Abstract
Feral cats (Felis catus) pose a significant global threat to biodiversity, primarily through predation, disease and competition. A key gap in parameterizing models for improving management decisions for feral cat control relates to factors that drive feral cat survival and movement in the wild. Our study objective was to conduct the first continental-scale analysis of survival rates and displacement distances for feral cats. We collated data on 528 feral cats from telemetry studies in naturally-vegetated landscapes across Australia. Using Cox-proportional hazards models, we investigated the effects of sex, presence of larger predators (dingoes, Canis familiaris and introduced foxes, Vulpes vulpes), presence of introduced prey (rabbits, Oryctolagus cuniculus), body mass, landscape productivity and feral cat density on feral cat survival. We also analysed the effects of sex, body mass and landscape productivity on feral cat displacement using linear mixed model analysis. Feral cat survival was positively associated with presence of dingoes and increasing body mass, whereas there was no clear association between feral cat survival and sex, presence of rabbits, or cat density. Presence of foxes had a strong negative effect on feral cat survival, but the hazard ratio was associated with considerable uncertainty. Net displacement of male feral cats was nearly two times further than that of females, and the proportion of feral cats making long-distance movements was greater in landscapes with low productivity. Increasing body mass of feral cats was positively related to net displacement, with heavier cats moving further. Analysis of metadata from telemetry studies can provide valuable insights into wildlife survival rates and movement behaviour. Our findings will help inform the development of effective management strategies and improve feral cat management for biodiversity conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free-ranging cats (Felis catus) pose a global threat to biodiversity conservation (Doherty et al. 2015; Medina et al. 2011). Cats are widely distributed around the world, and their generalist behaviour and diet makes them adaptable and capable of surviving diverse and extreme conditions (Fitzgerald 1988; Lepczyk et al. 2023; Loss et al. 2013; Medina et al. 2011; Nogales et al. 2013). Free-ranging cats are considered feral (hereafter ‘feral cats’) if they persist in self-sustaining populations living independently of humans (Lepczyk and Calver 2022). Feral cats have been listed as one of the 100 worst invasive species in the world, being responsible for at least 14% of global bird, mammal and reptile extinctions on islands (Lowe et al. 2000; Medina et al. 2011). They negatively impact native species through predation (Medina et al. 2014), disease transmission (Dubey 2002) and competition (Glen and Dickman 2005; Medina et al. 2014).
Developing effective invasive predator management strategies requires robust estimates of the species’ survival, reproduction, and movement rates (e.g., Byers et al. 2019; Fleming et al. 2006; Moro et al. 2018). Reproductive behaviour and fecundity of feral cats are well documented; average litter sizes are similar worldwide, while the timing of breeding season varies with latitude (Woinarski et al. 2019). An increasing databank of GPS and radio-tracking studies also provide estimates of home range sizes in many regions (Bengsen et al. 2015; Kays et al. 2020; Nottingham et al. 2022).
In contrast, the survival rate and behaviour of feral cats are poorly understood. Studies of baseline survival rates for feral cats have focused either on cats in anthropogenic landscapes (excluding pet cat studies; Devillard et al. 2003; Gehrt et al. 2013; Nutter et al. 2004; Schmidt et al. 2007) or on remote islands (Danner et al. 2010; Van Aarde 1983). A summary of these studies and the estimated survival rate for each population is provided in Table S1. Alternatively, studies have examined the effects of management and diseases on feral cat survival (e.g., Andersen et al. 2004; Jones and Downs 2011). Very little is known about feral cat survival in naturally-vegetated landscapes, or how environmental conditions and demographic characteristics such as sex and body mass affect feral cat survival rates.
Telemetry studies (i.e., remote data collection from freely moving animals through radio or satellite collaring) have shown that feral cats can move long distances (10 to over 200 km, e.g., Jansen et al. 2021; McGregor et al. 2016; Roshier and Carter 2021). But the influence of sex, body mass and habitat types on the likelihood or frequency of these movements is not well known. Information on such long-range movements in telemetry studies may be limited by lack of consistent long-distance data, costs involved in collar deployment and data acquisition, operating life of collars, satellite signal reception, potential for technical failures and loss of collars (Matthews et al. 2013).
A better understanding of feral cat survival rates and long-distance movement behaviour is important for improving management decisions around feral cat control. Survival rates can be used to provide baseline rates for designing or improving management programs and to parameterize population models (Bull et al. 2009; Gaillard et al. 1998; Stenseth et al. 2001). Likewise, long-distance movements influence recolonization rates and recovery from lethal population management programs (e.g., Comer et al. 2018). Understanding movement between locations can provide information on how populations respond to habitat conditions and their distribution, as displacement and population dynamics are interlinked (Bowler and Benton 2005; Dunning et al. 1995).
Here we conduct the first continental-scale analysis of feral cat survival and long-distance displacements, with a focus on feral cats in naturally vegetated landscapes. Feral cats in Australia remain a major driver of species decline since their introduction by European settlers in the eighteenth century (Abbott 2008; Fisher et al. 2014; Woinarski et al. 2015). There are numerous examples of feral cats causing localised population declines and extirpations of prey species on both mainland Australia and offshore islands (Burbidge and Manly 2002; Risbey et al. 2000).
We hypothesised that feral cat survival rates are higher for female cats (e.g., Danner et al. 2010; Kaeuffer et al. 2004; Schmidt et al. 2007) and are negatively influenced by the presence of foxes and/or dingoes due to antagonistic interactions or dominance from these larger predators (Kennedy et al. 2012; Molsher et al. 2017). Conversely, we hypothesised that introduced rabbits benefit cat survival (McGregor et al. 2020), as does cat density (indirectly), as densities may reflect prey abundance (Legge et al. 2017; Read and Bowen 2001). Further, we hypothesised that cats survive better in more productive landscapes: cat home-ranges are larger in landscapes with lower productivity (Bengsen et al. 2015; Nottingham et al. 2022). We hypothesised that larger cats survive better, as they can hunt a greater range of prey sizes (Moseby et al. 2020; although see Fleming et al. 2020). Similarly, we hypothesised that larger male cats may move further due to their polygynous mating system and feral cats moving longer distances in landscapes with low to moderate level of resources (Bengsen et al. 2015; McGregor et al. 2015; Mirmovitch 1995; Say and Pontier 2004). We therefore predicted that survival rates would be higher for female cats, be lower in the presence of foxes and dingoes, higher in areas with introduced rabbits, be positively associated with landscape productivity, cat density and increasing body mass. We further predicted that the average distance between a feral cat’s first and final capture locations would be higher for males and vary among body mass classes and habitat types.
