Abstract
Local predators are often considered effective and environmentally-friendly control agents to limit invasive species. Such biological control, however, depends on both the predator prey preferences and performances, which are in turn affected by variations in the physical environment. This study investigated the predation of the swimming crab Thalamita danae on the invasive mussel Xenostrobus securis under different salinity and thermal conditions. Xenostrobus securis, which is native to Australia, has spread to Hong Kong since the last decade, causing adverse ecological and economic impacts. Laboratory experiments evaluated the prey preferences and feeding behaviours of the crab on both the native mussel Brachidontes variabilis and the invasive X. securis under different typical salinities (15, 25, and 35‰) and temperatures (22 and 28 °C). The crab did not show clear preference toward either the invasive or the native mussels. Although the shell morphology of the invasive mussels lowered handling time as compared to the native mussels, the crab consumption rate was similar between the mussel species. The survival and predation rate of the crab were, however, substantially reduced under low salinities (< 15‰) where X. securis could be found. Thalamita danae, therefore, is a potential predator of X. securis, but such predation is only possible under normal, oceanic conditions. In hyposaline, estuarine/ freshwater environments where X. securis can survive, however, T. danae performs poorly and, as a result, such physical conditions may represent a predator refuge for the mussels to invade local ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasion is considered one of the most important direct drivers for the decline of global biodiversity in recent decades (Kolar and Lodge 2001; Perrings 2001; Louette 2012). Invasive species can replace or even extirpate native species via niche displacement, hybridization and exploitation (i.e. predation and parasitism), altering community structure and ecological processes (Ruiz et al. 1997; Streftaris et al. 2005). Some of these ecological impacts can further disrupt ecosystem services causing adverse economic impacts (Perrings 2001). For example, the accidental introduction of the warty comb jellyfish Mnemiopsis leidyi A. Agassiz 1865 to the Black Sea led to a dramatic drop in local fish populations, causing a loss of around USD$250 million in the fisheries industry (Ruiz et al. 1997; Anil et al. 2002). Owing to the tremendous growth of international trade, the problem of biological invasion has worsened (Ruiz et al. 2000) and become a pressing issue prompting governments around the world to formulate preventive and mitigation measures for invasive species management (McNeely 2000; Law 2006).
Despite the fact that invasive populations can be rapidly removed by biocides over a large area, biocides have a high environmental footprint and may impact non-targeted species (Wittenberg and Cork 2005; Giakoumi et al. 2019). For this reason, the biological control role of native generalist predators has been increasingly investigated to develop sustainable and environmentally-friendly approaches for bioinvasion management (Wittenberg and Cork 2005; Myers and Cory 2017). According to the “enemy release hypothesis” (Keane and Crawley 2002), however, generalist predators may facilitate the establishment of invasive species by exerting a greater predation pressure on native rather than invasive prey (Colautti et al. 2004; Veiga et al. 2011; Naddafi and Rudstam 2014). Thus, to be able to adopt native predators as a biological control agent, quantifying predator prey preferences is a key consideration to assess if predators are facilitating or deterring invasive prey (Lai 1996; Veiga et al. 2011).
Prey preferences are dependent on prey profitability, which is traditionally defined as the energy gain per unit handling time when predators prey and feed on their prey (Elner and Hughes 1978; Hughes and Seed 1981). The profitability of a prey is influenced by a combination of factors, such as species (Dudas et al. 2005; Veiga et al. 2011), size (Elner and Hughes 1978; Hughes and Seed 1981), and habitat type (Sponaugle and Lawton 1990; Eggleston et al. 1992). These factors are considered important criteria for selecting prey based on optimal foraging theory (Krebs 1980). Selecting the optimal set of prey allows predators to maximize their energy gain per unit time, and to achieve that, there are two general strategies adopted by predators depending on the physical environment: energy maximization and time minimization (Elner and Hughes 1978; Hughes and Seed 1981; Seed and Hughes 1995). Energy maximizing predators spend more time on foraging and consume prey with acceptable profitability to maximize their net energy gain, particularly in benign environments, while time minimizers devote less time on predation and obtain less energy to minimize the time exposed to danger and environmental stresses (Seed and Hughes 1995). As a result, apart from prey preference, the physical environment is another key factor which may impact the effectiveness of native predators in biological control via indirect effects on their foraging strategies.
In the intertidal zone, temperature and salinity are two key physical environmental factors determining the performance, survival and distribution of predators and prey (Kinne 1966; Garton and Stickle 1980). The metabolism of ectothermic predators generally elevates with ambient temperature, causing an increase in energy demand and allowing them to attack, handle and process prey items at faster rates (Wallace 1973; Bergman 1987; Barbeau and Scheibling 1994). Similarly, changes in salinity also causes an elevation in predators’ metabolism to maintain internal osmotic balance, leading to increases in energy demand and food consumption (Taylor et al. 1977; Curtis et al. 2010). Although such changes in temperature and salinity can drive higher predation rates, extreme variations in these factors can, conversely, cause physiological stress and impact survival of predators (McGaw and McMahon 1996; McGaw 2007). Severe environmental stress also can lower predation rates indirectly by limiting locomotion, and thus decreasing the predator–prey encounter rate (Draper and Weissburg 2022).
The black pygmy mussel Xenostrobus securis (Lamarck 1819) is native to estuarine habitats of Oceania (Wilson 1968), but invasions of this species have been documented in Southern Europe and East Asia since the last century (Lazzari and Rinaldi 1994; Sabelli 1993; De Min and Vio 1997; Kimura et al. 1999; Shirafuji and Sato 2003; Garci et al. 2007; Pascual et al. 2010), and in the Mediterranean Sea this mussel is considered as one of the 100 “worst” marine invasive species (Streftaris and Zenetos 2006). Xenostrobus securis was first observed in the Shing Mun River and inner Tolo Habour of Hong Kong in 2010 and was suggested to be introduced via the ballast water of maritime vessels (Morton and Leung 2015). Xenostrobus securis has extremely wide thermal and osmotic tolerance ranges from 5 to 32 °C (Morton and Leung 2015; Astudillo et al. 2017) and 1–31‰ (Wilson 1968), allowing the population to spread rapidly across the hydrological gradient from the eastern (oceanic) to the western (estuarine) waters of Hong Kong (Astudillo 2015; Lau et al. 2018). The growth of X. securis in Hong Kong has resulted in this species outcompeting the native mussel, Arcuatula senhousia (Benson 1842), and also increasing the mortality of the cultured oyster, Magallana hongkongensis (Lam & Morton 2003), resulting in an estimated loss of more than HKD$2.4 million (approximately USD$304 thousands) to the local oyster production industry (Lau et al. 2018).
In spite of the rapid expansion and devastating effects of X. securis, there has been little discussion on how to manage invasion by this species in Hong Kong. Astudillo et al. (2018) examined the use of the whelk Reishia clavigera (Küster 1860) as a native predator to control X. securis. Although the whelk consumed more invasive mussels (X. securis and Mytilopsis sallei (Récluz 1849)) than the native species (Brachidontes variabilis (Krauss 1848)), the consumption rate was significantly decreased when the whelk was exposed to salinities below 22‰, conditions where X. securis is still able to survive and reproduce (Astudillo et al. 2018). As a result, the invasive mussels were effectively exempted from predation by the native whelk depending on the salinity of ambient waters.
