necessity for a multifaceted approach in studying and mitigating the impacts of invasive species on global cycad populations. Highlighting the importance of continued research, monitoring, and fostering international collaboration, particularly in areas such as biosecurity measures and conservation strategies, is essential.
References
Amaro G, Fidelis EG, da Silva RS, Marchioro CA (2023) Effect of study area extent on the potential distribution of species: a case study with models for Raoiella indica Hirst (Acari: Tenuipalpidae). Ecol Model 483:110454. https://doi.org/10.1016/j.ecolmodel.2023.110454
Baradevanal G, Chander S, Singh HS, Reddy DS, Rajan S (2023) Mapping the risk of quarantine pest Sternochetus mangiferae under different climate change scenarios through species distribution modelling. Int J Trop Insect Sci 43:919–932. https://doi.org/10.1007/s42690-023-01000-y
Bellard C, Thuiller W, Leroy B, Genovesi P, Bakkenes M, Courchamp F (2013) Will climate change promote future invasions? Global Change Biol 19:3740–3748. https://doi.org/10.1111/gcb.12344
Birkbeck F, Birkbeck J (2014) A palm paradise in the tropics. Palm Cycad 123:10–18
Boria RA, Olson LE, Goodman SM, Anderson RP (2014) Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol Model 275:73–77. https://doi.org/10.1016/j.ecolmodel.2013.12.012
Bösenberg JD (2022) Cycas taitungensis. The IUCN red list of threatened species 2022: e.T42067A69826816. https://doi.org/10.2305/IUCN.UK.2022-1.RLTS.T42067A69826816.en. Accessed 06 June 2023
Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FKA (2002) Evaluating resource selection functions. Ecol Model 157:281–300. https://doi.org/10.1016/S0304-3800(02)00200-4
Chang Y-C (1989) Morphology, life history and damage of cycas blue butterfly (Chilades pandava pandava) as well as pathogenicity of entomogenus fungus to its larva. Bull Taiwan Res Inst New Ser 4:43–50
Chang J-T, Chao C-T, Nakamura K, Liu H-L, Luo M-X, Liao P-C (2022) Divergence with gene flow and contrasting population size blur the species boundary in Cycas Sect. Asiorientales, as inferred from morphology and RAD-Seq Data. Front Plant Sci 13:824158. https://doi.org/10.3389/fpls.2022.824158
Chao JT, Lai PY (2005) Taiwan CAS invasion timeline. http://www.iucn.org/themes/ssc/sgs/csg/pages/CAS.htm. Accessed 6 Jan 2009
Covassi M (1974) Il Demyrsus meleoides Pascoe: un potenziale nemico delle cicadae ornamentali introdotto in Italia (Coleoptera, Curculionidae). Redia 55:211–217
da Silva JMM, Ramos RS, Souza PGC, JdaS P, Picanco MC, Silva GA, da Silva RS (2023) Mapping brazilian expansion risk levels of mango weevil (Sternochetus mangiferae Fabricius) based on MaxEnt. Neotrop Entomol 52:760–771. https://doi.org/10.1007/s13744-023-01041-5
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49. https://doi.org/10.1017/S0376892997000088
Franklin J (2010) Mapping species distributions: spatial inference and prediction. Cambridge University Press, Cambridge
Glass C (1980) Tranes beetle now a pest in the US. Cycad Newsl 3:7
Hayward P, Kuwahara S (2012) Sotetsu heritage—cycads, sustenance and cultural landscapes in Amami Islands. Aust Pac J Reg Food Stud 2:26–46
Hernandez PA, Graham C, Master LL, Albert DL (2006) The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29:773–785. https://doi.org/10.1111/j.0906-7590.2006.04700.x
Hijmans RJ, Phillips S, Leathwick J, Elith J (2022) Package ‘Dismo’. http://cran.r-project.org/web/packages/dismo/index.html. Accessed 20 Sep 2022
