Abstract
Biological control programs provide simplified systems in which to investigate the influence of temperature on trophic interactions. We investigated the interaction between the invasive thistle Carduus nutans and its specialist herbivore, the biological control agent weevil Rhinocyllus conicus, along a temperature gradient spanning an urban-to-rural urbanization gradient in central Pennsylvania. As expected, more urbanized sites were warmer than rural sites. Nevertheless, C. nutans phenology was only slightly earlier at warmer sites, and the timing of observations of adult R. conicus on thistles did not shift earlier, leaving synchrony apparently unchanged. Despite unchanged phenological matching, seasonal patterns in weevil damage varied with temperature: early-appearing capitula at warmer sites were more heavily damaged than early capitula at cooler sites, yet overall a lower proportion of capitula was damaged at warmer sites. Rising temperatures may therefore reduce effectiveness of R. conicus for C. nutans control, as more capitula escape damage. Biological control systems and naturally occurring temperature gradients are useful systems in which to investigate climate change impacts, but a focus on the phenology of organisms’ presence or absence may not be sufficient; our study demonstrates that consideration of the interaction itself may be necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate change alters the seasonal timing of organisms’ life cycle events (i.e., their phenology), which may in turn disrupt interactions between organisms (Fitter and Fitter 2002; Miller-Rushing and Primack 2008; Yang and Rudolf 2010; Simmonds et al. 2020; Twining et al. 2022). When interacting species have differing phenological responses to rising temperatures, their interactions may change in seasonal timing and duration (Fig. 1), which can in turn affect their performance (Visser and Holleman 2001; Post and Forchammer 2007; Blois et al. 2013). Phenological mismatches have been documented for mutualistic interactions, competitive interactions, and trophic interactions (Thomson 2010; Burkle et al. 2013; Ahola et al. 2007). Some plant-pollinator interactions, for instance, have been altered, with rising temperatures reducing total pollination for some flowering species (Thomson 2010). These changes in phenology have been implicated in the local extirpation of several specialist bee species, which effectively lost food resources as species providing essential floral resources shifted their flowering phenology beyond the bees’ foraging period (Burkle et al. 2013). Shifting phenology may also underlie changes in competition between species (Rudolf 2019). Some migratory birds are projected to face greater competition with non-migratory birds as a result of shifts in phenology (Ahola et al. 2007). Climate change-induced phenological shifts have also affected trophic interactions in diverse systems, including marine zooplankton consuming phytoplankton (Edwards and Richardson 2004), migrating caribou grazing on spring foliage in the arctic (Post and Forchhammer 2007), and birds foraging for caterpillars to feed their offspring in temperate forests (Visser et al. 2006). While phenological mismatch frequently indicates the disruption of species interactions, it is possible that warming may also perturb interactions even when synchrony is unchanged (Fig. 1).
Conceptual figure showing a possible phenological response to warming that would lead to phenological mismatch or potentially alter the interaction between a weed (black, solid) and biological control agent (grey, dashed lines). Panels on the left show seasonal patterns in abundance, while panels on the right show the cumulative probability that an observation occurs. Both species in this scenario have earlier phenology under warming than under ambient conditions, but the magnitude of their response is different, leading to reduced temporal overlap. Beneath each plot, the colored bar shows the frequency of interaction between the two species, which may or may not correspond with the two species' abundances, depending on behavior and other environmental cues. Warmer colors indicate more frequent interactions. In the conceptual example shown here, interactions occur with moderate frequency for a long time span under current conditions, but after warming, not only does phenology shift, but also interactions occur at very high frequency for only a short period, potentially leaving more late-appearing weeds unaffected by biological control. Note however, that climate change may alter phenology, the frequency of interaction, both or neither
In complex systems, the effect of phenological shifts on trophic interactions can be difficult to disentangle (Nakazawa and Doi 2012). Network analysis holds promise as one means to tackle this issue (Miranda et al. 2013). Alternatively, simple systems with relatively few interacting species provide tractable test cases and can help to inform more complex analysis (Murdoch 1994). Classical biological control of weeds, wherein an invasive weed’s herbivores are brought from the weed’s native range to the introduced range to reduce the weed’s fitness, is a widely used and economically valuable tool for land managers working to control the impact and spread of invasive weeds (Waage and Greathead 1988; Schwarzländer et al. 2018). This practice creates a simplified set of trophic interactions, with relatively few interacting species. Furthermore, the success of classical biological control programs depends in part on phenological synchrony between the weed species and the herbivore in the introduced range (McClay and Balciunas 2005). Thus, climate matching is frequently considered when sourcing biological control agents (Robertson et al. 2008). As climate change alters species’ phenology, these economically valuable interactions may be susceptible to change, potentially reducing the impact of introduced biological control herbivores on their target weeds. Thus, studying biological control system response to climate warming may both improve understanding and have useful applied ramifications.
Urban–rural temperature gradients provide an opportunity to study the influence of temperature on trophic interactions (Youngsteadt et al. 2015; Seress et al. 2018). Urban temperatures are elevated both by the physical characteristics of the urban environment (including albedo, heat capacity, and vertical structure) and by anthropogenic heating (waste heat generated by vehicles, buildings, and industrial activities) (Fan and Sailor 2005). Together, these features increase air temperatures by approximately 1–2 °C during the winter and 0.5–1 °C during the summer in mid-latitude areas in the United States (Botkin and Beveridge 1997). Urban heat islands significantly affect plant phenology, and have been shown to alter interactions between plants and insects (Dale and Frank 2014; Meineke et al. 2014; Jochner and Menzel 2015). Urban environments may therefore provide a useful tool with which to investigate the potential for global climate change to influence weed/herbivore interactions.
We observed the phenology of the invasive pasture weed Carduus nutans (musk thistle) and a biological control agent released for its control, the specialist weevil Rhinocyllus conicus, at several sites spanning an urban–rural gradient in central Pennsylvania. Musk thistle is an economically important herbaceous invasive species, and R. conicus is a successful, European-origin biological control agent that damages musk thistle capitula, reducing seed set (Kok 2001; Crawley 1989). Our study aimed to answer the following questions:
-
1.