Materials and methods
Data collection
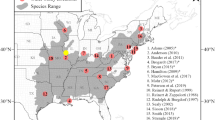
We compiled data on the fate and net displacement of individual feral cats from published and unpublished telemetry studies on 528 feral cats (354 male and 173 female) from thirty-one study areas across mainland Australia and offshore islands (Fig. 1). We define ‘net displacement’ as the linear distance between a feral cat’s first and final capture locations. This value is a conservative estimate of the maximum distance moved, and does not distinguish between dispersive and home ranging movements as we were unable to collate all movement locations for individuals.
The telemetry studies were conducted from 2006 to 2023, with the study area defined as the area where a researcher collared cats for their study. All studies had a minimum of three cats from a single study area. We collated information on the first and last recorded capture dates and location for cats, along with their sex, body mass, status at last capture (dead, alive or unknown), cause of death (if known), and the presence/absence of foxes, dingoes and rabbits in the study location (researcher reported, Figs. S1, S2, S3). For the studies that were trialling the effectiveness of feral cat baiting programs, we used the last known alive date before poison baits were deployed, if the cat died from bait intake. Similarly, cats that were trapped, shot or euthanized by the researchers were also considered to be alive until their date of death.
To test our predictions, we used two metrics for landscape productivity: (1) we classified Australia into distinct habitat types, with each study falling into one of these simplified categories: deserts, Mediterranean, Savannahs, temperate forests and temperate grasslands (Fig. 1; adapted from Australian Government Department of Sustainability, Environment, Water, Population and Communities 2012); (2) we obtained yearly rainfall data for the 12 months before a feral cat’s last capture and a 50-year rainfall average for the study locations using the R package ‘cropgrowdays’ (Baker and Mortlock 2022). We then derived the rainfall deviation for each cat as rainfall deviation = (rainfall in the last year – 50 year rainfall average)/100. We included rainfall deviation as cat population densities can fluctuate with varying rainfall conditions in different environments in Australia (Legge et al. 2017).
We categorised feral cats into body mass classes, based on their weight during initial capture and the groupings in Jones and Coman (1982). We also distinguished between the sexes where sample sizes allowed (> 125 individuals of each sex). This resulted in five classes: very small (male ≤ 2.2 kg, female ≤ 1.9 kg), small (male 2.2–3.2 kg, female 1.9–2.5 kg), medium male (3.2–4.5 kg), medium female (2.5–4.5 kg), and large cats (> 4.5 kg). Only four (0.7%) of the large cats were female. Cat density estimates were obtained from field observations for each study where available (n = 7). When unavailable, we derived predicted densities from published studies in the same area (n = 2) or from Legge et al. (2017) (n = 20). We removed very small cats from the compiled dataset as this class was not adequately represented across different habitat types (n = 23). There were no cats from temperate grasslands (Fig. 1). Cats with an unknown status at last capture were not included in survival analyses.
Data analysis
Survival analysis
We used two different metrics to evaluate the effects of covariates on feral cat survival. Hazard ratios (HR) can be considered as an estimate of relative risk, which is the risk or probability of an event happening (i.e., death of a feral cat) relative to a reference group. A HR of 1 implies equal hazard in each group (i.e., an equal likelihood of dying), a HR > 1 means increased risk relative to the reference group (i.e., an increased likelihood of dying) and HR < 1 means reduced risk in comparison to the reference group (i.e., a reduced likelihood of dying). For example, HR = Hazard female cats/Hazard male cats = 2 means that the risk of dying for female cats is two times higher than male cats (i.e., female cats have lower chance of surviving).
We used a mixed-effects Cox proportional hazard model (Therneau 2022) to evaluate the effects of sex, body mass (as a continuous variable), habitat type, presence/absence of foxes, dingoes and rabbits, rainfall deviation and cat densities on the hazard ratio for feral cats. We fitted an additive model with all predictors to test our hypotheses. Study area was fitted as a random effect (intercept) to account for potential clustering in the dataset. We accounted for differences in study duration by truncating the data to 52 weeks (Fig. 2). All feral cats that were collared for more than 52 weeks (9.2% of individuals) were classed as ‘alive’ at the end of the 52-week period.
We tested for the assumption of Cox models that the hazard ratio predicted by the model is proportional over time by plotting the scaled Schoenfeld residuals over time, where a non-zero slope over time can indicate a lack of proportionality (Cox and Oakes 1984; Kalbfleisch and Prentice 2002). The assumption of proportional hazards was supported for our predictor variables. We checked for correlations between predictor variables using pairwise Pearson’s correlation test. We removed habitat type from our hazard analysis as savannahs and presence of dingoes were moderately correlated (R = 0.54, Fig. S4) and including both caused model instability; all study sites in savannahs had dingoes present. We also used a generalised linear mixed model with a binomial distribution to test for any association between the monitoring duration of feral cats (in weeks) and their status during the last capture (0 = dead, 1 = alive, NA = unknown), study area was included as a random effect.
Annual feral cat survival rate is the proportion of feral cats alive at the end of 52 weeks. We estimated the annual survival rate of feral cats using the duration of monitoring and status at last capture for each collared individual. We estimated annual survival probabilities separately for sex, body mass class, presence/absence of foxes, dingoes and rabbits, and habitat types using Kaplan Meier estimates (Kaplan and Meier 1958). The estimates also account for feral cats that were collared for < 52 weeks by taking their survival into account until their last known event.
For both the hazard ratio and survival rate analyses, we considered that p values < 0.05 indicated that estimates were significantly different for categories within each predictor variable. Note that confidence intervals around the estimated survival rates tend to increase over time, due to the decreasing sample size (i.e., censored individuals that were monitored for < 52-week). We provide both metrics for the effects of covariates on survival, as the effects of covariates may be misinterpreted from providing just hazard ratios. Hazard ratios estimate relative differences in the ratio of risk involved among animals as a function of covariates but cannot estimate changes in survival over time. A high hazard ratio may not translate to a high difference in survival rates, as survival rates estimate the proportion of feral cats that survive after a particular time (Sashegyi and Ferry 2017; Spruance et al. 2004). Providing survival curves along with hazard ratios assists to interpret the effects of covariates on feral cat survival.
