Abstract
The global increase in frequency and severity of marine heatwaves (MHWs) is highlighting the impact these extreme climatic events can have on marine ecosystems. Throughout the summer of 2021/2022 northeastern Aotearoa/New Zealand suffered an unprecedented MHW. Worst impacted areas, such as the semi-enclosed Te Moananui-ā-Toi/Tīkapa Moana/Hauraki Gulf, experienced more than three continual months of temperatures at or above the climatological mean maximum (20.7 °C). During this period, we observed a rapid increase in the abundance and cover of the invasive, warm-affinity colonial ascidian Symplegma brakenhielmi on temperate rocky reefs. Population expansion of this species has not previously been linked to MHW events. Benthic monitoring as water temperatures cooled showed a sharp decrease in S. brakenhielmi abundance, but not complete disappearance, and the coverage of individually monitored colonies also declined. There was no observed increase in abundance or cover throughout the summer of 2022/2023, a potential consequence of cooler water temperatures and multiple cyclones. Observed impacts included the growth of S. brakenhielmi over other sessile invertebrate and macroalgal species, as well as on highly mobile spiny lobster, suggesting that this species can have a variety of impacts on temperate rocky reefs. In the future, as ocean temperatures continue to warm, these findings provide insight into what we can expect and highlight how MHW can facilitate the rapid expansion and integration of non-native, warm-affinity species into temperate reef ecosystems.
Similar content being viewed by others
Introduction
Over the past century the frequency and duration of marine heatwaves (MHWs), defined as ≥ 5 consecutive days of water temperature exceeding the seasonally varying 90th percentile for the historical baseline (Hobday et al. 2016), has increased (Oliver et al. 2018). This is leading to catastrophic changes within marine ecosystems including negatively impacting thermally sensitive native species (Arafeh-Dalmau et al. 2020; Hughes et al. 2018; Starko et al. 2023), and faciliating the spread and dominance of invasive or warm-affinity species through cooler regions (Arafeh-Dalmau et al. 2019; Félix-Loaiza et al. 2022).
In November 2021 (austral spring), northeastern Aotearoa/New Zealand entered an unprecedented MHW, with the semi-enclosed Te Moananui-ā-Toi/Tīkapa Moana/Hauraki Gulf experiencing 205 continuous days of MHW conditions (Bell et al. 2023). This included a period (mostly through the austral summer) where there were 107 continuous days of water temperatures at or above the climatological mean maximum temperature (average maximum temperature for the period 1980–2010) of 20.7 °C (December 2021–April 2022; Table 1). During this period, widespread and severe episodes of sponge necrosis and mortaility were observed within Te Moananui-ā-Toi/Tīkapa Moana/Hauraki Gulf (Bell et al. 2023), along with observations of increased abundance and sizes of the invasive colonial ascidian Symplegma brakenhielmi (authors per. obs.).
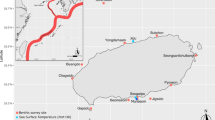
Symplegma brakenhielmi is a pantropical ascidian, which has invaded, likely through vessel movements, temperate regions such as the Mediterranean (Ramos-Esplá et al. 2020). Symplegma brakenhielmi was first detected in the temperate waters of northeastern Aotearoa/New Zealand in 2015 on vessel hulls and mooring pontoons within the Marsden Cove Marina, Whangārei Harbour as part of routine biosecurity surveys (Fig. 1; Page 2015). Following thise initial detection, S. brakenhielmi was then found further south within Auckland’s Waitematā Harbour in early 2016 and 2017 (Fig. 1; N.S. pers. obs., Seaward and Inglis 2018). There has previously been some confusion between S. brakenhielmi and closely related S. reptans, which has been documented as an invasive species in southern California (Lambert and Lambert 2003). Symplegma reptans was initially classified as S. brakenhielmi in California based on collected fragments of immature colonies (Lambert and Lambert 1998). To confirm that the New Zealand species is indeed S. brakenhielmi, samples collected in early 2024 from locations where it had previously been detected, were sent to the National Institute of Water and Atmospheric Research (NIWA) for morphological identification. The specimens examined had a long, curved caecum with two gastro-intestinal connectives, indicative of S. brakenhielmi (M. Page, pers. comms.) and agreeing with morphological descriptions separating S. brakenhielmi and S. reptans (Kott 1985).