Hsiao Y, Oberprieler RG (2020) A review of the trunk-boring cycad weevils in Australia, with description of a second species of Demyrsus Pascoe, 1872 (Coleoptera: Curculionidae). Austral Entomol 59:677–700. https://doi.org/10.1111/aen.12498
Hsiao Y, Oberprieler RG (2021) Bionomics and new host plant records of the Australian trunk-boring cycad weevils (Coleoptera: Curculionidae). Coleopt Bull 75:695–699. https://doi.org/10.1649/0010-065X-75.3.695
Hsiao Y, Oberprieler RG, Zwick A, Zhou YL, Ślipiński A (2023) Museomics unveil systematics, diversity and evolution of Australian cycad-pollinating weevils. Proc R Soc B Biol Sci 290:20231385. https://doi.org/10.1098/rspb.2023.1385
Hustache A (1934) Un curculionide [Col.] nouveau de l’Ile de Elbe. Bull Soc Entomol Fr 39:250–253
INVASIVE.ORG (2023) Macrozamia borer Tranes internatus Pascoe, 1870. https://www.invasive.org/index.cfm. Accessed 06 October 2023
IUCN (2023) The IUCN red list of threatened species. Version 2022–2. https://www.iucnredlist.org. Accessed 6 June 2023
Kennedy P (1992) Cycad–insect relationships. sestruction. Encephalartos 29:20–22
Kennedy P (2011) Trunk-boring weevils—Melanotranes internatus in New South Wales. Encephalartos 105:14–20
Kass JM, Muscarella R, Galante PJ, Bohl C, Buitrago-Pinilla GE, Boria RA, Soley-Guardia M, Anderson RP (2022) Package ‘ENMeval’. http://cran.r-project.org/web/packages/ENMeval/index.html. Accessed 20 Sep 2022
Kelley M, Schmidt GA, Nazarenko LS, Bauer SE, Ruedy R, Russell GL, Ackerman AS, Aleinov I, Bauer M, Bleck R, Canuto V, Cesana G, Cheng Y, Clune TL, Cook BI, Cruz CA, Del Genio AD, Elsaesser GS, Faluvegi G, Kiang NY, Kim D, Lacis AA, Leboissetier A, LeGrande AN, Lo KK, Marshall J, Matthews EE, McDermid S, Mezuman K, Miller RL, Murray LT, Oinas V, Orbe C, García-Pando CP, Perlwitz JP, Puma MJ, Rind D, Romanou A, Shindell DT, Sun S, Tausnev N, Tsigaridis K, Tselioudis G, Weng E, Wu J, Yao M-S (2020) GISS-E2.1: Configurations and climatology. J Adv Model Earth Syst 12:e2019MS002025. https://doi.org/10.1029/2019MS002025
Kobayashi A, Toya M, Fukunishi R, Yoshida A (1974) Safety evaluation of sotetsu-miso, cycad bean paste, chemical determination of toxic substances and long-term feeding test of the miso. J Jpn Soc Food Nutr 27:263–268. https://doi.org/10.4327/jsnfs1949.27.263
Liao JR, Chiu MC, Kuo MH (2023) Reassessing the presence of alien predatory mites and their prospects in the face of future climate change. Pest Manag Sci 79:5186–5196. https://doi.org/10.1002/ps.7722
Liu C, White MT, Newell G (2013) Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr 40:778–789. https://doi.org/10.1111/jbi.12058
Low BW, Zeng Y, Tan HH, Yeo DCJ (2021) Predictor complexity and feature selection affect Maxent model transferability: evidence from global freshwater invasive species. Divers Distrib 27:497–511. https://doi.org/10.1111/ddi.13211
Lu Y (2004) A technical study of the trunk of Cycas d[r]evolutaThunb. forms pluriceps landscape quickly. Chin Agric Sci Bull 20:168–169
Merow C, Smith MJ, Silander JA (2013) A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36:1058–1069. https://doi.org/10.1111/j.1600-0587.2013.07872.x
Montgomery DC, Peck EA (1992) Introduction to linear regression analysis. Wiley, New York
Naimi B, Gapinha C, Ribeiro J, Rahbek C, Strubbe D, Reino L, Araújo MB (2022) Potential for invasion of traded birds under climate and land-cover change. Glob Chang Biol 28:5654–5666. https://doi.org/10.1111/gcb.16310