Apart from whelks, swimming crabs in the family Portunidae are another common predator of bivalves in Hong Kong. In particular, the swimming crab, Thalamita danae Stimpson 1858, which is one of the most abundant and common predatory crabs in Hong Kong, has been shown to consume bivalves as its dominant prey (Lai 1996). Hence, considering the abundance of the swimming crab in the eastern and southern waters of Hong Kong (Lai 1996) and the fact that the crab preys primarily on bivalves, this paper investigates the prey preference of T. danae on the invasive mussel X. securis under different physical conditions (thermal and salinity stresses typically found in local waters). The predation rate on, and prey profitability of both the native B. variabilis and invasive X. securis were also compared to determine the differences in prey preference of the crab. By evaluating performances and feeding behaviours in different hydrological conditions, our study assessed the potential role of T. danae as a predator of the invasive X. securis in this newly colonised area, and how environmental conditions may modify such predation, in order to inform mitigation measures for bioinvasion management in the future.
Materials and methods
Sample collection and maintenance
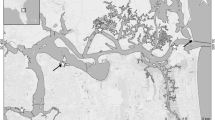
The invasive mussel Xenostrobus securis is found in the low intertidal to subtidal zones of Hong Kong, and is widespread among estuarine (Deep Bay, Lau et al. 2018) and sheltered shores (inner Tolo Harbour, Morton and Leung 2015; Kwun Tong typhoon shelter, Astudillo et al. 2018) where it is often sympatric with the native mussel Brachidontes variabilis in the low intertidal zone (Deep Bay, Tolo Harbour and Mirs Bay, Huang et al. 1992). In contrast, the native swimming crab, Thalamita danae, is mainly distributed along the oceanic shore of eastern and southern waters, such as Mirs Bay, Tolo Harbour, Port Shelter and Tai Tam Bay (Lai 1996) and there are no records of this crab in the western estuarine waters. The eastern oceanic coast, therefore, is the only region where the distribution of the crab and the two mussels overlap. For this study, adult crabs were collected in Starfish Bay, Hong Kong (22°26′N, 114°14′E, Fig. 1) at around 0.7–1.0 m above Chart Datum (C.D.) during low tide periods from September to December 2021 for different experiments. Starfish Bay is a sheltered sandy shore fringed by rocky, boulder substrates that are habitats for T. danae (Lai 1996). In Starfish Bay where the crab was abundant, however, both native and invasive mussels were uncommon and, therefore, mussels were collected from Island House, Hong Kong (22°26’N, 114°10’E, 6.8 km from Starfish Bay) at approximately 1.0–1.5 m above C.D. (Figs. 1 and S1).
Hong Kong showing the distributions and sampling sites of the targeted animals. Black triangles represent the native predatory crab Thalamita danae (after Lai 1996) and the grey triangle Starfish Bay (the sampling site for T. danae). Black circles represent the invasive mussel Xenostrobus securis (after Morton and Leung 2015; Astudillo et al. 2017; Lau et al. 2018) and the grey circle Island House (the sampling site for X. securis and Brachidontes variabilis)
All collected animals were maintained in the Swire Institute of Marine Science, The University of Hong Kong until experimentation. Brachidontes variabilis and X. securis were cleaned of epibionts, sorted and held in plastic containers (19.5 cm × 12.5 cm × 11 cm each) at a density of 40 individuals per container. The mussels were maintained under aerated, natural seawater (22 ± 2 °C and 32 ± 2‰, changed twice a week) and a photoperiod of 12 h:12 h day-night cycle. The mussels were fed with a commercial algae-based soft coral food (Reef Phytoplankton, Seachem, USA) every other day. A total 42 crabs were collected (12 for tolerance limits experiment, 18 for prey preference experiments, and 12 for the handling time experiments). Collected crabs were maintained in conditions as above, except in larger containers (26 cm × 16 cm × 13 cm) at a density of 1 individual per container. Instead of a fixed thermal and salinity regime, crabs in each container were randomly assigned to one of the acclimation treatments (see below). Ample amounts of commercial dried shrimp turtle food (Sun Dried Red Shrimp, Zoo Med, USA) instead of collected mussels were offered every other day to the crabs to avoid influencing their prey preference for different mussels (Cunningham and Hughes 1984).
Effects of acute temperature and salinity stresses on T. danae
The tolerance limits of T. danae were assessed under the combined stresses of temperature and salinity in conditions representative of habitats in Hong Kong (temperature: ranging from 17.5 to 31.0 °C, salinity: ranging from 5.4 to 33.2‰, EPD 2022), in order to determine the conditions used in the predation experiments. Only salinities above the lethal level (as indicated by the salinity where 50% of mortality occurred within 7 days) and temperatures below the Arrhenius breakpoint temperature (ABT) were selected, such that foraging activities are plausible without the crabs being killed by extreme environmental conditions. To achieve this, each of the 12 crabs were measured (30.1–58.2 mm carapace width) and randomly assigned to one of the four salinity treatments (5, 15, 25, and 35‰, n = 3 per treatment, all maintained at 22 °C) for a week and subsequently exposed to simulated thermal stress. Salinities were adjusted to the target levels by diluting natural seawater with Milli-Q water. The mortalities of T. danae in each treatment were checked daily to determine their survival under different salinities during the acclimation. The crabs were then exposed to an acute thermal ramp after the acclimation and their heart rates were measured to assess their physiological performances (Marshall et al. 2011; Burnett et al. 2013). Specifically, the crabs were taken out from the acclimation containers and introduced into a temperature-controlled waterbath (GP200, Grant Instruments, UK). The crabs were held separately in small plastic containers, in air, inside the waterbath, which heated the water from room temperature (25 °C) to 45 °C at a ramping rate of 1 °C per 5 min (total duration ~ 100 min) to simulate the natural rate of rock temperature increase in the intertidal zone of Hong Kong during emersion periods (after Luk 2014), in order to examine physiological responses under the maximum heating rate the crab could experience on the shore. A fine-tipped thermocouple (K-type, Omega) was inserted into the thorax coxopodite joint of an additional crab inside the waterbath to measure the crabs’ body temperatures with a digital thermometer (± 0.01 °C, TM-947SD, Lutron, Taiwan). Body temperatures of the crabs increased from 22 to 25 °C over 24 min when introduced into the containers and, during the thermal ramp increased at a rate of 0.6 °C per 5 min.
A non-invasive infrared sensor technique was adopted to monitor the heart rates of the crabs during the thermal ramp (after Depledge and Andersen 1990; see Burnett et al. 2013). A reflective optical sensor (CYN70, Vishay Semiconductors, USA) was glued (using cyanoacrylate glue) onto the crabs’ carapaces directly above the heart for real-time heart rate acquisition. An amplifier (AMP03, Newshift, Portugal) was used to amplify and filter the signals from the sensor, which were then transmitted to a PC-based oscilloscope (Picoscope 2204, Pico Technology, UK) before being recorded in the computer. The acute thermal ramp was ended when heartbeats of the crabs ceased at high temperatures. Recorded heartbeats were counted every twenty seconds to obtain heart rate expressed in hertz (beats per second, Σn = 4 salinity treatments × 3 replicates = 12 crabs in total).
Prey preference
Consumption rates and prey preference on the native B. variabilis and the invasive X. securis by T. danae were quantified for crabs acclimated under different environmental conditions. Each of 18 crabs were measured (36.6–67.5 mm carapace width) and randomly assigned to six treatments: crossing two temperatures (22 and 28 °C) and three salinities (15, 25, and 35‰, n = 3 per treatment) for a week (in acclimation containers as described above). Salinities and temperatures were adjusted to the target levels by diluting seawater with Milli-Q water and aquarium immersion heaters (Aquael, Poland) inside the acclimation containers, respectively. The crabs were fed as previously described but food supply was stopped 72 h prior to predation tests to standardize the satiation level of the crabs.
