Abstract
Trapping is an effective strategy for control of invasive wild pig (Sus scrofa), but eradication of populations via trapping alone typically is impractical throughout much of the species’ introduced range. Consequently, recovery of wild pig populations to pre-trapping levels following control is a major impediment to success of long-term control. In this study, we conducted camera-trap surveys to monitor recovery of two wild pig populations immediately following density reductions achieved via trapping on two sites on the Savannah River Site, SC. We used spatial capture-recapture models to estimate wild pig density during seven 3 week periods spanning 12 months to: (1) determine the time required for populations to recover to pre-trapping levels; and (2) evaluate trends in population growth, density, and distribution during recovery. Recovery of both wild pig populations started immediately after trapping ceased, with populations reaching pre-trapping levels in 3 and 5 months. Threeweek population growth rates for each wild pig population during recovery averaged 1.28 (range = 1.11–1.39 on site 1) and 1.24 (range = 0.64–1.92 on site 2), which corresponded to a 345% and 132% increase in abundance (an increase of 141 and 126 individual wild pigs on site 1 and 2, respectively) of each population by the last survey period. Rapid recovery and growth beyond pre-trapping levels of wild pig populations likely were driven by residual wild pigs not removed by trapping and compensatory reproduction and immigration. Our study is one of the first to provide information on how quickly wild pig populations can recover following overall density reductions of 54–68% and indicates practitioners should be prepared to reinitiate trapping efforts in as little as 3 months to prevent recovery of wild pig populations following control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive wild pigs (Sus scrofa) have become a major management concern due to their extensive negative economic and ecological impacts throughout their introduced range. Over the past several decades, the magnitude of negative impacts of wild pigs have increased concurrently with the species’ abundance and distribution throughout their introduced range (Seward et al. 2004; Barrios-Garcia and Ballari 2012; Bevins et al. 2014; Risch et al. 2021; McDonough et al. 2022). As one of the 10 most globally important non-native invasive species, the ongoing expansion of wild pig populations consequently has stimulated greater focus on management and control aimed at mitigating their negative impacts (Lowe et al. 2000; Crystal-Ornelas et al. 2021; Cuthbert et al. 2022).
Throughout much of their introduced range, eradication of invasive wild pigs is impractical given their extensive distribution, high fecundity, and ability to disperse. Trapping is often a primary strategy adopted for wild pig control due to its efficiency and accessibility to most managers (Choquenot et al. 1993). However, despite demonstrated success of trapping in achieving substantial local reductions in wild pig density (e.g., 40–90% reduction; Choquenot et al. 1993; Ditchkoff et al. 2017; Davis et al. 2022; Lewis et al. 2022; Kilgo et al. 2023), and recent technological advances that further increase trapping removal rates (e.g., whole-sounder removal; Gaskamp et al. 2021; Kilgo et al. 2023), eradication of wild pig populations via trapping alone typically is not feasible (but see Lewis et al. 2022). In addition to the sheer extent of established populations (e.g., every county in South Carolina is occupied by wild pigs), providing a nearly endless source of immigrants, locating and trapping the last remaining wild pigs after density has been reduced by trapping often is not cost effective relative to effort required (Garcelon et al. 2005; Davis et al. 2016; Fischer et al. 2020; Gaskamp et al. 2021). Additionally, the last remaining wild pigs often are conditioned to avoid traps due to previous exposure to traps and thus can be more difficult to trap (Kilgo et al. 2023). Consequently, many wild pig populations recover to or surpass pre-trapping density levels after cessation of trapping, thus requiring trapping to be implemented in perpetuity to maintain reduced density conditions and mitigate negative impacts of this invasive species (With 2002; Lurgi et al. 2016).
Recovery of wild pig populations following control represents a pervasive challenge to control efforts throughout the species’ introduced range. Compensatory in situ reproduction and immigration by wild pigs in response to local density reductions achieved via trapping are major impediments to long-term success of control programs (Hanson et al. 2009; Servanty et al. 2011; Gamelon et al. 2011), as these mechanisms should enable rapid recovery of wild pig populations to pre-trapping levels. Further, compensatory in situ reproduction and immigration may operate simultaneously and result in growth rates of recovering wild pig populations exceeding that observed during pre-control time periods (Morelle et al. 2016; Snow et al. 2017). The time required for wild pig population recovery to pre-control levels may also be influenced by the magnitude of density reduction achieved during control, such that removing a greater proportion of the wild pig population should increase the time required for population recovery. However, trapping at spatial scales sufficiently large to limit immigration is logistically difficult and often cost-prohibitive (Epanchin-Niell and Hastings 2010; Pepin et al. 2017, 2020). Compensatory immigration and reproduction following density reduction can facilitate rapid population growth, thereby reducing the time required to recover to pre-control levels (Pepin et al. 2017).
Guidance on when to reinitiate trapping efforts following density reduction is essential for control programs aimed at maintaining reduced wild pig density to minimize damage and public safety hazards. Although the efficacy of different trapping strategies has been studied (Choquenot et al. 1993; Gaskamp et al. 2021; Lewis et al. 2022; Kilgo et al. 2023), we are unaware of research that has provided empirical data to support when practitioners must reinitiate trapping efforts after density has been reduced or after reaching targets for proportion of the wild pig population removed (Pepin et al. 2017). Information needed to guide decisions on when to reinitiate trapping could be based on objectives of wild pig control programs. The time for a wild pig population to reach pre-trapping density levels is critical information for control programs aimed at reducing density to mitigate damage and risks to human safety. Similarly, trends in wild pig population growth rates relative to the magnitude of density reduction achieved are critical for success of control programs aimed at preventing growth and expansion of wild pig populations (Pepin et al. 2017). However, existing research on recovery of wild pig populations following control is limited and only focuses on longer time scales (i.e., > 1 year; Hone and Pedersen 1980; Hone 1983; Saunders et al. 1990; Twigg et al. 2006). Empirical data describing wild pig density and distribution at fine temporal scales following control would provide invaluable decision support for maximizing success of control efforts (Pepin et al. 2017, 2019, 2020).