Location of study sites within northeastern New Zealand relative to initial detection locations (Whangārei and Waitematā harbours) and sea surface temperature (SST) logger along with observations of S. brakenhielmi within the Te Moananui-ā-Toi/Tīkapa Moana/Hauraki Gulf, northeastern Aotearoa/New Zealand prior to (A, B) and during the 2021/2022 summer MHW (C–F). A First recorded detection of the species within the Waitematā Harbour, adjacent to the Hauraki Gulf, in 2016; B First recorded observation of the species at Ōtata Island in February 2021, C S. brakenhielmi covering the reef within the Tāwharanui Marine Reserve during the 2021/2022 summer MHW, D S. brakenhielmi colonies beginning to overgrow live sponge and occupying bare space left by the death and detachment of another sponge, E S. brakenhielmi growing on the carapace and tail of a red rock lobster (Jasus edwardsii) within the Tāwharanui Marine Reserve, F S. brakenhielmi colonies under kelp, including growing up kelp stipes near Ōtata Island
Limited surveillance of this species has been conducted outside of harbour and port areas, but small, isolated colonies have been detected at long-term monitoring sites in and adjacent to the Waitematā Harbour since 2017 (Fig. 1.; N.S. unpublished data). Little is known about S. brakenhielmi’s extent beyond the harbour surveillance locations, or its potential impacts in Aotearoa/New Zealand. Following increased observations of S. brakenhielmi in more open coast areas in Te Moananui-ā-Toi/Tīkapa Moana/Hauraki Gulf during the 2022 MHW, we utilised existing photographic benthic survey data from rocky reef habitat and established permanent photo plots of colonies to quantify colony abundance and track changes in the cover of individual colonies. Here we present evidence of the first documented population expansion of this species associated with a MHW event.
Methods
The abundance of S. brakenhielmi was assessed using a series of photomosaic transects and drop camera imagery captured at Ōtata Island within Te Moananui-ā-Toi/Tīkapa Moana/Hauraki Gulf (Fig. 1) between November 2020 and March 2023. Photomosaic transects (two per time point) and drop camera imagery were initially captured as part of monitoring for an unrelated project assessing kelp forest recovery following sea urchin removal (Miller et al. 2024). All imagery was captured from the same general area of rocky reef and included primarily urchin barren habitat from approximately − 1 m (mean low water spring) to − 12 m water depth (Table 1).
Each drop camera image captures approximately 3 m2 of reef. Images were manually checked for the presence of S. brakenhielmi and, where present, the number of individual colonies counted (considered as individual colonies where there was clear separation between identified patches). Colony counts were used because it was not possible to obtain accurate area measurements for each identified colony from the drop camera imagery. Using ArcGIS, a virtual transect line was laid down the centre of each photomosaic and a spatial buffer used to generate a survey area running the length of the transect with a width of two meters (one meter either side of the centreline). For consistency with the drop camera imagery, the photomosaic survey areas were then split into 3 m2 sections and all visible S. brakenhielmi colonies identified. As the dataset contained a high number of zeros (i.e. images or transect segments containing no S. brakenhielmi), and consequently failed the assumptions of normality, univariate PERMANOVA (in PRIMER v7) was used to evaluate changes in the number of S. brakenhielmi colonies through time. The PERMANOVA design consisted of one fixed factor (Time) and a random factor (Transect nested within Time). As the drop camera imagery overlapped with the area of reef where the photomosaic transects were captured, each image was assigned to the transect area it was closest to. Count data (number of colonies) were log-transformed prior to analysis to correct for heterogeneity of variance and the analysis was based on Euclidean distance matrices. Because no S. brakenhielmi colonies were detected in imagery collected in 2020 or 2021, these datasets (all zeros) were excluded from the analysis.