To quantify the crabs’ prey preferences, individuals were taken out from the acclimation containers and subjected to both with-choice and without-choice tests with the presence of both (with-choice) or one species (without-choice) of mussels (Underwood and Clarke 2005). The logic of comparing with- and without-choice tests is to quantify preferences unconfounded by consumption of prey when they are presented to the predator under the laboratory setting (as derived from the without-choice test, see Underwood and Clarke 2005). Control tests with only the mussel prey but not the crabs were also conducted to assess natural mortalities of the prey during the experimental period (Astudillo et al. 2018).
In the without-choice test, the crabs were provided with only one type of mussel prey: either ten native B. variabilis or ten invasive X. securis per container (Σn = 2 predator presence conditions (with or without crabs) × 2 prey × 2 temperatures × 3 salinities × 3 replicates = 72 tests in total). In the with-choice test, the same crabs were exposed to both types of mussel prey simultaneously: five native B. variabilis and five invasive X. securis per container (Σn = 2 predator presence conditions (with or without crabs) × 2 temperatures × 3 salinities × 3 replicates = 36 tests in total). Overall, ten mussel individuals were offered to the crab in each test. All predation tests were conducted in feeding arenas (26 cm × 16 cm × 13 cm each, with an opaque covering to avoid visual disturbance) with seawater at the same temperature and salinity as the crabs experienced in the acclimation containers. The tests lasted for 24 h, and the number of mussels being consumed was counted (defined as the complete absence of flesh in the shell; partial consumption was not counted). Every crab in this experiment underwent three predation tests in a randomized manner over time, including a without-choice test for B. variabilis, a without-choice test for X. securis, and a with-choice test for both mussel prey. After every test, the crabs were transferred back to the acclimation container and allowed to rest for at least three days between tests.
Handling time and mussel profitability
Predatory behaviour and handling time (breaking time plus feeding time) of the crabs were quantified to assess profitability of each type of mussel prey using timelapse videos. Each of the 12 crabs were measured (37.6–64.8 mm carapace width) and randomly assigned to acclimate in one of the four treatments crossing two temperatures (22 and 28 °C) and two salinities (25 and 35‰, n = 3 per treatment) for a week. Individuals were then taken out from the acclimation containers and provided with either one native B. variabilis or one invasive X. securis in the feeding arena setup as described above (Σn = 2 prey × 2 temperatures × 2 salinities × 3 replicates = 24 tests in total) with water temperature and salinity set as the same conditions as in the acclimation containers. Total shell lengths of the offered mussels were measured (± 0.5 mm) and to standardize the prey size of the two mussel species, only mussels with shell lengths between 15 and 20 mm were used (see Fig. S2, supplementary information for mussel size distributions on the shore). Breaking time and feeding times of the crabs preying on the mussels were recorded using a timelapse camera (T45A, Campark, China) positioned over the top of the arenas. Breaking time was defined as the period from which the crab seized the mussel prey until the mussel shell was broken, while feeding time was defined as the period from the shell being opened to the point where empty shells or shell fragments were discarded (Hughes and Seed 1981). The experiment lasted for two hours during which the crabs completed feeding and abandoned the emptied shells. The handling times for both native B. variabilis and invasive X. securis were quantified for each crab once, which was then returned to the acclimation containers and rested for at least three days between feeding measurements. Profitability of each consumed mussel was then calculated as the ratio between estimated dry flesh weight (as estimated from shell length, see supplementary information) and recorded handling time (= breaking + feeding times, Fig. S3).
Data analysis
Acclimation mortality of the crabs used in the tolerance limit experiment was tested between salinity treatments via a generalized linear model with binomial distribution (logit link function). The variation in thermal tolerance of T. danae between different salinity treatments was compared using their Arrhenius breakpoint temperature (ABT) and flatline temperature (FLT) under the acute thermal ramp. ABT is considered an indicator of rapid alteration or decline in metabolic performances under heat stress (Cossins and Bowler 1987), while FLT represent the limits of physiological tolerance when the heart ceases to function. To determine ABT for each crab, segmented regressions were conducted on an Arrhenius graph with the natural logarithmic of heart rate (ln (HR)) as the response variable and 1000/(T (temperature measured in Kelvin scale)) as the explanatory variable, and typically at least one breakpoint would be shown in each crab depending on their thermal sensitivity. Segmented regression models with 1–7 breakpoints were fitted to these graphs and the Akaike information criteria (AIC) of the fitted models were used for model comparison. For each crab, the model with the lowest AIC value was adopted after which ABT was determined as the final breakpoint (Dahlhoff and Somero 1993; Stillman and Somero 1996). FLT was estimated using the normal plot (HR (Hz) vs T (temperature measured in °C)) by extrapolating the fitted regression line after the ABT to the x-intercept (i.e. when HR = 0, Dahlhoff and Somero 1993). ABT and FLT were compared among the salinity treatments using one-way ANOVAs followed by Tukey’s HSD post-hoc tests (variances within treatments were homogenous, Levene’s tests P > 0.05 in all cases).
The prey preference of the crabs on the native and invasive mussels was analysed following Underwood and Clarke (2005). Chi-squared tests were adopted to test for differences between observed and expected crab consumptions of each mussel species based on with- and without-choice tests. In the without-choice tests, the effects of salinity (fixed, three levels), temperature (fixed, two levels), crab size (fixed, continuous), mussel species (fixed, two levels), and their interactions on the consumption rate of the crabs (= proportion of mussels being consumed, as the response variable) were analysed with a linear mixed model using the lme4 package (Bates et al. 2015) in R. The effect of crab size was assessed by comparing models with or without crab size, and the model was adopted based on whether crab size significantly improved the model fit. This was a balanced repeated measures design, in which each crab underwent a without-choice test for B. variabilis, a without-choice test for X. securis, and a with-choice test for both mussel species. Due to the repeated use of the same crab between treatments, the crab identity was treated as a random factor (measurements using the same crabs were considered independent, however, due to at least three days of recovery time). Using the same set of explanatory variables (salinity, temperature, crab size, mussel species and their interactions), the breaking, feeding and handling times of the crabs on, as well as the profitability (estimated by dry weight/ handling time) of both mussel prey, were analysed using generalized linear mixed models with gamma distribution (log link function, via the glmmTMB package, Brooks et al. 2017). Gamma distribution was used since more variable responses were observed during data exploration when the mean responses were higher. This model was also a balanced repeated measures design, in which each crab was used once in each of the B. variabilis and X. securis handling time tests. Goodness-of-fit of the model was evaluated based on standardized residual diagnostics (using the package DHARMa in R, Florian 2022).
Results
Tolerance limits
The mortality rate of Thalamita danae acclimated in 5‰ salinity was significantly higher than in 15‰ salinity or above (Generalized linear model with binomial distribution, χ23 = 12.17, P < 0.01, Fig. 2). No mortality was noted in 35‰ during the seven days acclimation and > 75% of crabs in 25 and 15‰ survived throughout the acclimation. All the crabs exposed to 5‰, however, died within 48 h. Heart rates of crabs acclimated in 35 and 25‰ increased steadily with temperature following the log-linear pattern until the ABT was reached, after which the heart rate dropped abruptly to flatline. In contrast, the heart rates of all crabs acclimated to 15‰ depressed twice before reaching the final breakpoint under the acute thermal ramp (Fig. S4). Neither ABT nor FLT, however, differed between salinity treatments (ABT: one-way ANOVA, F2,6 = 1.44, P > 0.05; FLT: F2,6 = 0.84, P > 0.05, Fig. 3). On average, heart rates of the crabs increased with rising body temperatures but started to decline at 34.6 °C (ABT) and reached zero at 39.7 °C (FLT).