In this study, we evaluated spatiotemporal patterns in density and distribution of two wild pig populations immediately following local density reductions achieved via an intensive trapping effort using whole-sounder removal strategies on the Savannah River Site, South Carolina. Our objectives were to: (1) determine the time required for each wild pig population to reach pre-trapping density levels; and (2) evaluate spatial patterns in density and distribution of wild pigs in each area across seven 3 weeks periods covering a 10 months period beginning immediately following cessation of trapping.
Methods
Study site
The Savannah River Site (SRS), an 80,267-ha National Environmental Research Park owned and operated by the U.S. Department of Energy, is located on the Upper Coastal Plain and Sandhills physiographic provinces in South Carolina. The landscape of the SRS is characterized by sandy soils and gently sloping hills dominated by pines with scattered hardwoods and, although forest rotation lengths are longer than average for the area, the site is generally representative of habitat in the region (Kilgo and Blake 2005). The climate of SRS is humid subtropical with annual precipitation and temperature averaging 1225 mm and 18 °C, respectively (Blake et al. 2005). Planted stands of loblolly (Pinus taeda), longleaf (P. palustris), and slash (P. elliottii) pines comprise 66% of the SRS and are managed on 50–120-year rotations. Bottomland hardwood, forested wetlands, and cypress (Taxodium distichum)–tupelo (Nyssa aquatica and N. sylvatica var biflora) comprise approximately 18,461 ha (23% of the SRS). The remaining 6421 ha of forested area on the site includes mixed-hardwood stands and water bodies/reservoirs (Imm and McLeod 2005). Developed areas (i.e., facilities) comprise 3% of the SRS (Imm and McLeod 2005).
Wild pigs have been known to exist on the SRS since 1950 but likely originated from free-ranging domestic pigs that went feral as early as the eighteenth century (Mayer 2005; Mayer and Brisbin Jr 2012). The size and distribution of the SRS wild pig population has expanded dramatically since the early 1950s (Mayer and Moore-Barnhill 2009), growing from a couple hundred individuals along the floodplain of the Savannah River in 1952 to between 4000 and 5000 individuals distributed throughout the entire SRS present day (Mayer et al. 2021). Wild pig density estimates on SRS range from 3 to 20 pigs/km2 (Davis et al. 2020; Bastille-Rousseau et al. 2021; Kilgo et al. 2021; Garabedian et al. 2023). The SRS wild pig population is demographically open with few barriers to dispersal and movement either within or outside the SRS, which presents a major obstacle to eradication of the SRS wild pig population (Mayer and Moore-Barnhill 2009).
Between 1956 and the present, the SRS wild pig population was managed through annual public deer hunts (during which wild pigs were legally harvested), a contract control program, and control activities conducted by the USDA Forest Service, Savannah River (Mayer 2005). Control activities, including lethal removal of wild pigs through shooting, trapping, and hunting with dogs (Mayer and Brisbin Jr 2012), were implemented sporadically since 1952 but more intensively beginning in 1985. From 1985 to 2022, control activities removed 26,059 wild pigs from the SRS, with annual removals averaging 696 wild pigs; from 2018 to 2022, annual wild pig removals averaged 1067 (T. Mims, USDA Forest Service, unpublished data).
Despite decades of intensive control activities, the SRS wild pig population has continued to increase in size (Mayer and Moore-Barnhill 2009; Mayer et al. 2021; Kilgo et al. 2021). Accordingly, the SRS wild pig control program is aimed at population reduction, rather than eradication, with the goal of minimizing damage and public safety hazards (i.e., pig-vehicle collisions; Mayer 2005; Mayer and Moore-Barnhill 2009; Beasley et al. 2014). Given the size of the SRS and the site-wide distribution of the SRS wild pig population, control activities necessarily are conducted on smaller management units with control operators shifting effort to different units once wild pig density has been reduced to the extent that removal of remaining wild pigs is not cost effective relative to effort required to remove them (Choquenot et al. 1993; Fischer et al. 2020). As a result, most units subjected to wild pig control on the SRS require follow-up control effort to varying degrees in order to maintain reduced density conditions achieved during initial control activities (Kilgo et al. 2023).
Data acquisition
Wild pig study populations
We addressed our study objectives using camera trap data collected on two sites on the SRS that were part of a previous study evaluating relative efficacy of whole-sounder removal and traditional removal strategies for reducing wild pig density (Kilgo et al. 2023). Sites 1 and 2 were 20.1 km2 and 26.3 km2, respectively (Fig. 1). We selected these two sites for the present study because whole-sounder removal strategies reduced wild pig density on sites 1 and 2 by 54% (90% CI 53–55%; reduced from 3.2 individuals/km2 [90% CI 2.7–3.7 individuals/km2] to 1.4 individuals/km2 [90% CI 1.2–1.7 individuals/km2]) and 68% (90% CI 65–70%; reduced from 5.5 individuals/km2 [90% CI 4.8–6.1 individuals/km2] to 1.8 individuals/km2 [90% CI 1.4–2.1 individuals/km2]; Kilgo et al. 2023), respectively, between 2017 and 2019. In Sep 2019, after two years of whole-sounder removal efforts that were as intensive as possible (Kilgo et al. 2023), post-control wild pig density estimates were 1.47 individuals/km2 (90% CI 1.23–1.72 individuals/km2) and 1.77 individuals/km2 (90% CI 1.44–2.11 individuals/km2) on sites 1 and 2, respectively. See Kilgo et al. (2023) for additional details on the range of wild pig density reductions achieved, the proportion of initial wild pig populations removed prior to our study, control effort, and whole-sounder removal strategies implemented, including selection of trap locations, on the two study sites selected for the present study.