Within the Tāwharanui Marine Reserve (Fig. 1), coverage of individual colonies of S. brakenhielmi was assessed using a series of five photo plots. Survey areas were first established in July 2022 for S. brakenhielmi following its detection during unrelated survey work in May 2022. Five discrete areas of reef with S. brakenhielmi (all less than 1 m2) were selected and photographed and distinctive features within the image used to establish the plot boundaries around S. brakenhielmi colonies. Plot size ranged from 0.085 to 0.35 m2 and each was photographed at five separate time points between July 2022 and May 2023. Spatial extent of S. brakenhielmi cover was assessed using ImageJ software. Each image was loaded into ImageJ and scaled using a scalebar that was present within the image footprint. A 10 cm2 grid was then overlaid over the image and all grid squares within the defined plot area containing S. brakenhielmi were labelled. This gave a total count of the number of grid squares containing S. brakenhielmi per plot; however, to account for the difference in size between plots, counts were converted to percent of total plot area and analysed using a repeated-measures ANOVA in R v4.2.3. The ANOVA design had one fixed factor (Time) and an error term (Plot/Time). Normality was checked using a Shapiro-Wilks test and sphericity tested by using the R function anova_test(). Pairwise comparisons were made using the R function emmeans.
Water temperature trends were examined for the period between November 2020 and May 2023 alongside the climatological data (based on mean daily temperatures between 1980 and 2010) using temperature data from the Leigh Marine Laboratory’s long-term sea surface temperature (SST) logger. This logger was situated approximately 10 km from the Tāwharanui Marine Reserve and 50 km from Ōtata Island (Fig. 1). The mean daily SST, maximum SST, number of days above 20.7 °C (climatological mean maximum), and longest continuous period above 20.7 °C were calculated between 01 November and 31 May for each of the 2020/2021, 2021/2022 and 2022/2023 summer periods. These date ranges correspond to that of the observed 2021/2022 MHW (Bell et al. 2023). As this MHW primarily occurred through the austral summer-autumn months it is termed the ‘summer MHW’ from here on. Differences in mean daily SST across these three summers were analysed using a non-parametric Kruskal–Wallis Test (R v4.2.3) as the data failed the assumption of normality. The test included Year (2020/2021, 2021/2022, 2022/2023) as a fixed factor; a Wilcoxon test with Benjamini–Hochberg adjusted p-values was used to explore significant difference between years.
Results
Water temperature trends
The mean daily SST between November 2021 and May 2022 (summer MHW period) of 20.5 °C ± 0.1 °C was significantly warmer than the same date range the year before (Table 2, p < 0.001) or the year following (Table 2, p < 0.001). Mean daily temperature during all three time periods was above the climatological mean of 18.8 °C, however the difference was greatest for 2021/2022 where mean temperature was 1.7 °C above the climatological mean (Table 2). The 2021/2022 also had the highest maximum temperature, which reached more than 2 °C above the climatological mean maximum (20.7 °C), and the greater number of overall and continual days above 20.7 °C (Table 2).
Symplegma brakenhielmi observations
We first detected the presence of S. brakenhielmi as small or isolated colonies at several rocky reef monitoring sites within Te Moananui-ā-Toi/Tīkapa Moana/Hauraki Gulf (immediately adjacent to the Waitematā Harbour) between 2016 and 2021, including observations at Ōtata Island in February 2021 (Fig. 1).
During the summer MHW event in 2021/2022, we observed a noticeable increase in the coverage of this species at sites that are routinely monitored, including Ōtata Island and the Tāwharanui Marine Reserve (Fig. 1). Symplegma brakenhielmi colonies were observed growing on rocky reef and shell hash, overtop marine sponges and other species of solitary and colonial ascidian, within natural and restored green-lipped mussel (Perna canaliculus) beds, on kelp stipes and on the carapace, pleopods and tail of the spiny lobster Jasus edwardsii (Fig. 1).
Temporal monitoring of Symplegma brakenhielmi at Ōtata
At Ōtata, S. brakenhielmi was not recorded in photographic surveys in 2020 or 2021 and was first recorded in February 2022, when a small number of colonies were observed on the reef (0.17 ± 0.07 colonies/m2; Fig. 2). Symplegma brakenhielmi abundance increased to 1.22 ± 0.43 colonies/m2 by April 2022 (still during the MHW), representing a sixfold increase over the two-month period (t = 7.39, p < 0.001; Fig. 2). Abundance then declined sharply (t = 6.43, p < 0.001) between April and September 2022 as water temperatures dropped over winter, falling back to 0.25 ± 0.11 colonies/m2 (Fig. 2). Symplegma brakenhielmi continued to be present within the monitoring area through March 2023, with the abundance remaining relatively low through the 2022/2023 summer (Fig. 2).