Net displacement
We used generalised linear mixed models to estimate mean net displacement for feral cats. Study area was included as a random effect (intercept). We compared net displacement for sex, body mass classes and habitat types. We log-transformed displacement to improve normality. We then back-transformed the modelled net displacement along with confidence intervals (CIs) to interpret differences between feral cat demographics. We used 95% confidence intervals to provide a range of possible mean distances and not as a dichotomous inference of significance versus non-significance (Austin and Hux 2002; Payton et al. 2003).
We also looked at the proportion of feral cats that moved long distances between the first and last capture date. Using the largest observed home-range for a feral cat in a recent global meta-analysis (32.32 km2 stable home-range, Nottingham et al. 2022), we calculated the diameter or maximum distance moved by a feral cat within its home-range to be 6.41 km (assuming a circular range). We therefore defined long-distance movement by feral cats as any displacement over 7 km. We acknowledge that we may have incorporated some intra-range movements in our ‘long distance movements’ as some cats may have home-ranges larger than 32 km2 or non-circular ranges (e.g., Comer et al. 2018; Roshier and Carter 2021). Furthermore, range size is influenced both by the analytic approach and definition of a stable home range. We tested for any association between monitoring duration and net displacement using Pearson’s coefficient and generalised linear models. All analyses were carried out in ‘survival’ (v 3.5-0; Therneau 2014), ‘coxme’ (v 2.2-18.1; Therneau 2022) and lme4 (v 1.1-31; Bates et al. 2015) in R (v4.2.1; R Core Team 2021).
Results
Survival analysis
We ran Cox-proportional hazards analysis on 498 cats. Sixty-three cats died within 52 weeks of collaring (12.6%), with most of these deaths attributed to unknown causes (n = 56). Some showed signs of being depredated (n = 4) or possibly killed by snakes (n = 3). A further 389 cats (78%) were monitored for < 52 weeks but were alive at the conclusion of the tracking period (Fig. 2). There was a significant but weak negative association between the monitoring duration of feral cats and their status during the last capture (R2m = 0.36, p < 0.01).
There was no association between sex and relative risk of mortality for feral cats (p = 0.77; Fig. 3). Fox presence was estimated to double the risk to feral cats, but the associated uncertainty was high, and the effect was not significant (HR = 2.04, p = 0.12; Fig. 3). In contrast, there was strong evidence of an association between dingo presence and relative risk for feral cats (p = 0.03; Fig. 3). The presence of dingoes was strongly associated with reduced risk to feral cats (i.e., positively associated with survival, HR = 0.38; Fig. 3). There was no clear association between rabbit presence and relative risk of feral cats (p = 0.30, Fig. 3).
Model summary for the additive Cox proportional-hazards model on feral cat survival. Data are from cats across Australia (n = 498). Reference in factor refers to the reference levels for each predictor. Hazard ratios are plotted with confidence intervals. Hazard ratios > 1 indicates increased hazard (lower survival) for the predictor compared to the reference level whereas hazard ratios < 1 indicates reduced risk (higher survival). P indicates the level of significance
Body mass of feral cats had a strong negative association with the risk of feral cats dying, with relative risk decreasing with every kilogram increase in weight (HR = 0.66, p = 0.01; Fig. 3). The model also supported a small but significant effect of increasing yearly rain deviation on relative risk to feral cats, with relative risk increasing when rainfall in the last 12 months was higher than the long-term average (HR = 1.17, p = 0.05). There was no relationship between cat density and relative risk to feral cats (p = 0.32, Fig. 3).
The average annual survival rates for feral cats in these telemetry studies was 0.75. Consistent with our hazard ratio analysis, survival rates were similar for male and female cats (p = 0.74, Fig. 4a). Survival rates for feral cats were significantly different between landscapes with and without foxes (p = 0.01, Fig. 4b). An opposite relationship was observed for dingoes: feral cat survival was significantly higher in landscapes with dingoes present (p = 0.02; Fig. 4c). There were no significant differences in survival rates for landscapes with rabbits present compared to landscapes with no rabbits (p = 0.78, Fig. 4d).
Annual survival curves and confidence intervals for feral cats based on their sex, age categories, presence/absence of foxes, dingoes and rabbits, and habitat types. Lines indicate the survival function (i.e., the proportion of individuals surviving). Crosses indicate when an individual was ‘censored’ (due to conclusion of the tracking period or dropping out of the study)
Feral cat survival rates were similar across all habitat types (p = 0.29, Fig. 4e). Cats in deserts tended to have the higher average survival rates, but confidence intervals around all estimates overlapped substantially throughout the monitoring period (Fig. 4e). There were no significant differences (p = 0.14, Fig. 4f) in feral cat survival rates among body mass categories (classed based on their sex and weight). Small cats tended to have the lowest survival rate, but confidence intervals were wide and substantially overlapped the estimates for the other body mass classes.
Net displacement
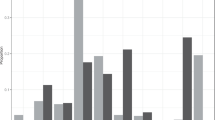
First and last capture locations were available for 447 cats. We found a significant but weak positive log–log relationship between the monitoring duration and net displacement of feral cats (R = 0.17, p < 0.01, Fig. S5). Displacement of cats over their tracking period varied from 0.01 to 164 km. The distribution of displacement was right skewed, with approximately 78% of the cats moving < 5 km and only 7% moving more than 25 km. The mean displacement of male feral cats was nearly two times further (2.33 km, CI: 1.48–2.47) than that of female cats (1.21 km, 0.90–1.63). Displacement distances varied among habitat types. Feral cats in Savannah and Mediterranean habitat had overall higher mean displacement compared to desert and temperate forests. The effect of life-stage class also differed among different habitat types (Fig. 5).
Mean displacement was lowest for medium female cats in all habitat types. For this class, mean displacement was < 2 km in all habitat types, with the lowest estimate in temperate forests (0.61 km, Fig. 5). Except in Mediterranean habitats, small cats also had lower mean displacement compared to adult male cats and large cats (Fig. 5). The highest mean displacement was for medium male cats in savannahs (5.06 km); this was nearly two times further than the distances for other body mass categories in the same habitat type. Mean displacement estimates and confidence intervals for feral cats in all habitat types and classes are provided in Table S2.
Among the feral cats that moved long distances between their first and final locations (more than 7 km, n = 74), a much higher proportion were male (75.6%). When categorised by body mass and sex, the smallest proportion were medium female cats (12.1%) followed by small cats (22.9%), large cats (27%) and medium male cats (37.8%).