Does temperature increase with increased urbanization in our study area?
-
2.
Does C. nutans phenology vary with temperature?
-
3.
Does R. conicus phenology vary with temperature?
-
4.
Does synchrony between C. nutans and R. conicus vary with temperature?
-
5.
Do differences in temperature lead to differences in the pattern of R. conicus damage on C. nutans?
Together, the answers to these questions enable improved predictions for the future effectiveness of R. conicus as a biological control agent for C. nutans and illuminate potential effects of warming on economically important trophic interactions.
Methods
Study species
Carduus nutans L. (Asteraceae) (musk thistle) is an introduced Eurasian weed and is a commonly listed noxious weed in the United States (Skinner et al. 2000). Carduus nutans was first documented in the United States in 1853, near Harrisburg, PA (Desrochers et al. 1988). Since then, it has spread to at least 46 continental American states and 9 Canadian provinces (USDA NRCS 2015). Musk thistle is a short-lived monocarpic perennial. After growing as rosettes for one to several years, individuals bolt, flower, set seed, and then die. In Pennsylvania, flowering is usually completed by early August (Rhoads and Block 2007). Carduus nutans thrives in disturbed areas, open fields, roadsides, and, notably, pastures (Desrochers et al. 1988). Its spiny leaves inhibit grazing, significantly reducing forage utilization by livestock (Seefeldt et al. 2005). A single individual per 1.5 m2 can reduce pasture productivity by as much as 23% (Trumble and Kok 1982). Prior field experiments show that musk thistle phenology is responsive to temperature, with both stem elongation and flowering phenology advancing when plants were warmed (Zhang et al. 2012). Additionally, musk thistle fecundity increased under experimental warming, as did the rate of annualism, both of which contribute to faster projected population growth rates (Zhang et al. 2011; Keller and Shea 2021).
Rhinocyllus conicus (Froelich) (Coleoptera: Curculionidae) (the thistle head weevil), was introduced to the U.S. from Europe for the biological control of C. nutans in 1969 (Surles et al. 1974). R. conicus is univoltine (Zwölfer and Harris 1984). Adults emerge from overwintering sites in April/May and lay eggs on the bracts of thistle capitula, leaving characteristic egg cases on the surfaces of the bracts (Kok 2001). Male R. conicus emerge earlier in spring than female R. conicus (Smith and Kok 1987). Larvae burrow into the flower head, feed on receptacle tissue, and prevent seed formation. Individuals form cysts within the flower head as they begin to pupate (Zwölfer and Harris 1984). Rhinocyllus conicus was actively distributed in mass releases across the country and has been widely credited with successful control of C. nutans, limiting population growth and occasionally even eliminating local populations (Rees 1977; Laing and Heels 1978; Crawley 1989; Roduner et al. 2003). Rhinocyllus conicus also reduces musk thistle spread by reducing the numbers of C. nutans’ wind-dispersed seeds released from capitula and reducing seeds’ mean dispersal distances (Marchetto et al. 2014). Field studies and lab experiments show that the timing of R. conicus oviposition and adult mortality are sensitive to temperature (Smith et al. 1984; Smith and Kok 1987; Russell and Louda 2004).
The success of this biological control agent in reducing C. nutans fitness depends in part on the synchronization of weevil emergence and thistle development (Surles and Kok 1977). R. conicus exerts the strongest control over C. nutans in years when peak oviposition coincides with peak availability of thistle buds (Smith et al. 1984). While the strength of the interaction in different locations, and the many factors that may influence it, has not been studied systematically, the success of biological control appears to vary considerably across the invaded range (Li et al. 2021). For example, R. conicus is reported to be highly effective in the USA (Crawley 1989; Julien 1992), but less so in New Zealand (Shea and Kelly 1998) and Australia (Shea et al. 2006).
Study sites
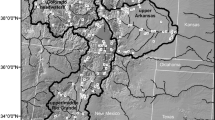
In 2013, we observed thistle and weevil phenology at 6 sites (Figure S1a) in central Pennsylvania. In 2014, we observed thistle and weevil phenology at 11 sites (Figure S1b) including some sites where we made observations in the year prior (see Table 1 for details on data collection at each site). We selected accessible sites that spanned a range of surrounding urbanization intensity. Sites were identified based on a prior survey of musk thistle distribution in central Pennsylvania (Allen and Shea 2006). In all cases, we selected sites with at least 10 flowering individuals. Musk thistle populations are present along roadsides and in disturbed areas throughout the area included in this study, despite the presence of R. conicus.
Land use
We analyzed land use around our study sites in ArcMap (ArcGIS10) using data from the 2011 National Land Cover database (Homer et al. 2012). This dataset had a spatial resolution of 30 m. For each study site, we generated a 1 km buffer around that site and calculated the percent of the area within that buffer that was categorized as “Developed and other human use” (attribute levels 580–584 in the NLCD) by dividing the area in those categories by the total area of the buffer.
Site temperature
We extracted site-specific mean spring temperatures for each site and each year of observation from the software package ClimateNA (Wang et al. 2016). This software provides scale-free historical climate data based on dynamic downscaling from gridded climate data, and has been demonstrated to provide highly accurate values, especially for temperature data (Stern et al. 2022; Ye et al. 2022).
We additionally placed HOBO pendant loggers (Onset Computer Corporation Part# UA-002–08) at the soil surface at each site, though issues with extreme values when loggers were exposed to direct sunlight and lost loggers make this temperature data more difficult to interpret. These loggers recorded temperature hourly from May through July. We lost temperature loggers at three sites due to human interference, though one was replaced early in the season. For these reasons, results based on temperature data from on-site temperature loggers are presented in the supplemental material only (Tables S19-S32).