Daily temperature observations of SST from the Leigh Marine Laboratory and periodic photographic survey data on S. brakenhielmi colonies at Ōtata Island between November 2020 and March 2023. In the top panel the red line corresponds to 20.7 °C, the climatological mean maximum temperature. Dashed lines indicate the start and end dates for the 2021/2022 summer MHW
Targeted sampling of Symplegma brakenhielmi colonies
The cover of individual S. brakenhielmi colonies gradually declined between July 2022 and May 2023 (Fig. 3). Average cover per plot when first mapped was 56.67 ± 8.53%. Average cover in August 2022 was 38.82 ± 7.28%, though this was not significantly lower than the July 2022 observation (t = 1.892, p = 0.318), and remained stable through until December 2022. Surveys in March and May 2023 showed declines in percent cover and were significantly lower than the original survey (t = 3.546, p = 0.019 and t = 4.912, p = 0.001). Cover in May 2023, at 12.42 ± 3.74%, was approximately one fifth of that recorded during the initial surveys in July 2022.
Discussion
Our findings are the first documented report of MHW-facilitated population expansion of S. brakenhielmi. Given the global increase in frequency and duration of MHWs (Oliver et al. 2018), this represents a mechanism that will likely enable further S. brakenhielmi establishment and competitive advantage within temperate waters into the future. The reproductive cycles of several species of ascidian have been positively linked to warmer water temperatures, including quicker zooid development and maturation and extended reproductive windows (Gasparini et al. 2015; Harris et al. 2017; Valentine et al. 2009). No information exists specifically as to how S. brakenhielmi reproduction relates to water temperature; however, this pantropical species has invaded temperate regions and we observed rapid population increases during a period where summer water temperatures were elevated (at or above the climatological mean maximum for three continual months). These findings indicate there is likely to be a positive relationship in New Zealand between water temperature and S. brakenhielmi reproductive function. Future summer MHW are likely to promote further rapid population increases and expansion.
Following the 2021/2022 summer MHW, as water temperatures cooled within Te Moananui-ā-Toi/Tīkapa Moana/Hauraki Gulf, we saw a significant decline in the abundance of S. brakenhielmi and individually tracked colonies showed signs of regression. Regression is a regular component of a colonial ascidians’ seasonal cycle and involves a disintegration of individual zooids and an overall contraction of the colony (Berrill 1951). Regression is essentially a form of hibernation, whereby colonies are able to persist during periods of adverse environmental conditions, including cooler water, before quickly regenerating and becoming active once conditions improve (Epelbaum et al. 2009; Valentine et al. 2009). This can allow colonies to persist in new locations once settled even if conditions are not continually favourable. This has previously been reported for Eudistoma elongatum, another non-native colonial ascidian found in northern New Zealand, whereby colony regression occurred during winter before regrowing the following spring once water temperatures reached 14 °C (Page et al. 2011). Although all of the individually monitored S. brakenhielmi colonies within the Tāwharanui Marine Reserve regressed, none disappeared entirely from the reef. Similarly at Ōtata Island, some S. brakenhielmi colonies remained visible on the reef even during the 2022 winter months, meaning that despite lower temperatures, a greater number persisted after than prior to the MHW. Given the continued presence of S. brakenhielmi within the reef community at these sites following the MHW, it is likely that when environmental conditions are next favourable (i.e. a prolonged periods of warm summer water temperatures), there will be a greater base population to facilitate further local establishment and potentially wider spread.