Post-control monitoring and density estimation
After whole-sounder removal efforts ceased in Sep 2019 on sites 1 and 2, we conducted camera-trap surveys to document wild pig detections on each site during seven 21 day periods between Nov 2019 and Aug 2020. We deployed 51 and 67 cameras (Reconyx Hyperfire 2 Professional White Flash, Holmen, WI) on grids of 636 × 636 m cells in sites 1 and 2, respectively (Fig. 1); average distance between cameras was 616 m (range = 216–798 m) and 635 m (range = 284–798 m) on sites 1 and 2, respectively. We chose a grid cell size of 636 × 636 m (40 ha) based on estimated home range radii of wild pigs on SRS (Kilgo et al. 2021, Kilgo, unpublished data), which maximized the likelihood of detecting individuals at multiple camera stations as well as the proportion of the population detected (Keiter et al. 2017; Moqanaki et al. 2021). Additionally, this camera spacing also was consistent with long-term camera-trap surveys conducted for white-tailed deer (Odocoileus virginianus) and wild pig population monitoring on the SRS (USDA Forest Service-Savannah River, unpublished data). Camera grids and deployment locations within grid cells were consistent throughout the study. We set cameras to capture a single image per trigger with no delay between consecutive triggers and did not use bait or lures to increase wild pig detection rates. Within each grid cell, we deployed cameras at locations with wildlife trails and/or evidence of wild pigs and where dense vegetation would not obscure camera viewsheds. We mounted cameras on trees at a height of approximately 60 cm and parallel with the ground, facing north unless vegetation obscured the camera viewshed, in which case we oriented cameras in the direction with the best visibility. Using images of wild pigs captured during surveys, we assigned individual identities to as many wild pigs as possible based on pelage color, pattern, natural markings, and/or uniquely numbered ear tags. Approximately 62% of the SRS population contains such markings (Kilgo et al. 2023).
We estimated wild pig density during each survey period using spatial capture-recapture models for partially marked populations (Royle et al. 2014) and data augmentation (Tanner and Wong 1987; Royle et al. 2007) using a half normal function to model detections on both marked (i.e., individually identifiable, hereafter, marked individuals) and unmarked wild pigs from each survey period. Following Kilgo et al. (2023), we fit models for each of the seven survey periods using a 1 km buffer around camera grids and ran three Monte Carlo Multiple Chain (MCMC) chains in parallel for 500,000 iterations with a burn-in of 100,000. We evaluated convergence of MCMC chains using the Brooks-Gelman-Rubin convergence diagnostic (i.e., potential scale reduction factor [PSRF]; Brooks and Gelman 1998) and considered MCMC chains to have converged if PSRF was < 1.1. We ran spatial capture-recapture models in the R statistical environment (v. 4.2.1; R Core Team 2022) using the “scrPID” MCMC algorithm from the contributed R package ‘scrbook’ (Royle et al. 2020) and evaluated convergence using the contributed R package ‘mcmcOutput’ (Meredith 2022). See Kilgo et al. (2023) for additional details describing our approach to fitting spatial capture-recapture models for partially marked populations and data augmentation.
Statistical analyses
We used two complementary approaches to assess recovery of local wild pig populations on sites 1 and 2. First, we evaluated changes in wild pig density across successive survey periods by comparing wild pig density estimated for survey period t against wild pig density estimated for survey period t + 1. Similarly, we evaluated changes in wild pig population growth rates (λ; calculated as Nt+1/Nt) across successive survey periods. We evaluated changes in density across survey periods based on overlap of confidence intervals and considered estimates with non-overlapping confidence intervals to be different.
Second, we used the degree of individual turnover and the number of marked piglets (piglet age class assigned if body mass was estimated at < 10 kg) detected during each survey period as indices of immigration and in situ reproduction, respectively. We assessed the degree of individual turnover of wild pigs in each study site based on changes in the number of marked wild pigs detected across survey periods. For this analysis, we cross-referenced images of uniquely marked pigs (i.e., markings were sufficient for assigning individuals a unique identity) obtained during a given survey period against images of uniquely marked pigs from preceding survey periods and enumerated the number of uniquely marked wild pigs that were detected across multiple survey periods. We considered individual turnover to be low if the number of marked wild pigs increased across successive survey periods and the majority of marked wild pigs (i.e., > 50%) were detected in a given study site during 3 or more other survey periods. In other words, this scenario suggests that new individuals that move into the study site following control remain in the area. On the other hand, we considered individual turnover to be high if the number of new uniquely marked pigs (i.e., uniquely marked pigs that were not matched with uniquely marked pigs detected during previous survey periods) increased across successive survey periods but the majority of new uniquely marked pigs were detected during fewer than 3 survey periods. This scenario suggests that new individuals moving into the study area following control are transient and do not remain in the area. Other sources of wild pig mortality (e.g., roadkill) were negligible and no removal events occurred on either of our study sites (T. Mims, USDA Forest Service, personal communication), and therefore these factors did not introduce measurable bias in our assessment of individual turnover or reproduction.
Finally, we enumerated the number of marked piglets that were detected during each survey period as an index of reproduction on each site. Given piglet striping patterns disappear by 3–4 months of age in the SRS population (Mayer et al. 2021, J. Mayer, personal communication), we could not track individual piglets across survey periods that spanned 10 months. Therefore, we used counts of uniquely marked piglets within survey periods as an index of reproduction.