Prey preference
All mussels survived throughout the experiment in the control tests (i.e. in the absence of crabs). Thalamita danae predated and fed on both native Brachidontes variabilis and invasive Xenostrobus securis when offered these prey. The proportion of each mussel prey consumed during the without-choice tests was, however, similar to that of with-choice tests in nearly all 18 comparisons, indicating that the crab did not show any preference towards either the native or invasive mussels (Table 1). The only significant difference between observed and expected consumption was shown in one individual held at 15‰ and 28 °C (Table 1). For prey consumption in the without-choice tests, the consumption rate did not vary with crab size and, as a result, a more simple model involving only temperature, salinity, prey type and crab identity was adopted (see Table S1). No significant variation in the consumption rate between the native and the invasive mussels was found in the without-choice tests (linear mixed model, χ22 = 2.32, P > 0.05, see Table S1 for results). The proportion of mussels consumed, however, decreased significantly when the crabs were exposed to 15‰ (χ22 = 18.30, P < 0.01). The crabs consumed more than 55% of mussel prey under salinities of 25 and 35‰, whereas only around 6% of mussel prey were eaten when exposed to 15‰ (Fig. 4). There was a significant interaction among mussel species, temperature, and salinity in without-choice tests (χ22 = 6.25, P < 0.05, see Table S2 for post-hoc test results). Water temperature, however, did not significantly influence the consumption rate of the crabs (see Table S1).
Number of mussels consumed by various-sized crabs in without-choice tests for a period of 24 h after the crabs were acclimated to one of the combinations of two temperatures (22 and 28 °C) and three salinities (15, 25, and 35‰). The colour and shape of a point represent the temperature and salinity that the crab experienced, respectively (n = 3)
Handling time and mussel profitability
Crab spent nearly twice as long to handle the native than the invasive mussel (generalized linear mixed model, χ21 = 8.12, P < 0.01, Fig. 5c). This discrepancy was attributed to differences in both the breaking (χ21 = 6.87, P < 0.01, Fig. 5a) and feeding (χ21 = 27.04, P < 0.01, Fig. 5b) times. As a result, the invasive mussel had a significantly greater profitability to the crabs, producing approximately 0.03 mg/s higher rate of energy gain than the native mussel (χ21 = 71.43, P < 0.01, Fig. 5d). The breaking time of the crabs increased significantly when they were exposed at 28 °C (χ21 = 4.21, P < 0.05, Fig. 5a). The feeding time increased substantially when crabs were exposed to 25‰ (χ21 = 18.53, P < 0.01, Fig. 5b). The mussels, therefore, presented significantly lower profitability to the crabs under hypo-osmotic stress (χ21 = 3.93, P < 0.05, Fig. 5d). Significant interactions between crab size and the three fixed factors (species, temperature and salinity) were, however, found on the feeding time, in which larger crabs consumed the native mussel at a faster rate, especially under 28 °C or 35‰ (see Table S3 for the results of generalized linear mixed model, Fig. 5b). Correspondingly, the profitability of native mussels was more dependent on crab size as compared to invasive mussels (see Table S4 for results of generalized linear mixed model, Fig. 5d).
a Breaking time, b feeding time, c handling time and d profitability of various-sized crabs, Thalamita danae on both native and invasive mussels under the combination of two temperatures (22 and 28 °C) and two salinities (25 and 35‰). The colour and shape of a point represent the temperature and salinity that the crab experienced, respectively (n = 3)
Discussion
Physiological tolerance limits of Thalamita danae
The thermal and salinity tolerance limits of Thalamita danae were around 35 °C and 15‰, which are similar to those of other portunoid crabs (e.g. T. crenata, Rüppell 1830; Kannupandi et al. 1997; Azra et al. 2020; Liocarcinus depurator, Linnaeus 1758; Hopkin et al. 2006; and Necora puber, Linnaeus 1767; Hopkin et al. 2006). Such tolerance levels appear to explain the distribution of T. danae on the more oceanic east coast of Hong Kong (Lai 1996, see also Fig. 1), but not the estuarine west coast where salinity can drop to 5.4‰ during the summer due to monsoon rains and freshwater discharge from the Pearl River (Morton and Morton 1983; EPD 2022). The invasive mussel Xenostrobus securis, in contrast, is euryhaline and has been found in both eastern and western waters of Hong Kong (Astudillo 2015; Morton and Leung 2015; Lau et al. 2018). Given these environmental conditions, T. danae does not appear to be an appropriate biological control agent for X. securis in estuarine environments due to its low survival.
In contrast to previous studies on other crab species, thermal tolerance limits of T. danae as measured by ABT and FLT did not decline when salinity decreased from 35 to 15‰. In contrast, Tagatz (1969) found that hypo-osmotic stress significantly reduced thermal tolerance of the blue crab Callinectes sapidus Rathbun 1896, which is less tolerant to high temperatures when exposed to water at 6.8‰ as compared to 34‰. Such trade-off was apparently driven by the increased energy demand for osmoregulation under hypo-osmotic conditions (Todd and Dehnel 1960; Taylor et al. 1977), which reduces the physiological ability to cope with thermal stress and eventually leads to lower thermal tolerances (Todd and Dehnel 1960; Rome et al. 2005). This trade-off, however, does not seem to occur in T. danae, possibly because of differences in energy allocation. The heart rates of the crabs reduced when they were exposed to low salinity (15‰), and this reduction in metabolism may conserve energy to support physiological functions at high temperatures without compromising the crab’s thermal limits. Such a trait is potentially important for intertidal and shallow water species such as T. danae, where environmental fluctuations in salinity can be rapid and contingent upon local weather conditions. As such, being able to adjust physiological rates and energy allocation under such variable conditions may offer an adaptive advantage when compared to pelagic and/ or stenohaline conditions.
When exposed to low salinities, T. danae demonstrated a unique heart rate profile under an acute thermal ramp (heart rate dropped and increased again before the final breakpoint, in contrast to the typical unimodal profiles for crabs in 25 and 35‰). Such depressed heart rates upon thermal stress might result from metabolic depression which has been reported in the littorinid, Echinolittorina malaccana (Philippi, 1847), oyster, Isognomon nucleus (Lamarck 1819) and decapods such as Austrothelphusa transversa (von Martens 1868) (MacMillen nd Greenaway 1978; Alekseev and Starobogatov 1996; Marshall et al. 2011; Hui et al. 2020). In those cases the suppression of aerobic metabolism has been suggested to minimize energy expenditure during or when anticipating adverse environmental conditions (Marshall and McQuaid 1991; Guppy and Withers 1999). In the case of T. danae, heart rate was depressed at low salinity (15‰). Whilst hyposaline stress may directly impact circulatory functions leading to bradycardia (Uglow 1973; McGaw and McMahon 1996), energy conservation may offer a possible explanation for such metabolic depression at hyposaline conditions (Guppy and Withers 1999), as short term fluctuations in salinity are common in the intertidal and subtidal zones (Firth and Williams 2009; Williams et al. 2011).
Although heart rates of the crabs increased with temperature in air, handling time was longer for crabs feeding in warm (28 °C) as compared to cooler water (22 °C). The decline in activity rate at 28 °C may indicate the behavioural thermal limits of the crabs, which are ~ 7 °C lower than the mean ABT at 34.6 °C in T. danae. The decoupling of physiological performance and behaviour could be driven by the behavioural partitioning of the crabs that feed primarily when immersed by the tide and become less active during tidal emersion. In intertidal gastropods such partitioning across tidal phases has been suggested to result in a decoupling of thermal performance curves, where behavioural performance has lower thermal limits and peak at temperatures close to seawater temperature (Monaco et al. 2007; Marshall et al. 2011). The consumption rates of the crabs, however, remained similar between 22 and 28 °C, suggesting that despite the differences in handling time the crabs are consuming a similar number of prey to fulfill their energy demands under different water temperatures. Water temperature, therefore, appears to play a less important role than salinity in determining the predation pressure of the crabs on the mussels.