The proportion of cats that made long-distance movements was lowest in temperate forests, with only 8% of cats in temperate forests moving more than 7 km (10 of 124 individuals). This was followed by 12.7% of feral cats in desert (18 of 141 individuals) and 17.5% in Mediterranean forests (16 of 91 individuals) making long-distance movements. The highest proportion of long-distance movements were observed in savannahs (32.9%, 30 of 91 individuals).
Discussion
Our study is among the first to conduct a continental-scale study of feral cat survival rates and long-distance movements. Contrary to our predictions, the presence of dingoes had a positive relationship with the survival of feral cats in telemetry studies. Greater body mass also had a positive relationship, whereas higher than average rainfall deviation had a negative relationship with feral cat survival. There was also some evidence that fox presence had a large negative effect, but this was associated with considerable uncertainty when the influence of other factors was also accounted for. Our predictions that net displacement would vary between body mass classes and habitat types were supported, with male cats in our study showing nearly two times higher net displacement than female cats and feral cats moving further in landscapes with lower productivity.
A better understanding of predator interactions and their functional roles in varying ecosystems can improve conservation outcomes (Ritchie and Johnson 2009). Not accounting for these complex interactions may reduce the efficacy of management actions or result in adverse outcomes such as the ‘release’ of other invasive mesopredators or prey species (Bergstrom et al. 2009; Courchamp et al. 1999). In many parts of Australia, lethal control of dingoes and foxes is widely conducted, with the aim of protecting livestock (both species) or wildlife (foxes). In contrast, feral cat control is primarily aimed at protecting native fauna with regulations for lethal control varying from state to state. Hence, it is important to understand whether these dominant predators affect feral cat survival.
There was some evidence for a negative association between fox presence and feral cat survival: the survival rate for feral cats was significantly higher in the absence of foxes (univariate survival analysis). Similarly, the multivariate hazard model estimated that the average relative risk to feral cats doubled when foxes were present, but this effect was associated with considerable uncertainty and so was non-significant. Foxes and feral cats are opportunistic predators and are sympatric over much of their range in Australia (Woinarski et al. 2019). Foxes may potentially affect feral cat survival through direct predation or competition for food due to their large overlap in diet (Fleming et al. 2022; Molsher et al. 2017; Risbey et al. 2000). Our finding of higher feral cat survival rates in the absence of foxes is consistent with studies that have shown increased cat activity in areas with reduced fox activity (Marlow et al. 2015; Read and Bowen 2001; Risbey et al. 2000) and higher cat densities in areas where foxes are controlled (Rees et al. 2023). The discrepancy between the univariate survival model and multivariate hazard model may be because the hazard model simultaneously accounted for the effects of other predictors. As discussed below, fox presence was associated with several other predictors; further controlled experiments are needed to better quantify the effects of foxes and fox management on feral cat survival.
Contrary to our predictions, the presence of dingoes was associated with lowered risk to feral cats, with risk of mortality for feral cats in the telemetry studies nearly 2.5 times lower in landscapes with dingoes. Likewise, survival rates for feral cats were significantly higher in landscapes with dingoes. Note that we cannot infer any relationship between dingo presence and the survival of young or small feral cats, because these animals were not present in our telemetry dataset. Previous studies on interactions between dingoes and feral cats have mainly reported negative relationships (Brawata and Neeman 2011; Kennedy et al. 2012) or no clear relationships (Fancourt et al. 2019; Hunter et al. 2018; Letnic et al. 2009). A possible explanation might be that dingoes indirectly benefit feral cats through their negative effects on foxes. Moseby et al. (2019) tested potential mesopredator release using field data and prior information on predators and found a strong negative association between foxes and dingoes, but only a weak negative effect of dingoes on cats. Fancourt et al. (2019) also found no significant negative impact of dingoes on feral cat presence in the absence of foxes. The predator cascade hypothesis predicts that top-down suppression of mesopredators by large predators may indirectly benefit smaller predators (Levi and Wilmers 2012; Prugh and Sivy 2020). For example, foxes in North American can be released from top-down control by coyotes when wolves suppress coyote populations (Levi and Wilmers 2012; Newsome and Ripple 2014). Similar agonistic interactions may occur between competing predators in Australia. Concurrent estimates of fox and dingo abundance in our study areas (Stobo-Wilson et al. 2022) would provide more insights into how these dominant predators influence feral cat survival.
We found no relationship between rabbit presence and relative risk to feral cats. While survival rates were slightly lower for feral cats in landscapes without rabbits, absence of rabbits may not pose a strong relative risk to feral cats as they are known to switch their diet to native prey when rabbit populations are low (McGregor et al. 2020). Our correlative approach meant that we were unable to fully distinguish the effects of foxes, dingoes and rabbit presence from each other, or from habitat type. For example, there are no foxes or rabbits in the tropics in northern Australia, whereas all study areas within savannahs in our study had dingoes present. Similarly, foxes and rabbits are common in south-east Australia where dingoes are heavily controlled through exclusion fencing and various lethal population management programs (Fleming et al. 2006). This highlights the need for more causal experiments to test the effects of manipulating the predator and introduced prey guild on native fauna.
We found that heavier feral cats were at significantly lower risk of dying. Likewise, small cats in our study had the lowest survival rate compared to large cats, and to medium male and female cats (albeit non-significantly). High levels of mortality among small cats or cats under 1 year old have also been observed in other studies (Jones and Coman 1982; Van Aarde 1983). Small cats may be vulnerable to territorial aggression from dominant feral cats (Corbett 1979; Langham and Porter 1991). However, our inference for this demographic is limited by our telemetry-based dataset (juvenile cats are usually too small to trap and fit with tracking collars). A growing body of evidence also shows that the individual traits of feral cats, particularly body mass, can influence their hunting behaviour and prey size (Dickman and Newsome 2015; Kutt 2012; Moseby et al. 2020). Note, however that Fleming et al. (2020) found that while older cats might pose a greater risk to large and ‘dangerous’ prey, small cats were also capable of hunting big prey. Nonetheless, their greater hunting experience and ability to handle larger prey may enhance the survival rate of heavier cats.