Results
Recovery to pre-control density
For both study sites, PSRF estimates were < 1.1 for MCMC chains across all survey periods indicating convergence. Overall, following density reductions of 54% and 68% on sites 1 and 2, respectively, wild pig populations increased in density by 345% (an increase from 1.4 individuals/km2 [90% CI 1.2–1.7 individuals/km2] to 6.1 individuals/km2 [90% CI 4.8–7.4 individuals/km2]) and 132% (an increase from 3.1 individuals/km2 [90% CI 2.8–3.5 individuals/km2] to 7.3 individuals/km2 [90% CI 6.5–8.5 individuals/km2]) between the first and seventh survey periods on sites 1 and 2, respectively (Fig. 2). During survey periods when wild pig density was increasing on site 2 (survey periods 1 through 5), wild pig density increased by 366% (an increase from 3.1 individuals/km2 [90% CI 2.8–3.5 individuals/km2] to 14.6 individuals/km2 [90% CI 13.2–16.2 individuals/km2]). Detection probability estimates on site 1 across survey periods averaged 0.75 (range = 0.63–0.88). On site 2, detection probability averaged 0.39 (range = 0.20–0.60).
Population growth rates (λ) among successive 3 weeks survey periods averaged 1.28 (range = 1.11–1.39) and 1.24 (range = 0.64–1.92) on sites 1 and 2, respectively (Table 1). During survey periods when wild pig density was increasing on site 2 (survey periods 1 through 5), average 3 weeks growth rate was 1.50 (range = 1.08–1.91; Table 1). The recovery trend on site 1 increased linearly over time, with the population surpassing pre-control density (3.2 individuals/km2, 90% CI 2.73–3.66 individuals/km2) in 5 months following trapping (Fig. 2). On Site 2, the recovery trend was more variable, with density increasing during the first 5 survey periods but declining during the last 2 survey periods (Fig. 2). On Site 2, the wild pig population surpassed pre-trapping density (5.5 individuals/km2; 90% CI 4.8–6.1 individuals/km2) in 3 months following control (Fig. 2).
Spatial distribution and spread during recovery
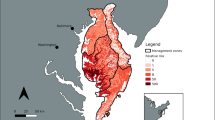
Overall, the distribution of wild pigs on both sites varied spatially across all survey periods (Figs. 3 and 4). Across all survey periods, the highest wild pig densities were located in or adjacent to bottomland hardwood areas (Figs. 3 and 4). No clear directional patterns in wild pig distributions on either of the two sites were evident (Figs. 3 and 4), although wild pig density appeared to be more concentrated along the eastern and western boundaries of site 2 during survey periods 1 and 2 (Fig. 4).
Individual turnover and in situ reproduction
The total number of marked wild pigs detected throughout the entire study on both sites was 353 (n = 216 for Site 1, n = 137 for Site 2). The number of marked wild pigs overall increased across survey periods on site 1 (Table 1). On site 2, the number of marked wild pigs increased consistently between survey periods 1 and 4, but then declined during survey periods 5 and 6 before increasing during survey period 7 (Table 1).
Individual turnover was generally high on both study sites. The percentage of marked wild pigs that were detected across 3 or more surveys was < 50% for both sites. The majority of marked wild pigs on both sites (overall 58% of marked pigs) were detected only during a single survey period, followed by 18% detected during two survey periods, 11% detected across 3 survey periods, 5% across four survey periods, and < 5% detected across more than 4 survey periods.
The number of marked piglets detected across survey periods on site 1 increased between survey periods one and five, but then slightly declined during survey periods six and seven (Table 1). On site 2, the number of marked piglets increased between survey periods one and five, but then declined during survey periods six and seven (Table 1).
Discussion
Our study indicates that recovery of wild pig populations following density reduction occurs over short time periods. Based on our results, recovery started immediately after control had ceased and rapid growth of both populations over short time intervals (approximately 3 weeks, equivalent to the time between survey periods in our study) resulted in recovery to pre-control levels in as little as 3 months. To our knowledge, no other study has reported empirical data on recovery times, changes in density and/or growth rates during recovery of wild pig populations at such fine temporal scales (i.e., 3 week periods), but the rapid recovery to pre-control levels we observed was surprising. Residual wild pigs not removed during trapping efforts prior to our study combined with compensatory reproduction and immigration likely both drove the rapid recovery of wild pig populations in our study.
Several factors may influence time to recovery, including level of density reduction, potential for immigration from surrounding areas, habitat and environmental conditions that affect suitability of the area, and resource levels, which in turn affect in situ reproduction. The recovery we observed occurred after density reduction of 54% and 68% in our two wild pig populations. Greater or lesser initial density reductions should affect time to recovery. For example, the lesser the density reduction, the more residual individuals remain in trapped areas to reproduce, likely leading to more rapid recovery. Increasing control intensity by as much as two-fold may be the best strategy to counteract rapid immigration from surrounding uncontrolled areas for some species (e.g., red fox Vulpes vulpes; Porteus et al. 2018), but on the SRS wild pig control efforts are already as intensive as possible relative to available resources. Nevertheless, population growth rates in both our study areas were unexpectedly rapid and were sufficient for both areas to achieve recovery to pre-removal levels within 5 months. Further, the area with the greater density reduction recovered more rapidly, within 3 months.
Although the relative importance of immigration and compensatory reproduction in recovery of our populations is difficult to discern, both mechanisms were likely instrumental. In an Alabama, USA study where the wild pig population had been reduced by over 90%, Lewis et al. (2022) considered their population demographically open but expected density to recover slowly (e.g., over a period of 12 months or more) due to the presence of rivers on two sides of their study area that would minimize immigration. In contrast, our sites lacked any natural or anthropogenic barriers to limit immigration. Likewise, food availability in our sites following density reduction likely was greater than prior to reduction. Abundant food resources are known to positively affect litter size in wild pigs (Mayer et al. 2021). In addition, timing of normal reproductive cycles in the population also may have affected the time to recovery we observed. Mayer and Brisbin (2012) reported annual pulses in reproduction occur in the SRS wild pig population during December and January, falling within the first 3–4 months of our recovery period. However, Chinn et al. (2022) reported March through June as the peak farrowing months in the SRS population, with nearly 50% of all litters farrowed during those months and < 20% farrowed during December and January. Whether a normal peak in reproduction occurred coincident with cessation of trapping in our populations, increased reproduction by both residual residents and immigrants (Hone and Pedersen 1980; Saunders et al. 1990) clearly could explain why wild pig populations in our study recovered to pre-control density levels in as little as 3 months.