Nazarenko LS, Tausnev N, Russell GL, Rind D, Miller RL, Schmidt GA, Bauer SE, Kelley M, Ruedy R, Ackerman AS, Aleinov I, Bauer M, Bleck R, Canuto V, Cesana G, Cheng Y, Clune TL, Cook BI, Cruz CA, Del Genio AD, Elsaesser GS, Faluvegi G, Kiang NY, Kim D, Lacis AA, Leboissetier A, LeGrande AN, Lo KK, Marshall J, Matthews EE, McDermid S, Mezuman K, Murray LT, Oinas V, Orbe C, García-Pando CP, Perlwitz JP, Puma MJ, Romanou A, Shindell DT, Sun S, Tsigaridis K, Tselioudis G, Weng E, Wu J, Yao M-S (2022) Future climate change under SSP emission scenarios with GISS-E2.1. J Adv Model Earth Syst 14:e2021MS002871. https://doi.org/10.1029/2021MS002871
Nogué S, Rull V, Vegas-Vilarrúbia T (2009) Modeling biodiversity loss by global warming on Pantepui, northern South America: projected upward migration and potential habitat loss. Clim Change 94:77–85. https://doi.org/10.1007/s10584-009-9554-x
Oberprieler RG (1995) The weevils (Coleoptera: Curculionoidea) associated with cycads. 1. Classification, relationships, and biology. In: Vorster P (ed) Proceedings of the third international conference on cycad biology. The Cycad Society of South Africa, Stellenbosch, South Africa, pp 295–334
Oberprieler RG, Caldara R (2012) Siraton devillei Hustache (Coleoptera: Curculionidae), the mysterious weevil from the Isle of Elba: exiled no longer. Zootaxa 3573:55–58. https://doi.org/10.11646/zootaxa.3573.1.6
Pearson RG, Raxworthy CJ, Nakamura M, Townsend Peterson A (2006) Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr 34:102–117. https://doi.org/10.1111/j.1365-2699.2006.01594.x
Peterson AT, Papeş M, Soberón J (2008) Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Model 213:63–72. https://doi.org/10.1016/j.ecolmodel.2007.11.008
Peterson AT, Soberón J, Anderson RP, Pearson RG, Martínez-Meyer E, Nakamura M, Araújo MB (2011) Ecological niches and geographic distributions: a modeling perspective. Princeton University Press, Princeton
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026
Phillips SJ, Dudik M, Schapire RE (2023) Maxent software for modeling species niches and distribution (Version 3.4.4). https://biodiversityinformatics.amnh.org/open_source/maxent/. Accessed 20 Jan 2023
Ponti L, Gutierrez AP, de Campos MR, Desneux N, Biondi A, Neteler M (2021) Biological invasion risk assessment of Tuta absoluta: mechanistic versus correlative methods. Bio Invasions 23:3809–3829. https://doi.org/10.1007/s10530-021-02613-5
QGIS Development Team (2022) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org/. Accessed 20 Dec 2022
R Core Team (2023) R: a language and environment for statistical computing. Vienna, Austria. https://www.R-project.org/. Accessed 20 Sep 2023
Radosavljevi A, Anderson RP (2014) Making better Maxent models of species distributions: complexity, overfitting and evaluation. J Biogeog 41:629–643. https://doi.org/10.1111/jbi.12227
RStudio Team (2023) RStudio: integrated development environment for R. Boston, MA. http://www.rstudio.com/. Accessed 20 Sep 2023
Shao K, Chung K (2022) Endemic species in Taiwan. Version 8.5. Taiwan Biodiversity Information Facility (TaiBIF). https://doi.org/10.15468/tkdy8a. Accessed 1 June 2023
Smith AB, Santos MJ (2020) Testing the ability of species distribution models to infer variable importance. Ecography 43:1801–1813. https://doi.org/10.1111/ecog.05317