Prey preference of Thalamita danae
Vulnerability to crab predation was similar between the invasive and native mussels despite the significantly longer handling time for consuming the native mussel. The variation in handling time may be explained by a substantial difference in the shell morphology of the two species; the invasive mussel having a thinner subcylindrical shell (average shell thickness index = 0.59 mm) with straight dorsal and ventral margins (Astudillo et al. 2018; Garci et al. 2007), while the native mussel has a thicker triangular shell (shell thickness index = 0.72 mm) with a globular and inflated posterior margin (Astudillo et al. 2018; Morton et al. 2020, see Fig. S1). The crab, therefore, should need a greater compressive force to break the native mussel shells (Boulding 1984; Dudas et al. 2005). The round shell shape also makes handling of native mussels more difficult as they can easily slip from the crabs’ chelipeds (Dudas et al. 2005). As a consequence, the crab may shift from the common shell crushing method to the more time-consuming edge chipping method when feeding on native mussels (Fig. S5, Elner and Hughes 1978; Hughes and Seed 1981; Seed and Hughes 1995) and, therefore, the profitability of preying on the invasive mussel will be higher than the native mussel.
Previous studies indicated that crab size is another critical factor affecting the selection of prey items (Elner and Hughes 1978; Hughes and Seed 1981). The chela of larger crabs is bigger and more powerful, allowing them to open more resistant prey in a shorter time than smaller crabs (Hughes and Seed 1981; Seed and Hughes 1995). Hence, the size of mussels consumed should increase with crab size (Enderlein et al. 2003). There was, however, no crab size effect on mussel consumption or breaking time. The effects of crab size were only detected in their feeding time, where smaller crabs spent more time eating native mussels. This finding may be explained by the accumulation of shell fragments in the foregut (Hill 1976; Ap Rheinallt and Hughes 1985), which were ingested accidentally during mussel consumption. The clearance of such shell fragments is very slow, and the foregut of small crabs fills up quickly when feeding on prey with thick shells (Hill 1976). The average shell thickness index of the native mussel is 0.13 mm greater than that of the invasive mussel (Astudillo et al. 2018). More time, therefore, will be required for small crabs to eat the entire native mussel. This effect was enhanced when the crabs were exposed to high temperature or low salinity when food digestion poses extra physiological demands to the crab (McGaw 2006a, b). These demands can be fulfilled by increasing air uptake which, however, facilitates the exchange of water and ions, resulting in a high death rate (McGaw 2005, 2006b). To avoid mortality, the metabolic performance of crabs declines by reducing respiration and cardiac output when exposed to high temperature or low salinity, leading to a decrease in the foregut clearance rate (Draper et al. 2022).
Prey size also contributes to mussel selection of crabs. Seed and Hughes (1995), in a laboratory experiment, showed that crabs exhibit a strong preference for small mussels (10–15 mm), and more than one-third of the tested crabs failed to feed on mussels > 20 mm (Seed and Hughes 1995), probably due to long handling times and thus reduced profitability (Elner and Hughes 1978; Enderlein et al. 2003). Although mussel size was standardized in our experiments, on the shore, mussel consumption by crabs could be substantially influenced by prey size due to the co-existence of various-sized mussels (Elner and Hughes 1978; Huges and Seed 1981). The mean total length of the invasive X. securis (16.8 mm) is larger than the native Brachidontes variabilis (14.0 mm) at the Island House site (Fig. S2), which may allow for size escapes of the invasive species and result in greater consumption of the smaller native mussels by the crabs if prey size overrides species identity in determining profitability.
Apart from the prey species identity and predator size, anti-predatory responses of mussels, like byssal attachment and aggregation, will also influence predators’ prey selection and consumption on the shore. Cheung et al. (2004) found that the length and number of byssus threads increase under predation risk, which will enhance the mussels attachment strength and reduce the chance of them being removed from the substratum (Lin 1991). Reimer and Tedengren (1997) also reported that mussels growing in a clump have, overall, less exposed surface area which limits the handling strategies used by crabs, consequently reducing the efficiency of mussel consumption. The formation of clumps, however, depends heavily on the density of mussels (Kobak 2001; Bertolini et al. 2017). A high density of mussels will result in a complex aggregation with better protection against predation (Bertolini et al. 2017). The native B. variabilis and invasive X. securis exist in mixed patches and form multi-layered reefs with the local oyster Saccostrea cuccullata (Born 1778) at the study site (Fig. S6). Such mixed aggregations may further complicate the clump structure and alter predation by the crabs. Although we were unable to directly measure predation by T. danae on the two mussel species on the shore, we adopted a comparative approach to assess the handling time of crabs on each mussel species following Hughes (1979). Quantifying differences in handling time is key in determining the variation in profitability among prey items, an important consideration when predicting the diet of the crabs. The shorter handling time required and higher profitability of X. securis suggest that this mussel is likely to be one of the prey items of the crab. This paper, therefore, provides estimates of foraging parameters under a simplified scenario. Other location-specific information such as predator and prey densities, prey recognition, and consumption of aggregated individuals must, however, be further considered when extrapolating our laboratory results and considering using T. danae as a native biological control agent on the shore.
Predation under environmental stresses
The decline in predation rate when the crabs were exposed to low salinity (15‰) is consistent with previous data (Taylor et al. 1977; McGaw and McMahon 1996; Witman and Grange 1998). The substantial reduction in consumption and survival rate under hypo-osmotic stress lowers the potential for the crab to control the invasive mussel populations. Feeding and food processing pose additional physiological demands on crabs under hypo-osmotic conditions (McGaw 2006a, b). Increasing oxygen uptake by more frequent respiration to support these demands, however, can lead to higher rates of water and ion exchanges across respiratory surface which may be lethal in osmo-conforming crabs under hypo-osmotic conditions (McGaw 2005, 2006b).
Such conflicting processes between feeding and stress responses have been shown to be mediated by hormonal controls. Curtis et al. (2010) reported that hypo-osmotic stress stimulates the sinus gland in crabs to produce a putative hormone, feeding inhibition factor (FIF), which suppresses hunger and stimulates satiety, resulting in a loss of appetite (Curtis et al. 2010). In addition, hypo-osmotic stress modulates the stomatogastric ganglion, which lowers the peristalsis rate in the foregut and stimulates food regurgitation, in order to lessen food materials entering the midgut (Morris and Maynard 1970; Rezer and Moulins 1983). These stress responses substantially decrease food intake (McGaw 2006a; Curtis et al. 2010), and when coupled with the reduced survival of the crabs in hypo-osmotic conditions, further reduce the feasibility of the crab to be a biological control agent for the invasive mussels in estuarine environments. The invasive X. securis is distributed widely from the eastern oceanic zone to the western estuarine zone of Hong Kong. Both Tolo Harbour and Deep Bay have a similar range of water temperatures (17.5 to 31.0 °C) but lower salinities, however, are recorded in Deep Bay with an average of 17.7‰ between 2018 and 2022, as compared to Tolo Harbour, which has a mean level of 30.5‰ (EPD 2022). Due to the high freshwater discharge from the Pearl River, the salinity of inner Deep Bay even drops to below 10‰ during the wet season (EPD 2022). As such, the crab could be used as a biological control agent for the invasive mussels currently present in Tolo Harbour and other coastal habitats with normal salinities, but not in Deep Bay where salinity is low and beyond the tolerance limits of the crabs.
Although temperature and salinity are the main driving forces for physiological responses in intertidal animals, a variety of environmental factors, like water flow and habitat heterogeneity, also have significant influences on crab predation (for instance by influencing searching time, Sponaugle and Lawton 1990). Whilst this study has examined the joint influence of temperature and salinity stress in the laboratory, further research should be undertaken to test the predation of crabs under natural conditions in the field, in order to verify our experimental findings and the feasibility of using T. danae as a native biological control agent.