The potential ecological and economic impacts of S. brakenhielmi in Aotearoa/New Zealand and globally are poorly understood and have not been studied to date. Our observations, along with the documented impacts of many other invasive ascidians, suggest that it could negatively affect numerous reef associated species and communities within northeastern Aotearoa/New Zealand. In Brazil, S. brakenhielmi has been reported growing on cultured mussels (Rocha et al. 2009), while closely related S. reptans, underwent rapid population expansion in southern California between 1998 and 2000, resulting in extensive smothering of mussels and other encrusting invertebrates (Lambert and Lambert 2003). We observed S. brakenhielmi colonies growing within natural and restored mussel beds, including on live mussels. Bivalve smothering may result in economic losses for the aquaculture industry (Rocha et al. 2009), while restoration initiatives that involve moving mussels from aquaculture facilities to natural substrate risk spreading S. brakenhielmi into new areas. In general, the highly invasive characteristics of many ascidian species, and their consequent ability to prosper outside of their native range, have led to widespread reporting of losses to local biodiversity, reductions in native ecosystem function and impacts to aquaculture and fisheries following their invasion to new locations (Zhang et al. 2020). During the 2021/2022 summer MHW we observed S. brakenhielmi overgrowing other sessile marine invertebrates, including heat-stressed sponge species (see Fig. 1). Sponge mortality also occurred during the MHW (Bell et al. 2023) and S. brakenhielmi was one of the first species to colonise the bare space left behind as dying sponges detached from the reef (Fig. 1). In this way heat-tolerant, highly reproductive invasive species can quickly exploit newly vacant space, restricting the ability of native foundational species to reestablish following mortality events (Félix-Loaiza et al. 2022). Colonial ascidians overtopping other sessile marine invertebrates has been reported previously for MHW related events such as coral bleaching and can hinder heat-stressed individuals’ ability to recovery as water temperatures cool (Dalton et al. 2020). We also saw S. brakenhielmi colonies growing on the tail and carapace of native rock lobster, a previously undocumented observation for this species. Other invasive ascidians, for example Botrylloides violaceus and Botryllus schlosseri in the Gulf of St. Lawrence, have been documented growing on the carapaces of crabs and lobster (Bernier et al. 2009). It is uncertain whether these colonisation events negatively impact their decopod hosts; however, given the mobile nature of decopods, this does present an additional vector for spread within the local area.
These findings highlight an important mechanism by which MHWs can enable rapid increases in abundance and greater integration of invasive, warm-affinity species into temperate reef communities. The increase in frequency and duration of MHWs may enable more regular periods of favourable conditions which facilitate spread for such species both locally and into new areas. Despite lowered winter temperatures, the S. brakenhielmi colonies persisted as an enduring feature of the reef. This persistence can have serious consequences for native species, particularly those also impacted by the MHW and may ultimately contribute towards greater tropicalisation of temperate reef ecosystems.
Data availability
The field data that support the findings of this study are available in Figshare: https://doi.org/10.17608/k6.auckland.24405925.
References
Arafeh-Dalmau N, Montaño-Moctezuma G, Martínez JA, Beas-Luna R, Schoeman DS, Torres-Moye G (2019) Extreme marine heatwaves alter kelp forest community near its equatorward distribution limi. Front Mar Sci 6:499
Arafeh-Dalmau N, Schoeman DS, Montaño-Moctezuma G, Micheli F, Rogers-Bennett L, Olguin-Jacobson C, Possingham HP (2020) Marine heat waves threaten kelp forests. Science 367:635
Bell JJ, Smith RO, Micaroni V, Strano F, Balemi CA, Caiger PE, Miller KI, Spyksma AJP, Shears NT (2023) Marine heat waves drive bleaching and necrosis of temperate sponges. Curr Biol 33(1):158–163. https://doi.org/10.1016/j.cub.2022.11.013
Bernier RY, Locke A, Hanson MJ (2009) Lobsters and crabs as potential vectors for tunicate dispersal in the southern Gulf of St. Lawrence. Canada Aquat Invas 4:105–110
Berrill NJ (1951) Regeneration and budding in tunicates. Biol Rev 26:456–475
Dalton SJ, Carroll AG, Sampayo E, Roff G, Harrison PL, Entwistle K, Huang Z, Salih A, Diamond SL (2020) Successive marine heatwaves cause disproportionate coral bleaching during a fast phase transition from El Niño to La Niña. Sci Total Environ 714:136951