Although some studies have found higher survival rates for female cats (Danner et al. 2010; Schmidt et al. 2007), this was not supported in our analysis. The difference in results might be because some of these studies were in urban areas. Causes of mortality in urban areas often differ from natural environments, with some of the primary causes of mortality in urban areas being attacks by stray or owned dogs and road-kills (Devillard et al. 2003; Nutter et al. 2004). Furthermore, urban areas often support very high cat densities (e.g., 972 cats per km2 in Kaeuffer et al. 2004); cats in areas with such high densities have a different, matrilineal mating system (Crowell-Davis 2007) which might influence sex-related survival. Finally, the low sample sizes within some of these study populations may have increased the likelihood of sex-biased estimates (e.g., n = 28 in Danner et al. 2010).
To our knowledge, ours is the first study to compare feral cat survival rates across different habitat types. Somewhat surprisingly, survival rates were similar across all habitats. Feral cats in deserts tended to have higher average survival rates, but confidence intervals overlapped substantially. Feral cat home-range size is often negatively associated with landscape productivity (Bengsen et al. 2015). That is, they have much larger home ranges in less productive arid and semi-arid environments (e.g., Roshier and Carter 2021) compared to temperate areas (e.g., Buckmaster and Dickman 2012). While higher landscape productivity might facilitate smaller home-ranges, it does not appear to confer higher survival rates, perhaps due to increased disease risk in wetter conditions (Brazier et al. 2014). Consistently, we also found that relatively wetter conditions increased the relative risk to feral cats. We found no significant effect of increasing cat density on feral cat survival. This may suggest that in areas with higher feral cat densities, cats are still able to find enough prey such that their survival is not negatively affected by increased intraspecific competition for prey.
Consistent with our predictions, we found differences in net displacement for feral cats among sex and body mass classes. Male feral cat displacement in our study was nearly two times higher than female cats. These findings were consistent with studies showing larger home-ranges for male cats than females, and feral cats having a polygynous mating system where males often displace further (Bengsen et al. 2015; Liberg et al. 2000). The highest proportion of long-distance movements in our study were observed among medium male cats, followed by large cats and small cats. Heavier cats, particularly large male cats are known to have a significantly higher impact on native mammal populations (Moseby et al. 2020), and are more likely to travel beyond their territory to access areas of high resources (McGregor et al. 2016).
Displacement can also vary between environments, with cats often moving further in less productive landscapes (e.g., Edwards et al. 2001; Roshier and Carter 2021). A relatively high proportion of feral cats in Savannahs, Deserts and Mediterranean forests moved long distances. Although a lower proportion of cats in temperate forests moved long distances, some individuals still moved more than 10 km from their initial point of capture (maximum of 37.5 km). Such long-distance movements by feral cats may be driven by food resources (e.g., Edwards et al. 2001). McGregor et al. (2014) reported adult feral cats travelling up to 12 km to hunt on fire scars from intense burns and subsequently returning to their home-ranges. Long-distance movements can affect feral cat management programs as new individuals can return and re-occupy vacant territories even though populations may be suppressed in an area (e.g., Algar et al. 2013; Moseby et al. 2009). Management programs should perhaps occur at different scales in different environments given the high variability in long-distance movement among feral cats across different habitat types.
Conclusion
Spatial, temporal and individual variation in survival rates and movement behaviour have important implications for invasive predator management, but are often poorly understood and not included in management decisions. Our average estimated feral cat survival rates for Australia were similar or higher than some of those previously recorded in free-roaming cat populations in USA and Europe (refer to Table S1). Feral cats currently inhabit most of mainland Australia and many offshore islands, and are responsible for declines and extinctions of numerous native prey (Abbott 2008; Burbidge and Manly 2002; Woinarski et al. 2015). The rates we have calculated can be used to improve population models and simulate potential responses to management. Further we have shown that the population dynamics of invasive predators like feral cats are likely driven not just by their density, but also by other ecological factors such as the predator community and landscape productivity. Integrating knowledge on how these factors affect survival and movement behaviour of feral cats could help improve the design of single-species and integrated invasive species management programs in Australia.
Change history
23 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10530-024-03337-y
References
Abbott I (2008) The spread of the cat, Felis catus, in Australia: re-examination of the current conceptual model with additional information. Conserv Sci West Aust 7:1–17
Algar D, Onus M, Hamilton N (2013) Feral cat control as part of Rangelands Restoration at Lorna Glen (Matuwa), Western Australia: the first seven years. Conserv Sci West Aust 8:367–381
Andersen MC, Martin BJ, Roemer GW (2004) Use of matrix population models to estimate the efficacy of euthanasia versus trap-neuter-return for management of free-roaming cats. J Am Vet Med Assoc 225(12):1871–1876. https://doi.org/10.2460/javma.2004.225.1871
Austin PC, Hux JE (2002) A brief note on overlapping confidence intervals. J Vasc Surg 36(1):194–195. https://doi.org/10.1067/mva.2002.125015
Australian Government Department of Sustainability, Environment, Water, Population and Communities. (2012) Terrestrial Ecoregions in Australia. https://www.dcceew.gov.au/sites/default/files/env/pages/1716eb1c-939c-49a0-9c0e-8f412f04e410/files/ecoregions_1.pdf
Baker P, Mortlock M (2022) Package “cropgrowdays”. https://cran.r-project.org/package=cropgrowdays
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
Bengsen AJ, Algar D, Ballard G, Buckmaster T, Comer S, Fleming PJS, Friend JA, Johnston M, McGregor H, Moseby K, Zewe F (2015) Feral cat home-range size varies predictably with landscape productivity and population density. J Zool 298(2):112–120. https://doi.org/10.1111/jzo.12290
Bergstrom DM, Lucieer A, Kiefer K, Wasley J, Belbin L, Pedersen TK, Chown SL (2009) Indirect effects of invasive species removal devastate World Heritage Island. J Appl Ecol 46(1):73–81. https://doi.org/10.1111/j.1365-2664.2008.01601.x
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80(2):205–225. https://doi.org/10.1017/S1464793104006645
Brawata RL, Neeman T (2011) Is water the key? Dingo management, intraguild interactions and predator distribution around water points in arid Australia. Wildl Res 38(5):426. https://doi.org/10.1071/WR10169
Brazier I, Kelman M, Ward MP (2014) The association between landscape and climate and reported tick paralysis cases in dogs and cats in Australia. Vet Parasitol 204(3–4):339–345. https://doi.org/10.1016/j.vetpar.2014.05.018
Buckmaster T, Dickman CR (2012) Feral cats in the tall forests of Far East Gippsland, Australia. Proc Vertebr Pest Conf. https://doi.org/10.5070/V425110488
Bull J, Nilsen EB, Mysterud A, Milner-Gulland EJ (2009) Survival on the border: a population model to evaluate management options for Norway’s wolves Canis lupus. Wildl Biol 15(4):412–424. https://doi.org/10.2981/08-010
Burbidge AA, Manly BFJ (2002) Mammal extinctions on Australian islands: causes and conservation implications. J Biogeogr 29(4):465–473. https://doi.org/10.1046/j.1365-2699.2002.00699.x
Byers KA, Lee MJ, Patrick DM, Himsworth CG (2019) Rats about town: a systematic review of rat movement in urban ecosystems. Front Ecol Evol 7:13. https://doi.org/10.3389/fevo.2019.00013
Comer S, Speldewinde P, Tiller C, Clausen L, Pinder J, Cowen S, Algar D (2018) Evaluating the efficacy of a landscape scale feral cat control program using camera traps and occupancy models. Sci Rep 8(1):5335. https://doi.org/10.1038/s41598-018-23495-z
Corbett LK (1979) Feeding ecology and social organization of wildcats (Felis silvestris) and domestic cats (Felis catus) in Scotland. Dissertation, University of Aberdeen.