Conclusion
The predatory crab Thalamita danae exerts similar predation pressure on the invasive mussel, Xenostrobus securis and the native mussel, Brachidontes variabilis of the same size, suggesting that the crab may deter the establishment of X. securis in Hong Kong. Variations in the physical environment, however, strongly affect the predation pressure of the crabs on the invasive mussels, with survival and consumption rate of the crabs significantly decreasing when exposed to low salinity. Hence, although the invasive mussels may be preyed upon by T. danae in regions with water salinities above 15‰, both this and previous studies show that local predators (swimming crabs and whelks) are unable to effectively prey on the invasive mussels in more estuarine environments (Astudillo et al. 2018). There is, therefore, a pressing need to investigate the biological control potential of other predators in estuarine and freshwater habitats to manage invasive mussel populations in these environments.
Change history
04 February 2024
The Supplementary file1 has been replaced with clean version.
References
Alekseev VR, Starobogatov YI (1996) Types of diapause in Crustacea: definitions, distribution, evolution. Hydrobiologia 320:15–26. https://doi.org/10.1007/-BF00016801
Anil AC, Venkat K, Sawant SS, Dileepkumar M, Dhargalkar VK, Ramaiah N, Harkantra SN, Ansari ZA (2002) Marine bioinvasion: concern for ecology and shipping. Curr Sci 83(3):214–218
Ap Rheinallt T, Hughes RN (1985) Handling methods used by the velvet swimming crab Liocarcinus puber when feeding on molluscs and shore crabs. Mar Ecol Prog Ser 25:63–70
Astudillo JC (2015) Assessment of non-native marine invertebrates in fouling communities in Hong Kong. PhD Thesis, The University of Hong Kong
Astudillo JC, Bonebrake TC, Leung KM (2017) The recently introduced bivalve Xenostrobus securis has higher thermal and salinity tolerance than the native Brachidontes variabilis and established Mytilopsis sallei. Mar Pollut Bull 118(1–2):229–236. https://doi.org/10.1016/j.marpolbul.2017.02.046
Astudillo JC, Bonebrake TC, Leung KM (2018) Deterred but not preferred: Predation by native whelk Reishia clavigera on invasive bivalves. PLoS ONE 13(5):e0196578. https://doi.org/10.1371/journal.pone.0196578
Azra MN, Mohamad A, Hidir A, Taufik M, Abol-Munafi AB, Ikhwanuddin M (2020) Critical thermal maxima of two species of intertidal crabs, Scylla olivacea and Thalamita crenata at different acclimation temperatures. Aquacult Rep 17:100301. https://doi.org/10.1016/j.aqrep.2020.100301
Barbeau MA, Scheibling RE (1994) Temperature effects on predation of juvenile sea scallops [Placopecten magellanicus (Gmelin)] by sea stars (Asterias vulgaris Verrill) and crabs (Cancer irroratus Say). J Exp Mar Biol Ecol 182(1):27–47. https://doi.org/10.1016/0022-0981(94)90209-7
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/-jss.v067.i01
Bergman E (1987) Temperature-dependent differences in foraging ability of two percids, Perca fluviatilis and Gymnocephalus cernuus. Environ Biol Fishes 19(1):45–53. https://doi.org/10.1007/BF00002736
Bertolini C, Geraldi NR, Montgomery WI, O’Connor NE (2017) Substratum type and conspecific density as drivers of mussel patch formation. J Sea Res 121:24–32. https://doi.org/10.1016/j.seares.2017.01.004
Boulding EG (1984) Crab-resistant features of shells of burrowing bivalves: decreasing vulnerability by increasing handling time. J Exp Mar Biol Ecol 76(3):201–223. https://doi.org/10.1016/0022-0981(84)90189-8
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9(2):378–400. https://doi.org/10.32614/RJ-2017-066
Burnett NP, Seabra R, de Pirro M, Wethey DS, Woodin SA, Helmuth B, Zippay ML, Sarà G, Monaco C, Lima FP (2013) An improved noninvasive method for measuring heartbeat of intertidal animals. Limnol Oceanogr Meth 11(2):91–100
Cheung SG, Tong PY, Yip KM, Shin PKS (2004) Chemical cues from predators and damaged conspecifics affect byssus production in the green-lipped mussel Perna viridis. Mar Freshw Behav Physiol 37(2):127–135. https://doi.org/10.1080/10236240410001705798
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7(8):721–733. https://doi.org/10.1111/j.1461-0248.2004.00616.x
Cossins AR, Bowler K (1987) Rate compensations and capacity adaptations. In: Temperature Biology of Animals pp155–203. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-3127-5_5
Cunningham PN, Hughes RN (1984) Learning of predatory skills by shore crabs Carcinus maenas feeding on mussels and dogwhelks. Mar Ecol Prog Ser 16(2):1–26
Curtis DL, Vanier CH, McGaw IJ (2010) The effects of starvation and acute low salinity exposure on food intake in the Dungeness crab, Cancer magister. Mar Biol 157(3):603–612. https://doi.org/10.1007/s00227-009-1345-4
Dahlhoff EA, Somero GN (1993) Effects of temperature on mitochondria from abalone (genus Haliotis): adaptive plasticity and its limits. J Exp Biol 185(1):151–168. https://doi.org/10.1242/jeb.185.1.151
De Min R, Vio E (1997) Molluschi conchiferi del litorale sloveno. Ann Istrian Mediterr Stud (series Historia Naturalis) 4:241–258
Depledge MH, Andersen BB (1990) A computer-aided physiological monitoring system for continuous, long-term recording of cardiac activity in selected invertebrates. Comp Biochem Physiol Part A Physiol 96(4):473–477. https://doi.org/10.1016/0300-9629(90)90664-E
Draper AM, Weissburg MJ (2022) Differential effects of warming and acidification on chemosensory transmission and detection may strengthen non-consumptive effects of blue crab predators (Callinectes sapidus) on mud crab prey (Panopeus herbstii). Front Mar Sci 9:944237. https://doi.org/10.3389/-fmars.2022.944237
Dudas SE, McGaw IJ, Dower JF (2005) Selective crab predation on native and introduced bivalves in British Columbia. J Exp Mar Biol Ecol 325(1):8–17. https://doi.org/10.1016/j.jembe.2005.04.015
Eggleston DB, Lipcius RN, Hines AH (1992) Density-dependent predation by blue crabs upon infaunal clam species with contrasting distribution and abundance patterns. Mar Ecol Prog Ser 85:55–68
Elner RW, Hughes RN (1978) Energy maximization in the diet of the shore crab, Carcinus maenas. J Anim Ecol 47(1):103–116. https://doi.org/10.2307/3925
Enderlein P, Moorthi S, Röhrscheidt H, Wahl M (2003) Optimal foraging versus shared doom effects: interactive influence of mussel size and epibiosis on predator preference. J Exp Mar Biol Ecol 292(2):231–242. https://doi.org/10.1016/S0022-0981(03)00199-0
Environmental Protection Department (2022) Marine water quality in Hong Kong in 2022. Annual Marine Water Quality Reports. Retrieved from https://www.epd.gov.hk/epd/sites/default/files/epd/english/environmentinhk/water/hkwqrc/files/waterquality/annual-report/marinereport2022.pdf
Firth LB, Williams GA (2009) The influence of multiple environmental stressors on the limpet Cellana toreuma during the summer monsoon season in Hong Kong. J Exp Mar Biol Ecol 375(1–2):70–75. https://doi.org/10.1016/j.jembe.2009.05.011