While the rapid recovery to pre-control levels may have been driven by compensatory reproduction and immigration, continued growth well beyond pre-control density levels may have been largely driven by ongoing reproduction. The interplay between wild pig population density and food availability is a key driver of wild pig demographics through effects on reproduction (Gamelon et al. 2021; Mayer et al. 2021; Chinn et al. 2022). During periods of low wild pig density following control, reduced competition for food can accelerate growth rates of sows (Mayer et al. 2021), thereby reducing the time for sows to reach threshold size for breeding and resulting in a rapid increase in the number of breeding females and their reproductive output (Sabrina et al. 2009; Gamelon et al. 2021; Gethöffer et al. 2023). Such increases in female body mass, and by extension reproductive output (Mayer et al. 2021), could explain why number of piglets on site 1 rapidly increased during early survey periods and remained high through the final survey period in our study. Moreover, such increases in body mass can result in breeding by females as young as 3 months to 1 year old, thus accelerating the already fast life history of wild pigs (Giles 1980; Comer and Mayer 2009; Veylit et al. 2020; Mayer et al. 2021; Mayer 2021; Chinn et al. 2022). Thus, marked density reductions following control that create conditions that favor rapid increases in body mass of females in all age classes can dramatically increase reproductive output, thereby resulting in growth of recovering wild pig populations far beyond pre-control density levels.
Resource availability in areas surrounding our study sites could explain divergent recovery trends between our two wild pig study populations. Even when control units are relatively close in space, recovery of wild pig populations cannot be assumed to follow similar trends among control units. The decline in the recovery trend on our site 2 study population occurred during the hottest months of the year (Jul and Aug), suggesting thermoregulatory requirements of wild pigs may have driven this decline, rather than limited food resources, as wild pigs may have sought thermal refuge in nearby floodplain forests where water was more readily available. Such refugia were not present near site 1 where recovery increased linearly across survey periods. Temperature is a well-known driver of wild pig movements, whereby pigs reduce movement when temperatures are high to avoid overheating (Geisser and Reyer 2005; Kay et al. 2017). Reduced movement when temperatures are high also can entail declining abundance in an area when wild pig distributions shift to areas where thermoregulatory cover and food resources can be accessed without incurring costs of travel (Choquenot and Ruscoe 2003; Chinn et al. 2023). Indeed, the southern border of site 2 was the large bottomland hardwood swamp in the floodplain of the Savannah River. The eastern and western borders of this site were also along creeks supporting bottomland hardwoods, a vegetation type known to influence wild pig movements and home ranges, especially during the hottest months when thermoregulatory needs are most demanding (Schlichting et al. 2016; Garza et al. 2018; Clontz et al. 2021). The potential for movement of wild pigs in and out of refuges like the large bottomland hardwood swamp that bordered our study population reinforces the importance of ongoing monitoring of areas following wild pig control (Lewis et al. 2022).
That areas with increased wild pig density became more diffuse as density increased across survey periods likely reflects wild pigs’ ability to quickly adjust movement and territorial behaviors during periods of rapid population growth following control. When pigs are not constrained by high density and associated intraspecific competition, their distribution appears to be dynamic, likely reflecting increased movements to access resources previously defended by neighboring individuals removed by trapping (Fischer et al. 2020; Bastille-Rousseau et al. 2021). Space rendered free of wild pigs by control efforts was reoccupied in as little as three weeks in our study, similar to the rapid reoccupation of control areas with reduced density of Rattus spp. (Hein and Jacob 2015). Such reoccupation of vacated space required wild pigs to quickly adjust movements, which ultimately manifested as short-term and extensive spatial variation in density. This spatial variation in wild pig density over short periods of time presents a major challenge to preventing recovery of wild pig populations, because control efforts often are necessarily shifted among units across the landscape over time, rather than being implemented continuously on a given area. In some species (e.g., brushtail possum Trichosurus vulpecula; Morgan et al. 2006), recovery can be countered by prioritizing control along borders with adjacent populations, thus curbing immigration. Such an approach may be an effective strategy to prevent population recovery in areas where wild pigs from neighboring areas quickly immigrate and reoccupy vacated space (Cowled et al. 2006; Hanson et al. 2009; Delgado-Acevedo et al. 2013). Based on our results, in areas that have high likelihood of recovery due to immigration, information on wild pig distribution more than 2 months old may be unreliable for selecting trap locations when the goal is preventing recovery following control, and real-time information is preferable if possible.
We suspect that population growth rates as high as we observed are not sustainable over longer periods than we studied, as food resources will be depleted (limiting reproduction) and vacant space will be re-occupied (limiting immigration). Indeed, after an initial increase on our site 2, density there declined even during our monitoring period. Further, had control activities continued, growth could have been reversed. Nevertheless, in the absence of control, our data indicate the potential of wild pig populations to recover to and exceed pre-control densities rapidly, regardless of whether such growth is sustainable.
Population dynamics of wild pigs, in particular their reproductive potential and dispersal ability, are a major challenge for successful control of this species. Although eradication of demographically open wild pig populations may be possible under certain conditions (Lewis et al. 2022), in most cases eradication of established wild pig populations is generally not feasible. Thus, developing control strategies aimed at preventing and/or extending time to recovery of wild pig populations following control is paramount for mitigating their detrimental impacts. To our knowledge, our study is the first to report empirical data on density and/or growth rates during population recovery and the time required for recovery of wild pig populations to pre-control levels at such fine temporal scales following density reduction via intensive trapping efforts. Based on our results, recovery begins immediately following control, so practitioners should be prepared to reinitiate control of recovering wild pig populations in as little as 3 months following initial control. Importantly, our results indicate growth of wild pig populations will continue well beyond pre-control levels, placing greater emphasis on post-control monitoring at frequent intervals to adequately track changes in wild pig density and distribution. Managers can leverage information gained from more frequent or real-time monitoring to inform the decision of when to re-initiate control to maximize trapping success, thereby reducing long-term costs of ongoing control programs. Where wild pigs are present in surrounding areas, we recommend conducting post-control surveys at approximately 3 week intervals to detect incipient recovery of wild pig populations following control.