To investigate the relationship between surrounding urbanization and site temperature, we fitted a linear mixed effects model regressing mean spring temperature against year (categorical), proportion of the 1 km buffer categorized as developed, latitude, and their interactions. We fitted this regression in R using the lmer function from the lme4 package (Bates et al. 2015; R Core Team 2022). We included a random effect for plot to account for repeated observations at each site. We used the dredge function from the package MuMIn to fit all submodels and selected the best supported model based on AICc (Bartoń 2022).
Thistle phenology
At each site, we marked 10 bolting C. nutans individuals (seven at site PS in 2014) along a haphazard transect. In 2013, we made twice-weekly observations of capitula, noting:
-
1.
The number of capitula present
-
2.
The number of capitula that had undergone first anthesis (flower opening)
-
3.
The number of capitula that were senescent (Fig. 2)
In 2014, observation frequency was reduced to once each week in order to accommodate observations at additional sites.
To investigate the timing of musk thistle phenology, we calculated the proportion of individuals that had at least one capitulum reach the following stages on each observation date: 1. visible buds present, 2. first anthesis, 3. senescent capitula present. To assess whether temperature altered the timing of these events, we fitted generalized linear mixed effects model (GLMM) with binomial error structure and logit link function using the glmmTMB function from the glmmTMB package in R (Brooks et al. 2017). Responses were: proportion of individuals with visible buds present, proportion of individuals with at least one capitulum having undergone first anthesis, and proportion of individuals with at least one senescent capitulum. Predictors were: the mean spring temperature, the calendar day of year, and their interaction. We included a random effect for the plot x year combination. We used dredge to identify the best-support submodel of this full model, and we verified model assumptions by checking residuals using the DHARMa package (Hartig 2022). To evaluate the significance of temperature as a predictor in these models, we carried out likelihood ratio tests comparing the selected model against a model with this variable removed.
Adult weevil phenology
To measure weevil phenology, we counted adult weevils present on the individually marked thistles described above before observing thistle phenology. We searched the surface of the plant for 1 min per plant, counting all adult R. conicus individuals on the plant. While searching the plant surface, we took care not to physically contact the plant in order to avoid causing R. conicus’ to drop to the ground. We summed the number of weevils observed on all plants at each site on each day of observation. We then calculated the proportion of weevil observations made on or before each observation date at each plot by summing the plot’s number of weevils observed on that date and on all prior dates and dividing by the sum of all weevil observations made across all dates at that site. We fitted a GLMM with binomial error structure and logit link function with the cumulative proportion of weevil observations predicted by mean spring temperature, day of year, and their interaction. Plot x year combination was included as a random effect. We assessed model fit using the DHARMa package. We fitted all submodels and selected the best supported model based on AICc using the dredge function.
We also investigated whether the total number of weevils observed in plots was affected by mean spring temperature. We summed the total number of weevil observations made over the entire observation period in each plot, and fitted a GLMM with Poisson error structure and a log link function that included the following predictors: year of observation, mean spring temperature and their interaction. We included the number of observation periods as an offset to account for the fact that the number of observations and the number of plants observed was not constant year to year and plot to plot, respectively. We checked model assumptions using the DHARMa package in R. To assess the effect of temperature in this model, we performed a likelihood ratio test comparing the full model to a model with the temperature predictor excluded.
Thistle and weevil synchrony
We characterized synchrony between thistle capitulum first appearance and adult weevil phenology by comparing the timing of accumulation of thistle capitula and the timing of weevil observations. We used two metrics to assess synchrony between thistle flower head accumulation and weevil presence on plants. First, we used Kolmogorov–Smirnov tests (Corder and Foreman 2014) to describe the magnitude of differences between the empirical cumulative distribution of capitula appearing over time and the empirical cumulative distribution of weevil observations occurring over time. We calculated the Kolmogorov–Smirnov D-statistic, which measures the maximum difference between the two cumulative distributions, as a measure of thistle and weevil phenological synchrony. Large values of D indicate low synchrony, as the two distributions are widely separated, while small values of D indicate high synchrony, as the two distributions are only narrowly separated. Second, we used the area of overlap under the probability density functions describing the probability that weevil observations occurred over time and the probability that new capitula appeared over time. To fit these curves, we used the nlsLM function from the R package minpack.lm (Elzhov et al. 2013) to estimate parameters describing the logistic curve that best fitted the cumulative proportion of weevil observations that occurred over time and the logistic curve describing the proportion of capitula that accumulated over time. We took the derivative of these cumulative probability functions to get the probability density functions for weevil occurrence and thistle flower head appearance over time for each observation site. We then calculated the area of overlap between these two curves at each site using the midpoint rule for integration (Lax and Terrell 2014) as an alternative measure of phenological synchrony between R. conicus and C. nutans.
To evaluate whether synchrony varied with temperature, we used simple linear regression to regress both of these measures (Kolmogorov–Smirnov D and area of overlap) against mean spring temperature.
Seasonal weevil damage
To investigate weevil damage on C. nutans capitula that first appeared at different times throughout the season, we marked individual flower heads using colored plastic ties fastened loosely around stems directly below focal capitula. On each of the thistles for which we tracked phenology (see above), we marked the main primary capitulum when it first appeared, and then marked an additional capitulum once each week (if present), selecting the smallest if more than one was present. We made weekly observations of capitulum diameter, phenological stage, height from the soil surface, and the number of R. conicus egg cases present on marked capitula.
To characterize temperature’s effect on the number of R. conicus egg cases that accumulated on capitula that first appeared at different times during the season, we regressed the maximum number of R. conicus egg cases observed on a capitulum against site temperature and day of year on which that capitula was first observed to have a diameter greater than one cm. Capitulum diameter was excluded as a predictor because it was highly correlated with the date that capitula first reached 1 cm diameter. We fitted a GLMM with negative binomial error and log link and included random effects for each observed year and plot combination and for plant to account for non-independence between observations. We fitted this model using the function glmmTMB. We fitted all submodels and selected the best supported model based on AICc using dredge.
End-of-season weevil damage
To quantify weevil damage on musk thistle capitula at each study site, we harvested plants in early July, once we stopped seeing overwintered, reproductive adult weevils present on plants, but before newly emerged adult weevils appeared. We removed and individually stored all capitula from each plant. In the lab, we dissected all capitula, counting the number of distinctive R. conicus egg cases present on the bracts and the number of R. conicus cysts inside the flower head.