Courchamp F, Langlais M, Sugihara G (1999) Cats protecting birds: modelling the mesopredator release effect. J Anim Ecol 68(2):282–292. https://doi.org/10.1046/j.1365-2656.1999.00285.x
Cox DR, Oakes D (1984) Analysis of Survival Data, 1st edn. Chapman and Hall/CRC, New York. https://doi.org/10.1201/9781315137438
Crowell-Davis SL (2007) Cat behaviour: Social organization, communication and development. In: Rochlitz I (ed) The welfare of cats, vol 3. Springer, Netherlands, pp 1–22. https://doi.org/10.1007/978-1-4020-3227-1_1
Danner RM, Farmer C, Hess SC, Stephens RM, Banko PC (2010) Survival of feral cats, Felis catus (Carnivora: Felidae), on Mauna Kea, Hawai’i, based on tooth cementum lines. Pac Sci 64(3):381–389. https://doi.org/10.2984/64.3.381
Devillard S, Say L, Pontier D (2003) Dispersal pattern of domestic cats (Felis catus) in a promiscuous urban population: do females disperse or die? J Anim Ecol 72(2):203–211. https://doi.org/10.1046/j.1365-2656.2003.00692.x
Dickman CR, Newsome TM (2015) Individual hunting behaviour and prey specialisation in the house cat Felis catus: implications for conservation and management. Appl Anim Behav Sci 173:76–87. https://doi.org/10.1016/j.applanim.2014.09.021
Doherty TS, Davis RA, van Etten EJB, Algar D, Collier N, Dickman CR, Edwards G, Masters P, Palmer R, Robinson S (2015) A continental-scale analysis of feral cat diet in Australia. J Biogeogr 42(5):964–975. https://doi.org/10.1111/jbi.12469
Dubey JP (2002) A review of toxoplasmosis in wild birds. Vet Parasitol 106(2):121–153. https://doi.org/10.1016/S0304-4017(02)00034-1
Dunning JB, Stewart DJ, Danielson BJ, Noon BR, Root TL, Lamberson RH, Stevens EE (1995) Spatially explicit population models: current forms and future uses. Ecol Appl 5(1):3–11. https://doi.org/10.2307/1942045
Edwards GP, De Preu N, Shakeshaft BJ, Crealy IV, Paltridge RM (2001) Home range and movements of male feral cats (Felis catus) in a semiarid woodland environment in central Australia. Austral Ecol 26(1):93–101. https://doi.org/10.1111/j.1442-9993.2001.01091.pp.x
Fancourt BA, Cremasco P, Wilson C, Gentle MN (2019) Do introduced apex predators suppress introduced mesopredators? A multiscale spatiotemporal study of dingoes and feral cats in Australia suggests not. J Appl Ecol 56(12):2584–2595. https://doi.org/10.1111/1365-2664.13514
Fisher DO, Johnson CN, Lawes MJ, Fritz SA, McCallum H, Blomberg SP, VanDerWal J, Abbott B, Frank A, Legge S, Letnic M, Thomas CR, Fisher A, Gordon IJ, Kutt A (2014) The current decline of tropical marsupials in Australia: is history repeating? Glob Ecol Biogeogr 23(2):181–190. https://doi.org/10.1111/geb.12088
Fitzgerald BM (1988) Diet of domestic cats and their impact on prey populations. In: Turner D, Bateson P (eds) Domestic cat: the biology of its behaviour. Cambridge University Press, Cambridge, UK, pp 123–144
Fleming PJS, Allen LR, Lapidge SJ, Robley A, Saunders GR, Thomson PC (2006) A strategic approach to mitigating the impacts of wild canids: proposed activities of the Invasive Animals Cooperative Research Centre. Aust J Exp Agric 46(7):753. https://doi.org/10.1071/EA06009
Fleming PA, Crawford HM, Auckland CH, Calver MC (2020) Body size and bite force of stray and feral cats: are bigger or older cats taking the largest or more difficult-to-handle prey? Animals 10(4):707. https://doi.org/10.3390/ani10040707
Fleming PA, Stobo-Wilson AM, Crawford HM, Dawson SJ, Dickman CR, Doherty TS, Fleming PJS, Newsome TM, Palmer R, Thompson JA, Woinarski JCZ (2022) Distinctive diets of eutherian predators in Australia. R Soc Open Sci 9(10):220792. https://doi.org/10.1098/rsos.220792
Gaillard J-M, Festa-Bianchet M, Yoccoz NG (1998) Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol Evol 13(2):58–63. https://doi.org/10.1016/S0169-5347(97)01237-8
Gehrt SD, Wilson EC, Brown JL, Anchor C (2013) Population ecology of free-roaming cats and interference competition by coyotes in urban parks. PLoS ONE 8(9):e75718. https://doi.org/10.1371/journal.pone.0075718
Glen AS, Dickman CR (2005) Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol Rev 80(03):387. https://doi.org/10.1017/S1464793105006718
Hunter DO, Lagisz M, Leo V, Nakagawa S, Letnic M (2018) Not all predators are equal: a continent-scale analysis of the effects of predator control on Australian mammals. Mammal Rev 48(2):108–122. https://doi.org/10.1111/mam.12115
Jansen J, McGregor H, Axford G, Dean AT, Comte S, Johnson CN, Moseby KE, Brandle R, Peacock DE, Jones ME (2021) Long-distance movements of feral cats in semi-arid south Australia and implications for conservation management. Animals 11(11):3125. https://doi.org/10.3390/ani11113125
Jones Coman EBJ (1982) Ecology of the feral cat, Felis catus (L.), in south-eastern Australia III.* Home ranges and population ecology in semiarid north-west Victoria. Aus Wildl Res 9:409–420
Jones AL, Downs CT (2011) Managing feral cats on a university’s campuses: how many are there and is sterilization having an effect? J Appl Anim Welfare Sci 14(4):304–320. https://doi.org/10.1080/10888705.2011.600186
Kaeuffer R, Pontier D, Devillard S, Perrin N (2004) Effective size of two feral domestic cat populations (Felis catus L.): effect of the mating system. Mol Ecol 13(2):483–490. https://doi.org/10.1046/j.1365-294X.2003.02046.x