Abundance and density (individuals/km2) estimates for wild pig (Sus scrofa) populations recovering following intensive trapping on sites 1 (a) and 2 (b) between Nov 2019 and Aug 2020 on Savannah River Site, South Carolina, USA. Black horizontal lines reflect wild pig abundance and density estimates in 2017, prior to initiation of trapping efforts on sites 1 and 2, and gray shaded areas about each line represent 90% confidence intervals
Spatial variation in relative wild pig (Sus scrofa) density following intensive trapping on site 1 during 19 Nov 2019–10 Dec 2019 (a), 31 Dec 2019–21 Jan 2020 (b), 11 Feb 2020–3 Mar 2020 (c), 24 Mar 2020–14 Apr 2020 (d), 5 May 2020–26 May 2020 (e), 16 Jun 2020–7 Jul 2020 (f), and 28 Jul 2020–18 Aug 2020 (g) on Savannah River Site, South Carolina, USA
Spatial variation in relative wild pig (Sus scrofa) density following intensive trapping on site 2 during 19 Nov 2019–10 Dec 2019 (a), 31 Dec 2019–21 Jan 2020 (b), 11 Feb 2020–3 Mar 2020 (c), 24 Mar 2020–14 Apr 2020 (d), 5 May 2020–26 May 2020 (e), 16 Jun 2020–7 Jul 2020 (f), and 28 Jul 2020–18 Aug 2020 (g) on Savannah River Site, South Carolina, USA
Data availability
Supporting data for this study will be uploaded to the FigShare repository upon acceptance. Materials will be provided upon request during the review process.
References
Barrios-Garcia MN, Ballari SA (2012) Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol Invasions 14:2283–2300. https://doi.org/10.1007/s10530-012-0229-6
Bastille-Rousseau G, Schlichting PE, Keiter DA et al (2021) Multi‐level movement response of invasive wild pigs (Sus scrofa) to removal. Pest Manag Sci 77:77–95. https://doi.org/10.1002/ps.6029
Beasley JC, Grazia TE, Johns PE, Mayer JJ (2014) Habitats associated with vehicle collisions with wild pigs. Wildl Res 40:654–660. https://doi.org/10.1071/WR13061
Bevins SN, Pedersen K, Lutman MW et al (2014) Consequences associated with the recent range expansion of nonnative feral swine. BioScience 64:291–299
Blake JI, Hunter CH Jr, Bayle BA (2005) Climate and air quality. In: Kilgo JC, Blake JI (eds) Ecology and Management of a Forested Landscape: fifty years of natural resource stewardship on the Savannah River Site. Island Press, Washington, DC, pp 20–30
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Gr Stat 7:434–455
Chinn SM, Hepinstall-Cymerman J, Beasley JC (2023) Reproduction drives changes in space use and habitat selection in a highly adaptable invasive mammal. J Mammal 104(3):479–495
Chinn SM, Schlichting PE, Smyser TJ et al (2022) Factors influencing pregnancy, litter size, and reproductive parameters of invasive wild pigs. J Wildl Manag 86:e22304. https://doi.org/10.1002/jwmg.22304
Choquenot D, Kilgour RJ, Lukins BS (1993) An evaluation of feral pig trapping. Wildl Res 20:15–21. https://doi.org/10.1071/wr9930015
Choquenot D, Ruscoe WA (2003) Landscape complementation and food limitation of large herbivores: habitat-related constraints on the foraging efficiency of wild pigs. J Anim Ecol 72:14–26
Clontz LM, Pepin KM, VerCauteren KC, Beasley JC (2021) Influence of biotic and abiotic factors on home range size and shape of invasive wild pigs (Sus scrofa). Pest Manag Sci 78:914–928. https://doi.org/10.1002/ps.6701
Comer CE, Mayer JJ (2009) Wild pig reproductive biology. In: Mayer JJ, Brisbin IL (eds) Wild pigs: biology, damage, control techniques, and management. SRNL-RP-2009-00869. Savannah River National Laboratory, Aiken, pp 51–75
Cowled BD, Lapidge SJ, Hampton JO, Spencer PBS (2006) Measuring the demographic and genetic effects of pest control in a highly persecuted feral pig population. J Wildl Manag 70:1690–1697
Crystal-Ornelas R, Hudgins EJ, Cuthbert RN et al (2021) Economic costs of biological invasions within North America. NeoBiota 67:485–510. https://doi.org/10.3897/neobiota.67.58038
Cuthbert RN, Diagne C, Haubrock PJ et al (2022) Are the 100 of the world’s worst invasive species also the costliest? Biol Invasions 24:1895–1904. https://doi.org/10.1007/s10530-021-02568-7
Davis AJ, Hooten MB, Miller RS, Farnsworth ML, Lewis J, Moxcey M, Pepin KM (2016) Inferring invasive species abundance using removal data from management actions. Ecol Appl 26(7):2339–2346
Davis AJ, Farrar R, Jump B et al (2022) An efficient method of evaluating multiple concurrent management actions on invasive populations. Ecol Appl 32:e2623. https://doi.org/10.1002/eap.2623
Davis AJ, Keiter DA, Kierepka EM et al (2020) A comparison of cost and quality of three methods for estimating density for wild pig (Sus scrofa). Sci Rep 10:2047. https://doi.org/10.1038/s41598-020-58937-0
Delgado-Acevedo J, DeYoung RW, Campbell TA (2013) Effects of local-scale removals on feral swine populations in southern Texas. Int J Pest Manag 59:122–127. https://doi.org/10.1080/09670874.2013.776723
Ditchkoff SS, Holtfreter RW, Williams BL (2017) Effectiveness of a bounty program for reducing wild pig densities. Wildl Soc Bull 41:548–555. https://doi.org/10.1002/wsb.787
Epanchin-Niell RS, Hastings A (2010) Controlling established invaders: integrating economics and spread dynamics to determine optimal management. Ecol Lett 13:528–541. https://doi.org/10.1111/j.1461-0248.2010.01440.x
Fischer JW, Snow NP, Wilson BE et al (2020) Factors and costs associated with removal of a newly established population of invasive wild pigs in Northern U.S. Sci Rep 10:11528. https://doi.org/10.1038/s41598-020-68264-z
Gamelon M, Besnard A, Gaillard J et al (2011) High hunting pressure selects for earlier birth date: wild boar as a case study. Evolution 65:3100–3112. https://doi.org/10.1111/j.1558-5646.2011.01366.x
Gamelon M, Touzot L, Baubet É et al (2021) Effects of pulsed resources on the dynamics of seed consumer populations: a comparative demographic study in wild boar. Ecosphere 12:e03395. https://doi.org/10.1002/ecs2.3395
Garabedian JE, Cox KJ, Vukovich M, Kilgo JC (2023) Co-occurrence of native white-tailed deer and invasive wild pigs: evidence for competition? Ecosphere 14:e4435. https://doi.org/10.1002/ecs2.4435
Garcelon DK, Ryan KP, Schuyler PT (2005) Application of techniques for feral pig eradication on Santa Catalina Island, California. In: Proceedings of the Sixth California Islands Symposium. National Park Service Technical Publication, CHIS-05-01. Institute for Wildlife Studies, Arcata, California, pp 331–340.