We measured the effect of temperature on the proportion of capitula with egg cases present using a generalized linear model with binomial error distribution. This regression model included mean spring temperature, plant size (as measured by longest leaf length prior to bolt), year of observation, and their interactions as predictors. Plant size has been shown to influence R. conicus oviposition patterns (Sezen et al. 2021, Groentemen et al. 2008). Random effects for plant nested in plot x year combination were included to account for non-independence in our observations. This regression was fitted using the glmmTMB function in R and validation of assumptions was checked using the DHARMa package. We fitted all submodels and selected the best model based on AICc using the dredge function. We used the same predictors and approach to evaluate the influence of temperature on the proportion of flower heads with R. conicus larval cysts present.
Results
Urban–rural temperature gradient
Daily mean temperature significantly increased with increasing urbanization, as measured by the proportion of the area in a 1 km radius buffer around each study site categorized as developed (Fig. 3). Model selection retained the year of observation and the proportion of the buffer developed as predictors of mean spring temperature (Table S1, Table S2). Latitude was not retained as a significant predictor of temperature (but differed very little between the sites chosen, by design). Sites as urbanized as our most urban site (proportion of surrounding 1 km radius buffer developed = 0.79) had average spring temperature 0.32 °C warmer than those as urbanized as our least urban site (proportion of surrounding 1 km radius buffer developed = 0.08), on average. The warmest plot in the warmer year (2013) had average mean spring temperature of 10.5 °C, while the coolest plot in the cooler year (2014) had average mean spring temperature of 9.2 °C.
Mean spring temperatures at study sites increased with the proportion of a 1 km radius buffer that was developed. Temperature data are from ClimateNA, a scaleless interpolated temperature dataset (Wang et al. 2016). Fitted regression lines are shown, with shading to show the 95% confidence interval for the mean
Thistle phenology and temperature
Musk thistle flowering phenology was significantly earlier at warmer sites (Fig. 4, table D-2). The proportion of thistles with at least one visible capitulum increased over the course of the season, but the timing of this increase was not significantly affected by site temperature (Fig. 4A, χ2 = 1.50, d.f. = 1, P = 0.22, Table S3, Table S4). The proportion of plants with capitula having undergone first anthesis was affected by temperature, however (Fig. 4B, χ2 = 8.51, d.f. = 2, P = 0.01, Table S5, Table S6), as was the proportion of plants with senescent capitula (Fig. 4C, χ2 = 4.74, d.f. = 1, P = 0.03, Table S7, Table S8). The date on which 50% of individuals were predicted to have at least one capitulum having undergone first anthesis was 10.6 days earlier at the temperature of the warmest plot compared against that of the coolest. Similarly, the date on which 50% of individuals were predicted to have at least one senescent capitulum was 7.7 days earlier for sites with these temperatures.
Thistle phenology advanced with warmer temperatures at more urbanized sites, but we found no evidence that the timing of weevil presence on plants did the same, (see Fig. 5 for synchrony results). The proportion of thistles with visible flower buds increased over time (A), but the rate of increase was not significantly affected by temperature. The proportion of thistles with at least one flowering capitulum (B), however, was significantly affected by temperature: plants at warmer sites flowered significantly earlier. Similarly, the proportion of individuals with at least one senescent capitulum (C) increased significantly earlier with warmer temperatures. The cumulative proportion of weevil observations on thistles, however, did not have a significant relationship with temperature. In each panel, raw data points are colored based on the temperature for that season at that site. Fitted regression lines with 95% confidence intervals for the mean are included. Separate lines for the coolest observed mean spring temperature (9.2 °C) and warmest (10.5 °C) are shown in all panels regardless of temperature’s significance for visual consistency
Weevil phenology and temperature
We observed no significant effect of temperature on the number of weevils observed within plots (χ2 = 0.83, d.f. = 2, P = 0.66). The timing of observations of adult weevils was not affected by temperature (Fig. 4D, χ2 = 0.14, d.f. = 1, P = 0.71, Table S9, Table S10).
Thistle and weevil synchrony and temperature
We did not detect a significant relationship between temperature and thistle and weevil synchrony as measured by Kolmogorov–Smirnov D statistics (slope = 0.051, F1,14 = 0.68, P = 0.42, Fig. 5A, Table S11). Area of overlap between probability density functions for adult weevil observation and thistle head appearance ranged from 0.37 to 0.71, yet was not significantly related to site temperature (slope = 0.062, F1,14 = 2.63 P = 0.15, Fig. 5B, Table S12).
Two measures of synchrony between R. conicus adult presence on plants and C. nutans capitulum accumulation, plotted against mean temperature at observation sites: Kolmogorov–Smirnov D (panel A), and area of overlap under probability density functions for weevil observation and thistle flower head appearance (panel B). In panel A, high D values indicate lack of synchrony, while low D values indicate strong synchrony. We found no significant relationship between temperature and this measure of phenological synchrony. In panel B, high values for area of overlap indicate synchrony, while low value indicate asynchrony. Again, we found no significant relationship between this measure of synchrony and temperature
Weevil damage
We observed a significant, negative relationship between temperature and the proportion of capitula with weevil egg cases present in each year of the study (Fig. 6A, Table S13, Table S14). The model selection procedure retained year of observation, mean spring temperature, longest leaf length, and all interactions between these predictors. The effect of plant size as measured by longest leaf length was not consistent: in 2013, larger plants tended to have slightly higher proportion of capitula infested, while in 2014 this pattern was reversed. In each year, plants at warm sites had lower proportions of flower heads with R. conicus egg cases present. In 2013, plants with the median longest leaf length (27.5 cm) at sites with the warmest temperature observed that year (10.5 °C) were found to have 63.2% of heads infested, on average, while similarly sized thistles at the coolest temperature observed that year (10.0 °C) had 68.8% infested. In 2014, a similar pattern was found: plants with median longest leaf length at the warmest sites (9.7 °C) had 50.1% of heads infested, while those at the coolest sites (9.2 °C) had 70.0% infested, on average.