Kalbfleisch JD, Prentice RL (2002) The statistical analysis of failure time data, 2nd edn. John Wiley, New York
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53(282):457–481. https://doi.org/10.1080/01621459.1958.10501452
Kays R, Dunn RR, Parsons AW, Mcdonald B, Perkins T, Powers SA, Shell L, McDonald JL, Cole H, Kikillus H, Woods L, Tindle H, Roetman P (2020) The small home ranges and large local ecological impacts of pet cats. Anim Conserv 23(5):516–523. https://doi.org/10.1111/acv.12563
Kennedy M, Phillips BL, Legge S, Murphy SA, Faulkner RA (2012) Do dingoes suppress the activity of feral cats in northern Australia? Austral Ecol 37(1):134–139. https://doi.org/10.1111/j.1442-9993.2011.02256.x
Kutt AS (2012) Feral cat (Felis catus) prey size and selectivity in north-eastern Australia: implications for mammal conservation. J Zool 287(4):292–300. https://doi.org/10.1111/j.1469-7998.2012.00915.x
Langham N, Porter R (1991) Feral cats (Felis catus L.) on New Zealand farmland I. Home range. Wildl Res 18(6):741
Legge S, Murphy BP, McGregor H, Woinarski JCZ, Augusteyn J, Ballard G, Baseler M, Buckmaster T, Dickman CR, Doherty T, Edwards G, Eyre T, Fancourt BA, Ferguson D, Forsyth DM, Geary WL, Gentle M, Gillespie G, Greenwood L, Zewe F (2017) Enumerating a continental-scale threat: how many feral cats are in Australia? Biol Conserv 206:293–303. https://doi.org/10.1016/j.biocon.2016.11.032
Lepczyk CA, Calver MC (2022) Cat got your tongue? The misnomer of ‘community cats’ and its relevance to conservation. Biol Invasions 24(8):2313–2321. https://doi.org/10.1007/s10530-022-02788-5
Lepczyk CA, Fantle-Lepczyk JE, Dunham KD, Bonnaud E, Lindner J, Doherty TS, Woinarski JCZ (2023) A global synthesis and assessment of free-ranging domestic cat diet. Nat Commun 14(1):7809. https://doi.org/10.1038/s41467-023-42766-6
Letnic M, Koch F, Gordon C, Crowther MS, Dickman CR (2009) Keystone effects of an alien top-predator stem extinctions of native mammals. Proc R Soc B Biol Sci 276(1671):3249–3256. https://doi.org/10.1098/rspb.2009.0574
Levi T, Wilmers CC (2012) Wolves–coyotes–foxes: a cascade among carnivores. Ecology 93(4):921–929. https://doi.org/10.1890/11-0165.1
Liberg O, Sandell M, Pontier D, Natoli E (2000) Density, spatial organisation and reproductive tactics in the domestic cat and other felids. In: Turner D, Bateson P (eds) The domestic cat: the biology of its behaviour. Cambridge University Press, Cambridge, pp 119–147
Loss SR, Will T, Marra PP (2013) The impact of free-ranging domestic cats on wildlife of the United States. Nat Commun 4(1):1396. https://doi.org/10.1038/ncomms2380
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. The Invasive Species Specialist Group (ISSG).
Marlow NJ, Thomas ND, Williams AAE, Macmahon B, Lawson J, Hitchen Y, Angus J, Berry O (2015) Cats (Felis catus) are more abundant and are the dominant predator of woylies (Bettongia penicillata) after sustained fox (Vulpes vulpes) control. Aust J Zool 63(1):18. https://doi.org/10.1071/ZO14024
Matthews A, Ruykys L, Ellis B, FitzGibbon S, Lunney D, Crowther MS, Glen AS, Purcell B, Moseby K, Stott J, Fletcher D, Wimpenny C, Allen BL, Van Bommel L, Roberts M, Davies N, Green K, Newsome T, Ballard G, Wiggins N (2013) The success of GPS collar deployments on mammals in Australia. Aust Mammal 35(1):65. https://doi.org/10.1071/AM12021
McGregor HW, Legge S, Jones ME, Johnson CN (2014) Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS ONE 9(10):e109097. https://doi.org/10.1371/journal.pone.0109097
McGregor H, Legge S, Jones ME, Johnson CN (2015) Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS ONE 10(8):e0133915. https://doi.org/10.1371/journal.pone.0133915
McGregor HW, Legge S, Jones ME, Johnson CN (2016) Extraterritorial hunting expeditions to intense fire scars by feral cats. Sci Rep 6(1):22559. https://doi.org/10.1038/srep22559
McGregor H, Moseby K, Johnson CN, Legge S (2020) The short-term response of feral cats to rabbit population decline: are alternative native prey more at risk? Biol Invasions 22(2):799–811. https://doi.org/10.1007/s10530-019-02131-5
Medina FM, Bonnaud E, Vidal E, Tershy BR, Zavaleta ES, Josh Donlan C, Keitt BS, Corre M, Horwath SV, Nogales M (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Change Biol 17(11):3503–3510. https://doi.org/10.1111/j.1365-2486.2011.02464.x
Medina FM, Bonnaud E, Vidal E, Nogales M (2014) Underlying impacts of invasive cats on islands: not only a question of predation. Biodivers Conserv 23(2):327–342. https://doi.org/10.1007/s10531-013-0603-4
Mirmovitch V (1995) Spatial organisation of urban feral cats (Felis catus) in Jerusalem. Wildl Res 22(3):299. https://doi.org/10.1071/WR9950299
Molsher R, Newsome AE, Newsome TM, Dickman CR (2017) Mesopredator management: effects of red fox control on the abundance, diet and use of space by feral cats. PLoS ONE 12(1):e0168460. https://doi.org/10.1371/journal.pone.0168460
Moro D, Byrne M, Kennedy M, Campbell S, Tizard M (2018) Identifying knowledge gaps for gene drive research to control invasive animal species: the next CRISPR step. Glob Ecol Conserv 13:e00363. https://doi.org/10.1016/j.gecco.2017.e00363
Moseby KE, Stott J, Crisp H (2009) Movement patterns of feral predators in an arid environment: implications for control through poison baiting. Wildl Res 36(5):422. https://doi.org/10.1071/WR08098