Epelbaum A, Herborg LM, Therriault TW, Pearce CM (2009) Temperature and salinity effects on growth, survival, reproduction, and potential distribution of two non-indigenous botryllid ascidians in British Columbia. J Exp Mar Biol Ecol 369:43–52
Félix-Loaiza AC, Rodríguez-Bravo LM, Beas-Luna R, Lorda J, de La Cruz-González E, Malpica-Cruz L (2022) Marine heatwaves facilitate invasive algae takeover as foundational kelp. Bot Mar 65:315–319
Gasparini F, Manni L, Cima F, Zaniolo G, Burighel P, Caicci F, Franchi N, Schiavon F, Rigon F, Campagna D, Ballarin L (2015) Sexual and asexual reproduction in the colonial ascidian Botryllus schlosseri. Genesis 53:105–120
Harris AM, Moore AM, Lowen JB, DiBacco C (2017) Seasonal reproduction of the non-native vase tunicate Ciona intestinalis (Linnaeus, 1767) in Nova Scotia, Canada, in relation to water temperature. Aquat Invas 12:33–41
Hobday AJ, Alexander LV, Perkins SE, Smale DA, Straub SC, Oliver EC, Benthuysen JA, Burrows MT, Donat MG, Feng M (2016) A hierarchical approach to defining marine heatwaves. Prog Oceanogr 141:227–238
Hughes T, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Kott P (1985) The Australian Ascidiacea. Part 1, Phlebobranchia and Stolidobranchia. Memoirs Queensland Museum 23:1–439
Lambert CC, Lambert G (1998) Non-indigenous ascidians in southern California harbors and marinas. Mar Biol 130:675–688
Lambert CC, Lambert G (2003) Persistence and differential distribution of nonindigenous ascidians in harbors of the Southern California Bight. Mar Ecol Progr Ser 259:145–161
Miller KI, Balemi CA, Bell DR, Blain CO, Caiger PE, Hanns BJ, Kulins SE, Peleg O, Spyksma AJP, Shears NT (2024) Large-scale one-off sea urchin removal promotes rapid kelp recovery in urchin barrens. Restorat Ecol 32(1):e14060
Oliver EC, Donat MG, Burrows MT, Moore P, Smale DA, Alexander LV, Benthuysen JA, Feng M, Sen Gupta A, Hobday AJ, Holbrook NJ (2018) Longer and more frequent marine heatwaves over the past century. Nat Commun 9:1–12
Page M (2015) Notes on the colonial styelid ascidian Symplegma brakenhielmi (Michaelsen, 1904). Mar Exot Species Note 87:1–3
Page MJ, Morrisey DJ, Handley SJ, Middleton C (2011) Biology, ecology and trials of potential methods for control of the introduced ascidian Eudistoma elongatum (Herdman, 1886) in Northland, New Zealand. Aquat Invasions 6:515–517
Ramos-Esplá AA, Bitar G, Sghaier YR, Çinar ME, Deidun A, Ferrario J, Ulman A (2020) Symplegma (Ascidiacea: Styelidae), a non-indigenous genus spreading within the Mediterranean Sea: taxonomy, routes and vectors. Aquat Invas 15:44–62
Rocha RM, Kremer LP, Baptista MS, Metri R (2009) Bivalve cultures provide habitat for exotic tunicates in southern Brazil. Aquat Invas 4:195–205
Seaward K, Inglis G (2018) Long-term indicators for nonindigenous species (NIS) in marine systems [Report prepared for Ministry for the Environment]
Starko S, Fifer JE, Claar DC, Davies SW, Cunning R, Baker AC, Baum JK (2023) Marine heatwaves threaten cryptic coral diversity and erode associations amongst coevolving partners. Sci Adv 9:eadf0954
Valentine PC, Carman MR, Dijkstra J, Blackwood DS (2009) Larval recruitment of the invasive colonial ascidian Didemnum vexillum, seasonal water temperatures in New England coastal and offshore waters, and implications for spread of the species. Aquat Invas 4:153–168
Zhang Z, Capinha C, Karger DN, Turon X, MacIsaac HJ, Zhan A (2020) Impacts of climate change on geographical distributions of invasive ascidians. Mar Environ Res 159:104993
Acknowledgements
We are especially grateful to Paul Caiger for his assistance in collecting field data along with others including Celia Balemi, Brady Doak, Eliana Ferretti, Dallas LaFont, Anna-Roosa Vesanen and Hannah Williams. Thanks to James Frankham, New Zealand Geographic and the Seascape Project for support with camera equipment. Thank you to the two reviewers for providing useful comments to improve the manuscript and to Mike Page (NIWA) for examining S. brakenhielmi samples at such short notice.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funded for this project was provided by the Live Ocean Foundation and Foundation North – GIFT.
Author information
Authors and Affiliations
Contributions
AJPS and NTS conceived the study design. AJPS, KIM and NTS collected field data. AJPS analysed the data and wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spyksma, A.J.P., Miller, K.I. & Shears, N.T. Marine heatwave promotes population expansion of the invasive ascidian Symplegma brakenhielmi. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03296-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03296-4