Garza SJ, Tabak MA, Miller RS et al (2018) Abiotic and biotic influences on home-range size of wild pigs (Sus scrofa). J Mammal 99:97–107
Gaskamp JA, Gee KL, Campbell TA et al (2021) Effectiveness and efficiency of corral traps, drop nets and suspended traps for capturing wild pigs (Sus scrofa). Animals 11:1565
Geisser H, Reyer H-U (2005) The influence of food and temperature on population density of wild boar Sus scrofa in the Thurgau (Switzerland). J Zool 267:89. https://doi.org/10.1017/S095283690500734X
Gethöffer F, Keuling O, Maistrelli C et al (2023) Heavy youngsters—habitat and climate factors lead to a significant increase in body weight of wild boar females. Animals 13:898. https://doi.org/10.3390/ani13050898
Giles JR (1980) The ecology of feral pigs in western New South Wales. Dissertation, Sydney University
Hanson LB, Mitchell MS, Grand JB et al (2009) Effect of experimental manipulation on survival and recruitment of feral pigs. Wildl Res 36:185–191. https://doi.org/10.1071/WR08077
Hein S, Jacob J (2015) Recovery of small rodent populations after population collapse. Wildl Res 42:108–118. https://doi.org/10.1071/WR14165
Hone J (1983) A short-term evaluation of feral pig eradication at Willandra in Western New South Wales. Wildl Res 10:269–275. https://doi.org/10.1071/wr9830269
Hone J, Pedersen H (1980) Changes in a feral pig population after Poisoning. Proc Vertebr Pest Conf 9:176–182
Imm DW, McLeod KW (2005) Plant communities. In: Kilgo JC, Blake JI (eds) Ecology and Management of a Forested Landscape: fifty years on the Savannah River Site. Island Press, Washington, DC, pp 106–161
Kay SL, Fischer JW, Monaghan AJ et al (2017) Quantifying drivers of wild pig movement across multiple spatial and temporal scales. Mov Ecol 5:14
Keiter DA, Davis AJ, Rhodes OE et al (2017) Effects of scale of movement, detection probability, and true population density on common methods of estimating population density. Sci Rep 7:9446
Kilgo JC, Blake JI (eds) (2005) Ecology and management of a forested landscape: fifty years on the Savannah River Site. Island Press, Washington, DC
Kilgo JC, Garabedian JE, Vukovich M et al (2021) Food resources affect territoriality of invasive wild pig sounders with implications for control. Sci Rep 11:18821
Kilgo JC, Vukovich M, Cox KJ et al (2023) Assessing whole-sounder removal versus traditional trapping for control of invasive wild pigs, Sus scrofa. Pest Manag Sci 79:3033–3042
Lewis AA, Williams BL, Smith MD, Ditchkoff SS (2022) Shifting to sounders: whole sounder removal eliminates wild pigs. Wildl Soc Bull 46:e1260. https://doi.org/10.1002/wsb.1260
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. In: Encyclopedia of Biological Invasions. The Invasive Species Specialist Group (ISSG), Species Survival Commission (SSC), World Conservation Union (IUCN), Auckland, New Zealand, pp 12
Lurgi M, Wells K, Kennedy M et al (2016) A landscape approach to invasive species management. PLoS ONE 11:e0160417. https://doi.org/10.1371/journal.pone.0160417
Mayer JJ (2005) Wild hog. In: Kilgo JC, Blake JI (eds) Ecology and Management of a Forested Landscape: fifty years on the Savannah River Site. Island Press, Washington, D.C, pp 374–379
Mayer J (2021) Body mass variation in an introduced wild pig population with changing ancestry. Savannah River National Laboratory, Aiken, South Carolina, USA
Mayer J, Brisbin IL Jr (2012) Wild pigs of the Savannah River Site. Savannah River National Laboratory, Aiken, South Carolina, USA
Mayer JJ, Edwards TB, Garabedian JE, Kilgo JC (2021) Sanitary waste landfill effects on an invasive wild pig population. J Wildl Manag 85:868–879. https://doi.org/10.1002/jwmg.22042
Mayer J, Moore-Barnhill L (2009) Wild pig management case study: Savannah River Site. Savannah River National Laboratory, Aiken, South Carolina, USA. Report #2009-00869. pp 331–340
McDonough MT, Ditchkoff SS, Smith MD, Vercauteren KC (2022) A review of the impacts of invasive wild pigs on native vertebrates. Mamm Biol 102:279–290. https://doi.org/10.1007/s42991-022-00234-6
Meredith M (2022) mcmcOutput: functions to store, Manipulate and Display Markov Chain Monte Carlo (MCMC) Output. R package version 0.1.3. https://CRAN.R-project.org/package=mcmcOutputhttps://CRAN.R-project.org/package=mcmcOutput
Moqanaki EM, Milleret C, Tourani M et al (2021) Consequences of ignoring variable and spatially autocorrelated detection probability in spatial capture-recapture. Landscape Ecol 36:2879–2895
Morelle K, Fattebert J, Mengal C, Lejeune P (2016) Invading or recolonizing? Patterns and drivers of wild boar population expansion into Belgian agroecosystems. Agric Ecosyst Environ 222:267–275