Florian H (2022) DHARMa: residual diagnostics for hierarchical (Multi-Level /Mixed) regression models. R package version 0.4.6. https://CRAN.R-project.org/package=DHARMa
Garci ME, Trigo JE, Pascual S, González AF, Rocha F, Guerra A (2007) Xenostrobus securis (Lamarck, 1819)(Mollusca: Bivalvia): first report of an introduced species in Galician waters. Aquac Int 15(1):19–24. https://doi.org/10.1007/s10499-006-9062-1
Garton D, Stickle WB (1980) Effects of salinity and temperature on the predation rate of Thais haemastoma on Crassostrea virginica spat. Biol Bull 158(1):49–57. https://doi.org/10.2307/1540757
Giakoumi S, Katsanevakis S, Albano PG, Azzurro E, Cardoso AC, Cebrian E, Deidun A, Edelist D, Francour P, Jimenez C, Mačić V, Sghaier YR (2019) Management priorities for marine invasive species. Sci Total Environ 688:976–982. https://doi.org/10.1016/j.scitotenv.2019.06.282
Guppy M, Withers P (1999) Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol Rev 74(1):1–40. https://doi.org/10.1017/S0006323198005258
Hill BJ (1976) Natural food, foregut clearance-rate and activity of the crab Scylla serrata. Mar Biol 34:109–116. https://doi.org/10.1007/BF00390752
Hopkin RS, Qari S, Bowler K, Hyde D, Cuculescu M (2006) Seasonal thermal tolerance in marine Crustacea. J Exp Mar Biol Ecol 331(1):74–81. https://doi.org/10.1016/j.jembe.2005.10.007
Huang ZG, Yan SK, Lin S, Zheng DQ (1992) Biofouling communities on pier pilings in Mirs Bay. In: The Marine Flora and Fauna of Hong Kong and Southern China III. (ed Morton BS). Hong Kong University Press, Hong Kong, pp 529–543
Hughes RN (1979) Optimal diets under the energy maximization premise: the effects of recognition time and learning. Am Nat 113(2): 209–221. https://doi.org/10.1086/283380
Hughes RN, Seed R (1981) Size selection of mussels by the blue crab Callinectes sapidus: energy maximizer or time minimizer. Mar Ecol Prog Ser 6(1):83–89. https://doi.org/10.3354/meps006083
Hui TY, Dong YW, Han GD, Lau SL, Cheng MC, Meepoka C, Ganmanee M, Williams GA (2020) Timing metabolic depression: predicting thermal stress in extreme intertidal environments. Am Nat 196(4):501–511. https://doi.org/10.1086/710339
Kannupandi T, Krishnan T, Shanmugam A (1997) Effect of salinity on the larvae of an edible estuarine crab Thalamita crenata (Crustacea, Decapoda, Portunidae). Indian J Mar Sci 26(3):315-318.
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17(4):164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Kimura T, Tabe M, Shikano Y (1999) Limnoperna fortunei kikuchii Habe, 1981 (Bivalvia: Mytilidae) is a synonym of Xenostrobus securis (Lamarck, 1819): introduction into Japan from Australia and/or New Zealand. Venus (Jpn J of Malacol) 58(3):101–117. https://doi.org/10.18941/venusjjm.58.3_101
Kinne O (1966) Physiological aspects of animal life in estuaries with special reference to salinity. Neth J Sea Res 3(2):222–244. https://doi.org/10.1016/0077-7579(66)90013-5
Kobak J (2001) Light, gravity and conspecifics as cues to site selection and attachment behaviour of juvenile and adult Dreissena polymorpha Pallas, 1771. J Molluscan Stud 67(2):183–189. https://doi.org/10.1093/mollus/67.2.183
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16(4):199–204. https://doi.org/10.1016/S0169-5347(01)02101-2
Krebs JR (1980) Optimal foraging, predation risk and territory defence. Ardea 55(1–2):83–90. https://doi.org/10.5253/arde.v68.p83
Lai CS (1996) The feeding ecology of Thalamita danae Stimpson 1858 (Brachyura: Portunidae), with a review of the genus Thalamita in Hong Kong. PhD Thesis, The University of Hong Kong
Lau SC, Brettell DL, Astudillo JC (2018) Rapid assessment of the invasive Xenostrobus securis on cultured oysters in Hong Kong. Reg Stud Mar Sci 17:11–16. https://doi.org/10.1016/j.rsma.2017.11.008
Law HSS (2006) Invasive species—what can Hong Kong do?. MSc Thesis, The University of Hong Kong
Lazzari G, Rinaldi E (1994) Alcune considerazioni sulla presenza di specie extra Mediterranee nelle lagune salmastre di Ravenna. Boll Malacol 30(5–9):195–202
Lin J (1991) Predator-prey interactions between blue crabs and ribbed mussels living in clumps. Estuar Coast Shelf Sci 32(1):61–69. https://doi.org/10.1016/0272-7714(91)90028-A
Louette G (2012) Use of a native predator for the control of an invasive amphibian. Wildl Res 39(3):271–278. https://doi.org/10.1071/WR11125
Luk IM (2014) The ecophysiology and dynamic energy budget of Septifer virgatus. MPhil Thesis, The University of Hong Kong
MacMillen RE, Greenaway P (1978) Adjustments of energy and water metabolism to drought in an Australian arid-zone crab. Physiol Zool 51(3):230–240. https://doi.org/10.1086/physzool.51.3.30155740
Marshall DJ, Dong YW, McQuaid CD, Williams GA (2011) Thermal adaptation in the intertidal snail Echinolittorina malaccana contradicts current theory by revealing the crucial roles of resting metabolism. J Exp Biol 214(21):3649–3657. https://doi.org/10.1242/jeb.059899
Marshall DJ, McQuaid CD (1991) Metabolic rate depression in a marine pulmonate snail: pre-adaptation for a terrestrial existence? Oecologia 88:274–276. https://doi.org/10.1007/BF00320822
McGaw IJ (2005) Prioritization or summation of events? Cardiovascular physiology of postprandial Dungeness crabs in low salinity. Physiol Biochem Zool 79(1):169–177. https://doi.org/10.1086/498353
McGaw IJ (2006a) Feeding and digestion in low salinity in an osmoconforming crab, Cancer gracilis I. Cardiovascular and respiratory responses. J Exp Biol 209(19):3766–3776. https://doi.org/10.1242/jeb.02441
McGaw IJ (2006b) Feeding and digestion in low salinity in an osmoconforming crab, Cancer gracilis II. Gastric evacuation and motility. J Exp Biol 209(19):3777–3785. https://doi.org/10.1242/jeb.02442
McGaw IJ (2007) Interactive effects of low salinity and feeding on the physiology and behaviour of decapod crustaceans. Comp Biochem Physiol Part A 4(146): S75–S76. https://doi.org/10.1016/j.cbpa.2007.01.086
McGaw IJ, McMahon BR (1996) Cardiovascular responses resulting from variation in external salinity in the Dungeness crab, Cancer magister. Physiol Zool 69(6):1384–1401. https://doi.org/10.1086/physzool.69.6.30164265
McNeely JA (2000) Global strategy for addressing the problem of invasive alien species. A result of the global invasive alien species (GISP). IUCN-The World Conservation Union. Draft Version
Monaco CJ, McQuaid CD, Marshall DJ (2017) Decoupling of behavioural and physiological thermal performance curves in ectothermic animals: a critical adaptive trait. Oecologia 185(4):583–593. https://doi.org/10.1007/s00442-017-3974-5
Morris J, Maynard DM (1970) Recordings from the stomatogastric nervous system in intact lobsters. Comp Biochem Physiol 33(4):969–972. https://doi.org/10.1016/0010-406X(70)90044-7