Weevil damage declined with increasing temperature in each year of observation. The proportion of capitula with egg cases present declined with increasing temperature for plants at sites spanning the urban–rural temperature gradient in 2013 and 2014 (A). Similarly, the proportion of capitula holding cysts formed by R. conicus declined with temperature (B). In both panels, point size is scaled to reflect the total number of capitula produced by the individual. Fitted lines from a binomial GLMM are shown with 95% confidence intervals for the mean. Solid lines show estimated mean values for smaller plants (longest leaf length 22 cm) while dashed lines show estimated mean values for larger plants (longest leaf length 36 cm)
The proportion of capitula that were found to contain R. conicus cysts declined at warmer temperatures in each year of observation (Fig. 6B, Table S15, Table S16). Again, the model selection procedure retained all predictors. The effect of plant size varied from year to year again, with large plants slightly less likely to have cysts in their capitula in 2013, but slightly more likely in 2014. In both years, the proportion of capitula with cysts declined with increasing temperature. In 2013, plants of median size had an estimated mean proportion of heads holding cysts of 37.4% at the coldest observed temperature and 20.8% at the warmest observed temperature. In 2014, the mean proportion of heads with cysts declined from 40.2 to 21.0% over the range of temperatures sampled.
Seasonal weevil damage
Capitula that appeared earlier in the year accumulated significantly more R. conicus egg cases than later-appearing capitula (Fig. 7, Table S17, Table S18). The trend in number of accumulated egg cases on capitula appearing on different dates was significantly affected by temperature, as evidenced by a significant interaction term for the interaction between the date capitula reached 1 cm and site mean temperature (estimate = − 0.012, z = − 2.130, P = 0.03). The likelihood ratio test evaluating whether the model including temperature as a predictor explained significantly more variance than the model without this predictor was only marginally significant, however (χ2 = 4.62, d.f. = 2, P = 0.099). Early-appearing flower heads accumulated slightly more egg cases at warm sites than they did at cool sites, on average. Later appearing capitula, however, accumulated slightly fewer egg cases at warm sites than they did at cool sites.
The number of egg cases observed on musk thistle capitula was higher for capitula that first reached 1 cm diameter earlier in the year. This relationship was slightly influenced by temperature: early appearing flower heads had more egg cases at warm sites than at cooler sites. Fitted regression lines from a generalized linear model with negative binomial error are shown, evaluated at two temperatures: 9.2 °C (purple) and 10.5 °C (yellow)
Discussion
The interaction between the invasive weed C. nutans and the specialist biological control agent weevil R. conicus varied across sites spanning an urban–rural temperature gradient. Carduus nutans flowering and capitulum senescence occurred earlier at warmer sites, but we found no differences in the timing of adult R. conicus presence on thistles. Two measures of phenological synchrony based on the timing of adult weevil presence and the timing of thistle capitulum production also showed no trend with temperature. Seasonal oviposition patterns varied predictably with temperature, however. The earliest appearing capitula accumulated more egg cases at warm sites than they did at cooler sites. In all, the proportion of capitula that were damaged by R. conicus and the proportion that held cysts were both lower at warm sites in both years of observation. This finding suggests that rising temperatures may alter R. conicus damage rates on C. nutans in the future, changing the distribution of eggs across flower heads, and potentially changing the overall impact on C. nutans performance.
Crucially, our observations of adult R. conicus abundance over time did not show a trend with temperature, contrary to expectations from laboratory studies, but our observations of the seasonal patterns in R. conicus damage on flower heads did vary with temperature. While adult weevils must visit thistles in order to oviposit, adult presence on plants does not necessarily indicate active oviposition on that plant (Sezen 2007). The timing of adult presence on plants may therefore not be sufficiently informative to accurately characterize the timing of the interaction between C. nutans and R. conicus. Differences in R. conicus behavior, including mating and oviposition, may underlie these differences in oviposition timing, as these behaviors may occur at different times at different temperatures even when adult weevils are present over similar timespans. This finding suggests that, in some cases, even when seasonal abundances of interacting organisms are observed to remain synchronous, their interactions may nevertheless change in intensity or peak timing. Conversely, it is possible that changes in the relative timing of seasonal abundance may not lead to substantial changes in the interaction between two species. This second scenario has been observed in the interaction between the western tent caterpillar (Malacosoma californicum pluviale) and the red alder (Alnus rubra Bong.), for example (Kharouba et al. 2015). Under experimentally applied warming, caterpillars advanced spring emergence substantially, while red alder leaf emergence did not occur earlier. Early-emerging caterpillars survived without food until leaves did emerge, though, and their performance was not affected by the apparent change in synchrony. These findings emphasize the importance of observing the phenology of ecological interactions themselves, when possible, in addition to changes in the timing of the presence or absence of individuals.
Rhinocyllus conicus and C. nutans coexist across a broad range of climatic conditions in North America, from relatively warm conditions in Georgia, USA (Buntin et al. 1993) to substantially colder conditions in Alberta, Canada (Gassmann and Louda 2001). The two species also coexist in widely differing climates in Australia and New Zealand (Julien 1992; Shea et al. 2006) as well as in the native range (Sheppard et al. 1994). Future comprehensive comparisons of seasonal timing (i.e., phenology) of oviposition and of weevil impact across this broad geographic range could improve our understanding of the drivers of the species’ phenology and the effectiveness of R. conicus as a biological control agent, but would need to consider the effects of precipitation, photoperiod, etc., as well as temperature, at multiple locations.