Moseby KE, Crowther MS, Letnic M (2019) Ecological role of an apex predator revealed by a reintroduction experiment and Bayesian statistics. Ecosystems 22(2):283–295. https://doi.org/10.1007/s10021-018-0269-6
Moseby KE, McGregor H, Read JL (2020) The lethal 23%: predator demography influences predation risk for threatened prey. Anim Conserv 24(2):217–229. https://doi.org/10.1111/acv.12623
Newsome TM, Ripple WJ (2014) A continental scale trophic cascade from wolves through coyotes to foxes. J Anim Ecol 84(1):49–59. https://doi.org/10.1111/1365-2656.12258
Nogales M, Vidal E, Medina FM, Bonnaud E, Tershy BR, Campbell KJ, Zavaleta ES (2013) Feral cats and biodiversity conservation: the urgent prioritization of island management. Bioscience 63(10):804–810. https://doi.org/10.1525/bio.2013.63.10.7
Nottingham C, Buckley H, Case B, Glen A, Stanley M (2022) Factors affecting home range size of feral cats: A meta-analysis. N Z J Ecol 46(2):3476. https://doi.org/10.20417/nzjecol.46.26
Nutter FB, Levine JF, Stoskopf MK (2004) Reproductive capacity of free-roaming domestic cats and kitten survival rate. J Am Vet Med Assoc 225(9):1399–1402. https://doi.org/10.2460/javma.2004.225.1399
Payton ME, Greenstone MH, Schenker N (2003) Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci. https://doi.org/10.1093/jis/3.1.34
Prugh LR, Sivy KJ (2020) Enemies with benefits: integrating positive and negative interactions among terrestrial carnivores. Ecol Lett 23(5):902–918. https://doi.org/10.1111/ele.13489
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Read J, Bowen Z (2001) Population dynamics, diet and aspects of the biology of feral cats and foxes in arid South Australia. Wildl Res 28(2):195. https://doi.org/10.1071/WR99065
Rees MW, Pascoe JH, Le Pla M, Robley A, Birnbaum EK, Wintle BA, Hradsky BA (2023) Mesopredator release among invasive predators: controlling red foxes can increase feral cat density and alter their behaviour. J Appl Ecol 1365–2664:14402. https://doi.org/10.1111/1365-2664.14402
Risbey DA, Calver MC, Short J, Bradley JS, Wright IW (2000) The impact of cats and foxes on the small vertebrate fauna of Heirisson Prong, Western Australia. II. A field experiment. Wildl Res 27(3):223. https://doi.org/10.1071/WR98092
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12(9):982–998. https://doi.org/10.1111/j.1461-0248.2009.01347.x
Roshier DA, Carter A (2021) Space use and interactions of two introduced mesopredators, European red fox and feral cat, in an arid landscape. Ecosphere. https://doi.org/10.1002/ecs2.3628
Sashegyi A, Ferry D (2017) On the Interpretation of the hazard ratio and communication of survival benefit. Oncologist 22(4):484–486. https://doi.org/10.1634/theoncologist.2016-0198
Say L, Pontier D (2004) Spacing pattern in a social group of stray cats: effects on male reproductive success. Anim Behav 68(1):175–180. https://doi.org/10.1016/j.anbehav.2003.11.008
Schmidt PM, Lopez RR, Collier BA (2007) Survival, fecundity, and movements of free-roaming cats. J Wildl Manag 71(3):915–919. https://doi.org/10.2193/2006-066
Spruance SL, Reid JE, Grace M, Samore M (2004) Hazard ratio in clinical trials. Antimicrob Agents Chemother 48(8):2787–2792. https://doi.org/10.1128/AAC.48.8.2787-2792.2004
Stenseth NC, Leirs H, Mercelis S, Mwanjabe P (2001) Comparing strategies for controlling an African pest rodent: an empirically based theoretical study. J Appl Ecol 38(5):1020–1031. https://doi.org/10.1046/j.1365-2664.2001.00656.x
Stobo-Wilson AM, Murphy BP, Legge SM, Caceres-Escobar H, Chapple DG, Crawford HM, Dawson SJ, Dickman CR, Doherty TS, Fleming PA, Garnett ST, Gentle M, Newsome TM, Palmer R, Rees MW, Ritchie EG, Speed J, Stuart J, Suarez-Castro AF, Woinarski JCZ (2022) Counting the bodies: estimating the numbers and spatial variation of Australian reptiles, birds and mammals killed by two invasive mesopredators. Divers Distrib 28(5):976–991. https://doi.org/10.1111/ddi.13497
Therneau TM (2014) Package “survival”: survival analysis. https://cran.r-project.org/package=survival
Therneau TM (2022) Package “coxme”: mixed effects cox models. https://cran.r-project.org/package=coxme
Van Aarde RI (1983) Demographic parameters of the feral cat Felis catus population at Marion Island. S Afr J Wild Res 13(1):12–16
Woinarski JCZ, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proc Natl Acad Sci 112(15):4531–4540. https://doi.org/10.1073/pnas.1417301112
Woinarski J, Legge S, Dickman CR (2019) Cats in Australia: companion and killer. CSIRO Publishing, Melbourne
Acknowledgements
This project was generously supported by the Australian Government through the Australian Research Council's Linkage Projects funding scheme (LP170101134). The authors declare that they have no conflict of interest.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Australian Research Council, LP170101134, Bronwyn Hradsky.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Menon, V., McGregor, H., Giljohann, K. et al. Ecological factors influencing invasive predator survival and movement: insights from a continental-scale study of feral cats in Australia. Biol Invasions 26, 1505–1520 (2024). https://doi.org/10.1007/s10530-024-03254-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-024-03254-0