Morgan DR, Nugent G, Warburton B et al (2006) Benefits and feasibility of local elimination of possum populations. Wildl Res 33:605–614. https://doi.org/10.1071/WR06055
Pepin KM, Davis AJ, VerCauteren KC (2017) Efficiency of different spatial and temporal strategies for reducing vertebrate pest populations. Ecol Model 365:106–118. https://doi.org/10.1016/j.ecolmodel.2017.10.005
Pepin KM, Smyser TJ, Davis AJ et al (2020) Optimal spatial prioritization of control resources for elimination of invasive species under demographic uncertainty. Ecol Appl 30:e02126. https://doi.org/10.1002/eap.2126
Pepin KM, Wolfson DW, Miller RS et al (2019) Accounting for heterogeneous invasion rates reveals management impacts on the spatial expansion of an invasive species. Ecosphere 10:e02657. https://doi.org/10.1002/ecs2.2657
Porteus TA, Reynolds JC, McAllister MK (2018) Quantifying the rate of replacement by immigration during restricted-area control of red fox in different landscapes. Wildlife Biology 2018:1-9.https://doi.org/10.2981/wlb.00416
R Core Team (2022) R: A language and environment for statistical computing Risch DR, Ringma J, Price MR (2021) The global impact of wild pigs (Sus scrofa) on terrestrial biodiversity. Sci Rep 11:13256. https://doi.org/10.1038/s41598-021-92691-1
Royle JA, Chandler RB, Sollmann R, Gardner B (eds) (2014) Spatial capture-recapture. Academic Press, Oxford, UK; Malden, MA, USA
Royle JA, Chandler R, Sollmann R, Gardner B (2020) Scrbook Companion to the book: spatial capture Recapture (2014)
Royle JA, Dorazio RM, Link WA (2007) Analysis of multinomial models with unknown index using data augmentation. J Comput Graph Stat 16:67–85
Sabrina S, Jean-Michel G, Carole T et al (2009) Pulsed resources and climate-induced variation in the reproductive traits of wild boar under high hunting pressure. J Anim Ecol 78:1278–1290. https://doi.org/10.1111/j.1365-2656.2009.01579.x
Saunders G, Kay B, Parker B (1990) Evaluation of a warfarin Poisoning programme for feral pigs (Sus scrofa). Wildl Res 17:525–533. https://doi.org/10.1071/wr9900525
Schlichting PE, Fritts SR, Mayer JJ et al (2016) Determinants of variation in home range of wild pigs. Wildl Soc Bull 40:487–493. https://doi.org/10.1002/wsb.662
Servanty S, Gaillard J-M, Ronchi F et al (2011) Influence of harvesting pressure on demographic tactics: implications for wildlife management. J Appl Ecol 48:835–843
Seward NW, VerCauteren KC, Witmer GW, Engeman RM (2004) Feral swine impacts on agriculture and the environment. Sheep Goat Res J 19:34–40
Snow NP, Jarzyna MA, VerCauteren KC (2017) Interpreting and predicting the spread of invasive wild pigs. J Appl Ecol 54:2022–2032. https://doi.org/10.1111/1365-2664.12866
Tanner MA, Wong WH (1987) The calculation of posterior distributions by data augmentation. J Am Stat Assoc 82:528–540. https://doi.org/10.1080/01621459.1987.10478458
Twigg LE, Lowe T, Everett M et al (2006) Feral pigs in north-western Australia: population recovery after 1080 baiting and further control. Wildl Res 33:417–425. https://doi.org/10.1071/WR05098
Veylit L, Sæther B-E, Gaillard J-M et al (2020) How do conditions at birth influence early-life growth rates in wild boar? Ecosphere 11:e03167. https://doi.org/10.1002/ecs2.3167
With KA (2002) The landscape ecology of invasive spread. Conserv Biol 16:1192–1203. https://doi.org/10.1046/j.1523-1739.2002.01064.x
Acknowledgements
This work was funded by the U.S. Department of Energy–Savannah River Operations Office through the U.S. Forest Service–Savannah River under Interagency Agreement No. 89303720SEM000037 and by the U.S. Forest Service Southern Research Station. We thank T. Mims for assistance in facilitating the study, R. Sutton, C. Ruth, Jr., and D. Hammett for assistance with camera trap deployment, and M. Bellamy, C. Parenti, and M. Pavlosky, Jr. for assistance with camera trap image processing.
Author information
Authors and Affiliations
Contributions
JEG and JCK contributed equally to study conceptualization, methodology, and data collection. JEG ran analyses and visualized results. JEG wrote the first draft of the manuscript. Both JEG and JCK reviewed, edited, and contributed to subsequent revisions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garabedian, J.E., Kilgo, J.C. Rapid recovery of invasive wild pig (Sus scrofa) populations following density reduction. Biol Invasions 26, 1075–1089 (2024). https://doi.org/10.1007/s10530-023-03230-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03230-0