Disentangling the effects of altered R. conicus oviposition phenology on the population dynamics of its target weed, C. nutans, remains an important goal for future research. The timing of seed release can be a strong driver of musk thistle performance (Keller and Shea 2021). Seeds that are released earlier in the year can result in larger rosettes, which are more likely to survive the winter and more likely to flower as annuals rather than as biennials or longer-lived perennials. Prior work has indicated that damage by R. conicus delays the timing of seed set (Kelly and McCallum 1995). Here, we show that temperature may influence R. conicus’ impact on the timing of C. nutans seed set. Warmer temperatures were associated with increased weevil damage on the earliest flower heads, a pattern that may decrease the number of early-released seeds that survive weevil damage under warming. Reductions in the production of early-released seeds, which can contribute disproportionately to musk thistle population growth, may compensate for reduced overall weevil damage.
Changes in R. concius oviposition phenology may alter recommendations for integrated weed management plans for C. nutans. Musk thistle is generally managed using mechanical and chemical control in addition to biological control (Trumble and Kok 1982), though the effectiveness of mowing may be reduced by rising temperatures (Keller and Shea 2022). Prior work has shown that that 2,4-D application, if appropriately timed in relation to R. conicus oviposition, could prevent C. nutans seed production while still allowing R. conicus to complete development (Kok and Trumble 1979). Mowing compatibility with R. conicus is similarly dependent on phenology; mowing when C. nutans buds first appeared prevented R. conicus development, while mowing as C. nutans flowers reached senescence had no effect on R. conicus survival and most effectively reduced C. nutans seed set (Tipping 1991). Changes in the timing of R. conicus oviposition within the season and relative to musk thistle development may require reevaluation of these interactions between management options. Concentrated R. conicus oviposition early in the season at warmer temperatures may allow for earlier application of herbicide or mowing without significant harm to developing R. conicus individuals, for example, though this possibility would require testing.
Our observations occurred along an urban–rural temperature gradient. There is growing interest in using urban environments as proxies for future environments (Lahr et al. 2018). Our analyses focused on temperature differences between urban and rural sites, as temperature is known to affect musk thistle phenology and many aspects of R. conicus biology (Surles and Kok 1977; Smith et al. 1984; Zhang et al. 2012). Plant–herbivore interactions have been observed to have similar responses to temperature over decades of climate warming and across urban–rural gradients (e.g., Youngsteadt et al. 2015). Other environmental conditions are also known to vary with urbanization, including several that parallel environmental changes projected to occur in the future. In addition to elevated temperatures, urban environments generally have higher atmospheric carbon dioxide concentrations (George et al. 2007; Briber et al. 2013) and increased nitrogen deposition (Dentener et al. 2006; Fang et al. 2011). While these factors may also have contributed to differences in C. nutans and R. conicus interactions, assessing their influence was beyond the scope of this study. Future work should consider the potential for these factors and their interactions to alter R. conicus’ and C. nutans’ interactions.
Classic approaches in ecology use simplified applied systems to test and refine ecological theory (Murdoch 1994). Biological control systems have been useful model systems in which to investigate a variety of ecological questions related to population dynamics and the nature of trophic interactions (Murdoch et al. 1985; Crawley 1986). As the potential for climate-induced phenological mismatches threatens to disrupt mutualisms, alter trophic interactions (Fig. 1), and change competition between species (Renner and Zohner 2018), insights from simple systems may be especially valuable to inform analyses of more complex interaction networks. Here, we investigated temperature’s effect on the interaction between an invasive weed (C. nutans) and a biological control agent (R. conicus) and found that warmer sites had different oviposition patterns, even though measures of adult weevil synchrony with thistles did not significantly vary with temperature. This work demonstrates the potential for warming to affect the interaction between a specialist herbivore and its host plant, even if the relative phenologies of the interacting species do not appear to significantly change.
Data availability
Data are available from the corresponding author upon reasonable request.
References
Ahola MP, Laaksonen T, Eeva T, Lehikoinen E (2007) Climate change can alter competitive relationships between resident and migratory birds. J Anim Ecol 76:1045–1052
Allen MR, Shea K (2006) Spatial segregation of congeneric invaders in central Pennsylvania, USA. Biol Invasions 8:509–521
Bartoń K (2022) MuMIn: Multi-Model Inference. R package version 1.47.1, <https://CRAN.R-project.org/package=MuMIn>.
Bates D, Maechler M, Bolker B, Walker S (2015) lme4: linear mixed-effects models using Eigen and S4. R Package Version 1:1–15
Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S (2013) Climate change and the past, present, and future of biotic interactions. Science 341:499–504
Botkin DB, Beveridge CE (1997) Cities as environments. Urban Ecosyst 1:3–19
Briber BM, Hutyra LR, Dunn AL, Raciti SM, Munger JW (2013) Variations in atmospheric CO2 mixing ratios across a Boston, MA urban to rural gradient. Land 2:304–327
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9:378–400
Buntin GD, Hudson RD, Murphy TR (1993) Establishment of Rhinocyllus conicus (Coleoptera: Curculionidae) in Georgia for control of musk thistle. J Entomol Sci 28:213–217
Burkle LA, Marlin JC, Knight TM (2013) Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339:1611–1615
Corder GW, Foreman DI (2014) Nonparametric statistics: a step-by-step approach. Wiley, Hoboken
Crawley MJ (1986) The population biology of invaders. Phil Trans r Soc Lond B 314:711–731
Crawley MJ (1989) Insect herbivores and plant population dynamics. Annu Rev Entomol 34:531–562
Dale AG, Frank SD (2014) Urban warming trumps natural enemy regulation of herbivorous pests. Ecol Appl 24:1596–1607
Dentener F, Drevet J, Lamarque JF, Bey I, Eickhout B, Fiore AM, Hauglustaine D, Horowitz LW, Krol M, Kulshrestha UC, Lawrence M (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem Cycles 20(4):850. https://doi.org/10.1029/2005GB002672
Desrochers AM, Bain JF, Warwick SI (1988) The Biology of Canadian weeds. 89. Carduus nutans L. and Carduus acanthoides L. Can J Plant Sci 68:1053–1068
Edwards M, Richardson AJ (2004) Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881
Elzhov TV, Mullen KM, Spiess AN, Bolker B (2013) minpack. lm: R interface to the Levenberg-Marquardt nonlinear least-squares algorithm found in MINPACK, plus support for bounds. R package version 1.1–8.