Morton B, Leung KF (2015) Introduction of the alien Xenostrobus securis (Bivalvia: Mytilidae) into Hong Kong, China: interactions with and impacts upon native species and the earlier introduced Mytilopsis sallei (Bivalvia: Dreissenidae). Mar Pollut Bull 92(1–2):134–142. https://doi.org/10.1016/j.marpolbul.2014.12.046
Morton B, Leung PT, Wei J, Lee GY (2020) A morphological and genetic comparison of Septifer bilocularis, Mytilisepta virgata and Brachidontes variabilis (Bivalvia: Mytiloidea) from Hong Kong and erection of the Mytiliseptiferinae sub-fam. nov. Reg Stud Mar Sci 34:100981. https://doi.org/10.1016/j.rsma.2019.100981
Morton B, Morton J (1983) The sea shore ecology of Hong Kong. Hong Kong University Press
Myers JH, Cory JS (2017) Biological control agents: invasive species or valuable solutions? In: Vilà M, Hulme P (eds) Impact of biological invasions on ecosystem services pp 191–202. Invading Nature - Springer Series in Invasion Ecology vol 12. https://doi.org/10.1007/978-3-319-45121-3_12
Naddafi R, Rudstam LG (2014) Predation on invasive zebra mussel, Dreissena polymorpha, by pumpkinseed sunfish, rusty crayfish, and round goby. Hydrobiologia 721(1):107–115. https://doi.org/10.1007/s10750-013-1653-z
Pascual S, Villalba A, Abollo E, Garci M, González AF, Nombela M, Posada D, Guerra A (2010) The mussel Xenostrobus securis: a well-established alien invader in the Ria de Vigo (Spain, NE Atlantic). Biol Invasions 12(7):2091–2103. https://doi.org/10.1007/s10530-009-9611-4
Perrings C (2001) The economics of biological invasions. Land Use Water Resour Res 1(3):1–9
Reimer O, Tedengren M (1997) Predator-induced changes in byssal attachment, aggregation and migration in the blue mussel, Mytilus edulis. Mar Freshw Behav Physiol 30(4):251–266. https://doi.org/10.1080/-10236249709379029
Rezer E, Moulins M (1983) Expression of the crustacean pyloric pattern generator in the intact animal. J Comp Physiol 153(1):17–28. https://doi.org/10.1007/BF00610338
Rome MS, Young-Williams AC, Davis GR, Hines AH (2005) Linking temperature and salinity tolerance to winter mortality of Chesapeake Bay blue crabs (Callinectes sapidus). J Exp Mar Biol Ecol 319(1–2):129–145. https://doi.org/10.1016/j.jembe.2004.06.014
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Amer Zool 37(6):621–632. https://doi.org/10.1093/icb/37.6.621
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst 31(1):481–531. https://doi.org/10.1146/annurev.ecolsys.31.1.481
Sabelli B (1993) Rinvenimento di Xenostrobus sp. (Bivalvia: Mytilidae) nella laguna di Venezia. Boll Malacol 29:311–318
Seed R, Hughes RN (1995) Criteria for prey size-selection in molluscivorous crabs with contrasting claw morphologies. J Exp Mar Biol Ecol 193(1–2):177–195. https://doi.org/10.1016/0022-0981(95)00117-4
Shirafuji J, Sato S (2003) Benthic communities of tidal flats in Sacheon and Masan, Gyeongsangnamdo, Korea. In: Investigation report of tidal flat in cooperation with Japan and Korea, pp 20–25
Sponaugle S, Lawton P (1990) Portunid crab predation on juvenile hard clams: effects of substrate type and prey density. Mar Ecol Prog Ser 67(1):43–53. https://doi.org/10.3354/meps067043
Stillman J, Somero GN (1996) Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): correlation of physiology, biochemistry and morphology with vertical distribution. J Exp Biol 199(8):1845–1855. https://doi.org/10.1242/-jeb.199.8.1845
Streftaris N, Zenetos A (2006) Alien marine species in the Mediterranean-the 100 ‘Worst Invasives’ and their impact. Mediterr Mar Sci 7(1):87–118. https://doi.org/10.12681/mms.180
Streftaris N, Zenetos A, Papathanassiou E (2005) Globalisation in marine ecosystems: the story of non-indigenous marine species across European seas. Oceanogr Mar Biol Ann Rev 43:419–453.
Tagatz ME (1969) Some relations of temperature acclimation and salinity to thermal tolerance of the blue crab, Callinectes sapidus. Trans Am Fish Soc 98(4):713–716
Taylor EW, Butler PJ, Al-Wassia A (1977) The effect of a decrease in salinity on respiration, osmoregulation and activity in the shore crab, Carcinus maenas (L.) at different acclimation temperatures. J Comp Physiol 119(2):155–170. https://doi.org/10.1007/BF00686563
Todd ME, Dehnel PA (1960) Effect of temperature and salinity on heat tolerance in two grapsoid crabs, Hemigrapsus nudus and Hemigrapsus oregonensis. Biol Bull 118(1):150–172. https://doi.org/10.2307/1539065
Uglow RF (1973) Some effects of acute oxygen changes on heart and scaphognathite activity in some portunid crabs. Neth J Sea Res 7:447–454. https://doi.org/10.1016/0077-7579(73)90065-3
Underwood AJ, Clarke KR (2005) Solving some statistical problems in analyses of experiments on choices of food and on associations with habitat. J Exp Mar Biol Ecol 318(2):227–237. https://doi.org/10.1016/j.jembe.2004.12.014
Veiga P, Rubal M, Arenas F, Incera M, Olabarria C, Sousa-Pinto I (2011) Does Carcinus maenas facilitate the invasion of Xenostrobus securis? J Exp Mar Biol Ecol 406(1–2):14–20. https://doi.org/10.1016/j.jembe.2011.05.035
Wallace JC (1973) Feeding, starvation and metabolic rate in the shore crab Carcinus maenas. Mar Biol 20(4):277–281. https://doi.org/10.1007/BF00354271
Williams GA, De Pirro M, Cartwright S, Khangura K, Ng WC, Leung PT, Morritt D (2011) Come rain or shine: the combined effects of physical stresses on physiological and protein-level responses of an intertidal limpet in the monsoonal tropics. Funct Ecol 25(1):101–110. https://doi.org/10.1111/j.1365-2435.2010.01760.x
Wilson BR (1968) Survival and reproduction of the mussel Xenostrobus securis (Lam.) (Mollusca: Bivalvia: Mytilidae) in a Western Australian estuary: Part I. Salinity tolerance. J Nat Hist 2(3):307–328. https://doi.org/10.1080/00222936800770341
Witman JD, Grange KR (1998) Links between rain, salinity, and predation in a rocky subtidal community. Ecol 79(7):2429–2447. https://doi.org/10.1890/0012-9658(1998)079[2429:LBRSAP]2.0.CO;2
Wittenberg R, Cock MJ (eds) (2005) Invasive alien species: a toolkit of best prevention and management practices. CABI
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MFFA conceived the study. MFFA, GAW and TYH carried out the sample collection; MFFA conducted the experiments; MFFA and TYH contributed to the data analysis. MFFA drafted the initial manuscript. GAW and TYH reviewed and edited the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Timelapse video showing the predation behaviour of the crab, Thalamita danae, on the mussel prey in prey preference experiment trials (MP4 5834 KB)
10530_2023_3234_MOESM3_ESM.r
R code analysing the linear mixed effect models used in the prey preference experiment and generalized linear mixed effect models used in the handling time experiment (R 9 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Au, M.F.F., Hui, T.Y. & Williams, G.A. Can the native crab Thalamita danae be an effective biological control agent of the invasive mussel Xenostrobus securis in Hong Kong?. Biol Invasions 26, 1139–1155 (2024). https://doi.org/10.1007/s10530-023-03234-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03234-w