Swart C, Donaldson J, Barker N (2018) Predicting the distribution of Encephalartos latifrons, a critically endangered cycad in South Africa. Biodivers Conserv 27:1961–1980. https://doi.org/10.1007/s10531-018-1519-9
Takagi S (1977) A new species of Aulacaspis associated with a cycad in Thailand (Homoptera: Coccoidea). Insecta Matsumurana New Series 11:61–72
Tan C-X, Ji J-M (2010) Production of cycad bonsai. Chinese Countryside Well-off Tech 10:48–49
Tang W, Oberprieler R (2006) Insect pests of cycads. http://www.iucn.org/themes./ssc/sgs/csg/publications/CAS/Cycad-Aulacaspis-Scale-Pest-Alert.pdf. Accessed 27 Mar 2020
Thompson CG, Kim RS, Aloe AM, Becker BJ (2017) Extracting the variance inflation factor and other multicollinearity diagnostics from typical regression results. Basic Appl Soc Psychol 39:81–90. https://doi.org/10.1080/01973533.2016.1277529
Velasco JA, Gonzalez-Salazar C (2019) Akaike information criterion should not be a “test” of geographical prediction accuracy in ecological niche modeling. Ecol Inform 51:25–32. https://doi.org/10.1016/j.ecoinf.2019.02.005
Warren DL, Seifert SN (2011) Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecol Appl 21:335–342. https://doi.org/10.2307/29779663
Westwood JO (1886) Observations upon species of Curculionidae injurious to Cycadeae, especially to plants of the genus Zamia. Ann Soc Ent Belg 30:125–130
Whitelock LM (2002) The cycads. Timber Press, Portland
Whiting MG (1963) Toxicity of cycads. Econ Bot 17:270–302
Wickham H, Chang W, Henry L, Pedersen TL, Takahashi K, Wilke C, Woo K (2019) ggplot2: Create elegant data visualisations using the grammar of graphics. R package version 3.1.1. https://CRAN.R-project.org/package=ggplot2 Accessed 20 Dec 2023
Wondafrash M, Wingfield MJ, Wilson JRU, Hurley BP, Slippers B, Paap T (2021) Botanical gardens as key resources and hazards for biosecurity. Biodivers Conserv 30:1929–1946. https://doi.org/10.1007/s10531-021-02180-0
Yeh HT, Cheah HY, Chiu MC, Liao JR, Ko CC (2021) Assessment of potential invasion for six phytophagous quarantine pests in Taiwan. Sci Rep 11:10666. https://doi.org/10.1038/s41598-021-89914-w
Acknowledgements
We express our gratitude to RG Oberprieler (CSIRO, Australia) for his invaluable suggestions and photos regarding weevils. We thank anonymous reviewers for help in improving the manuscript.
Funding
Open access funding provided by Tokyo Metropolitan University. The first author was funded by the grant Taiwan Australian National University Scholarship from Taiwanese Ministry of Education, and the second author was supported by the YF2022 Japan Society for the Promotion of Science (JSPS KAKENHI n°22P22380) Postdoctoral Fellowships, Japan.
Author information
Authors and Affiliations
Contributions
YH designed the study and drafted the manuscript; JRL analyzed the data and review the manuscript. All authors contributed to the study conception, design, and approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
All data generated and analyzed during this study are included in this published article and its supplementary information files.
Ethical approval
Not applicable.
Consent to participate
All authors agreed with the content and all gave explicit consent to submit this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsaio, Y., Liao, JR. Predicting habitat suitability for the Australian cycad-attacking weevil (Siraton internatus) under climate change. Biol Invasions 26, 2579–2594 (2024). https://doi.org/10.1007/s10530-024-03330-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-024-03330-5