Fan H, Sailor DJ (2005) Modeling the impacts of anthropogenic heating on the urban climate of Philadelphia: a comparison of implementations in two PBL schemes. Atmos Environ 39:73–84
Fang Y, Yoh M, Koba K, Zhu W, Takebayashi YU, Xiao Y, Lei C, Mo J, Zhang WEI, Lu X (2011) Nitrogen deposition and forest nitrogen cycling along an urban–rural transect in southern China. Glob Change Biol 17:872–885
Fitter AH, Fitter RSR (2002) Rapid changes in flowering time in British plants. Science 296:1689–1691
Gassmann A, Louda SM (2001) Rhinocyllus conicus: initial evaluation and subsequent ecological impacts in North America. In: Evaluating indirect ecological effects of biological control. Key papers from the symposium 'Indirect ecological effects in biological control', Montpellier, France, 17–20 October 1999 (pp 147–183). Wallingford UK: CABI Publishing.
George K, Ziska LH, Bunce JA, Quebedeaux B (2007) Elevated atmospheric CO2 concentration and temperature across an urban–rural transect. Atmos Environ 41:7654–7665
Groenteman R, Kelly D, Fowler SV, Bourdôt GW (2008) Factors affecting oviposition rate in the weevil Rhinocyllus conicus on non-target Carduus spp. in New Zealand. In: Proceedings of the XII international symposium on biological control of weeds, La Grande Motte, France, 22–27 April, 2007, 87–90. Wallingford UK: CAB International.
Hartig F (2022) DHARMa: Residual diagnostics for hierarchical (Multi-Level / Mixed) regression models. R package version 0.4.6, <https://CRAN.R-project.org/package=DHARMa>.
Homer CH, Fry JA, Barnes CA (2012) The national land cover database. US Geol Survey Fact Sheet 3020:1–4
Jochner S, Menzel A (2015) Urban phenological studies–past, present, future. Environ Pollut 203:250–261
Julien MH (1992) Biological control of weeds: a world catalogue of agents and their target weeds. In: CAB International/Australian centre for international agricultural research, Wallingford, Oxon, UK.
Keller JA, Shea K (2021) Warming and shifting phenology accelerate an invasive plant life cycle. Ecology 102:e03219
Keller JA, Shea K (2022) Pest management in future climates: warming reduces physical weed management effectiveness. Ecol Appl 32:e2633
Kelly D, McCallum K (1995) Evaluating the impact of Rhinocyllus conicus on Carduus nutans. In: New Zealand proceedings of the 8th international symposium on biological control of weeds: 205–11. Canterbury, New Zealand: Commonwealth Scientific and Industrial Research Organisation (CSIRO)/Lincoln University.
Kharouba HM, Vellend M, Sarfraz RM, Myers JH (2015) The effects of experimental warming on the timing of a plant–insect herbivore interaction. J Anim Ecol 84:785–796
Kok LT (2001) Classical biological control of nodding and plumeless thistles. Biol Control 21:206–213
Lahr EC, Dunn RR, Frank SD (2018) Getting ahead of the curve: cities as surrogates for global change. Proc Royal Soc B: Biol Sci 285:20180643
Laing JE, Heels PR (1978) Establishment of an introduced weevil Rhinocyllus (Col., Curculionidae) for the biological control of nodding thistle Carduus nutans (Compositae) in Southern Ontario. Proc Entomol Soc Ontario 109:3–8
Lax PD, Terrell MS (2014) Approximation of integrals. In: Calculus with applications. Undergraduate Texts in Mathematics Springer, New York, NY
Li SL, Keller J, Runge MC, Shea K (2021) Weighing the unknowns: value of Information for biological and operational uncertainty in invasion management. J Appl Ecol 58:1621–1630
Marchetto KM, Shea K, Kelly D, Groenteman R, Sezen Z, Jongejans E (2014) Unrecognized impact of a biocontrol agent on the spread rate of an invasive thistle. Ecol Appl 24:1178–1187
McClay AS, Balciunas JK (2005) The role of pre-release efficacy assessment in selecting classical biological control agents for weeds—applying the Anna Karenina principle. Biol Control 35:197–207
Meineke EK, Dunn RR, Frank SD (2014) Early pest development and loss of biological control are associated with urban warming. Biol Let 10:20140586
Miller-Rushing AJ, Primack RB (2008) Global warming and flowering times in Thoreau’s Concord: a community perspective. Ecology 89:332–341
Miranda M, Parrini F, Dalerum F (2013) A categorization of recent network approaches to analyse trophic interactions. Methods Ecol Evol 4:897–905
Murdoch WW (1994) Population regulation in theory and practice. Ecology 75:271–287
Murdoch WW, Chesson J, Chesson PL (1985) Biological control in theory and practice. Am Nat 125:344–366
Nakazawa T, Doi H (2012) A perspective on match/mismatch of phenology in community contexts. Oikos 121:489–495
Post E, Forchhammer MC (2007) Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philos Trans Royal Soc B: Biol Sci 363:2367–2373
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Rees NE (1977) Impact of Rhinocyllus conicus on thistles in southwestern Montana. Environ Entomol 6:839–842
Renner SS, Zohner CM (2018) Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu Rev Ecol Evol Syst 49:165–182
Rhoads AF, Block TA (2007) The plants of Pennsylvania: an illustrated manual. University of Pennsylvania Press, Philadelphia, p 194
Robertson MP, Kriticos DJ, Zachariades C (2008) Climate matching techniques to narrow the search for biological control agents. Biol Control 46:442–452
Roduner M, Cuperus G, Mulder P, Stritzke J, Payton M (2003) Successful biological control of the musk thistle in Oklahoma using the musk thistle head weevil and the rosette weevil. Am Entomol 49:112–120
Rudolf VH (2019) The role of seasonal timing and phenological shifts for species coexistence. Ecol Lett 22:1324–1338
Russell FL, Louda SM (2004) Phenological synchrony affects interaction strength of an exotic weevil with Platte thistle, a native host plant. Oecologia 139:525–534
Schwarzländer M, Hinz HL, Winston RL, Day MD (2018) Biological control of weeds: an analysis of introductions, rates of establishment and estimates of success, worldwide. BioControl 63:319–331
Seefeldt SS, Stephens JM, Verkaaik ML, Rahman A (2018) (2005) Quantifying the impact of a weed in a perennial ryegrass–white clover pasture. Weed Sci 53:113–120
Seress G, Hammer T, Bókony V, Vincze E, Preiszner B, Pipoly I, Sinkovics C, Evans KL, Liker A (2018) Impact of urbanization on abundance and phenology of caterpillars and consequences for breeding in an insectivorous bird. Ecol Appl 28:1143–1156
Sezen Z (2007) Interactions of the invasive thistle Carduus nutans and its biocontrol agent Rhinocyllus conicus in heterogeneous environments (Doctoral dissertation). Retrieved from ProQuest
Sezen Z, Bjørnstad ON, Shea K (2021) Oviposition response of the biocontrol agent Rhinocyllus conicus to resource distribution in its invasive host Carduus nutans. Biological Control 152:104369
Shea K, Kelly D (1998) Estimating biocontrol agent impact with matrix models: Carduus nutans in New Zealand. Ecol Appl 8: 824–832
Shea K, Sheppard A, Woodburn T (2006) Seasonal life-history models for the integrated management of the invasive weed nodding thistle Carduus nutans in Australia. J Appl Ecol 43:517–526
Sheppard AW, Cullen JM, Aeschlimann JP (1994) Predispersal seed predation on Carduus nutans (Asteraceae) in southern Europe. Acta Oecologica 15:529–541
Simmonds EG, Cole EF, Sheldon BC, Coulson T (2020) Phenological asynchrony: a ticking time-bomb for seemingly stable populations? Ecol Lett 23:1766–1775
Skinner K, Smith L, Rice P (2000) Using noxious weed lists to prioritize targets for developing weed management strategies. Weed Sci 48:640–644
Smith LM, Kok LT (1987) Influence of temperature on oviposition, quiescence, and mortality of Rhinocyllus conicus (Coleoptera: Curculionidae). Environ Entomol 16:971–974
Smith LM, Ravlin FW, Kok LT, Mays WT (1984) Seasonal model of the interaction between Rhinocyllus conicus (Coleoptera: Curculionidae) and its weed host, Carduus thoermeri (Campanulatae: Asteraceae). Environ Entomol 13:1417–1426
Stern MA, Flint LE, Flint AL, Boynton RM, Stewart JAE, Wright JW, Thorne JH (2022) Selecting the optimal fine-scale historical climate data for assessing current and future hydrological conditions. J Hydrometeorol 23:293–308
Surles WW, Kok LT (1977) Ovipositional preference and synchronization of Rhinocyllus conicus with Carduus nutans and C. acanthoides. Environ Entomol 6:222–224
Surles WW, Kok LT, Pienkowski RL (1974) Rhinocyllus conicus establishment for biocontrol of thistles in Virginia. Weed Sci 22:1–3
Thomson JD (2010) Flowering phenology, fruiting success and progressive deterioration of pollination in an early-flowering geophyte. Philos Trans Royal Soc London B: Biol Sci 365:3187–3199
Tipping PW (1991) Effects of mowing or spraying Carduus thoermeri on Rhinocyllus conicus. Weed Technol 5:628–631
Trumble JT, Kok LT (1982) Integrated pest management techniques in thistle suppression in pastures of North America. Weed Res 22:345–359
Twining CW, Shipley JR, Matthews B (2022) Climate change creates nutritional phenological mismatches. Trends Ecol Evol 37:736–739
USDA NRCS (2015) The PLANTS Database (http://plants.usda.gov, May 2011). National Plant Data Team, Greensboro.
Visser ME, Holleman LJ (2001) Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc Royal Soc London B: Biol Sci 268:289–294
Visser ME, Holleman LJ, Gienapp P (2006) Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147:164–172
Waage JK, Greathead DJ (1988) Biological control: challenges and opportunities. Phil Trans r Soc Lond B 318:111–128
Wang T, Hamann A, Spittlehouse D, Carroll C (2016) Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLoS ONE 11:e0156720
Yang LH, Rudolf VHW (2010) Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol Lett 13:1–10
Ye Z, O’Neill GW, Wang T (2022) Climate data for field trials: onsite micro stations versus ClimateNA. Can J for Res 52:1028–1041
Youngsteadt E, Dale AG, Terando AJ, Dunn RR, Frank SD (2015) Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob Change Biol 21:97–105
Zhang R, Jongejans E, Shea K (2011) Warming increases the spread of an invasive thistle. PLoS ONE 6:e21725
Zhang R, Post E, Shea K (2012) Warming leads to divergent responses but similarly improved performance of two invasive thistles. Popul Ecol 54:583–589
Zwölfer H, Harris P (1984) Biology and host specificity of Rhinocyllus conicus (Froel.)(Col., Curculionidae), a successful agent for biocontrol of the thistle, Carduus nutans L. 1. Zeitschrift Für Angewandte Entomologie 97:36–62
Acknowledgements
We thank C. Hovis, W. Fescemyer, D. Stephan, X. Ding, X. Gong, M. Bourguinon, and C. Shea for assistance in the field and in the lab. We thank S. Li, H. Inamine and E. Howerton for helpful comments on this manuscript. Financial support came from the Pennsylvania State University Center for Landscape Dynamics, Pennsylvania NASA Space Grant Consortium and from National Institute of Food and Agriculture, U.S. Department of Agriculture award number 2016-67011-24710. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author and do not necessarily reflect the views of the USDA.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were led by Joseph Keller. The first draft of the manuscript was written by Joseph Keller, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keller, J.A., Shea, K. Altered biological control species interactions despite phenological synchrony along an urban–rural temperature gradient. Biol Invasions 26, 2153–2169 (2024). https://doi.org/10.1007/s10530-024-03301-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-024-03301-w