Abstract
The European catfish (Silurus glanis) has been described as an invasive species exerting a relevant impact on the native fauna of the ecosystems where it is introduced. However, the lack of long-term data and the low catchability of this species with traditional methods have often made it difficult to evaluate its real impact. The main objective of this study was to investigate the effect of the invasive European catfish on the fish community of the Torrejón reservoir (Tagus River, Spain) using both direct fishing and indirect hydroacoustic methods. This study is the result of eleven years of monitoring. The results evidence the impact of European catfish on the reservoir fish assemblage, especially on the Iberian barbel (Luciobarbus bocagei) which significantly decreased its abundance and biomass from 2010 to 2020. The size structure of the fish assemblages in the reservoir allowed the use of hydroacoustic methodology to discriminate and monitor the population of European catfish. Throughout the 11 years of study, statistically significant differences were identified in the abundance of European catfish as a function of the reservoir´s area (dam - tail axis) and of the limnological period (summer vs. winter), while no significant differences were found in relation to bathymetry (surface - bottom axis). We can conclude that S. glanis has currently established in the Torrejón reservoir leading to the decrease of the Iberian barbel population, the only autochthonous species that persisted in the reservoir and dominated the fish community before the appearance of European catfish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater ecosystems are among the most threatened worldwide and the introduction of invasive species represents one of the main drivers of the decline in freshwater biodiversity (Dudgeon et al. 2006; Simberloff et al. 2013; Doherty et al. 2016). Invasive species can have a plethora of impacts on ecosystems where they are introduced, contributing to diversity loss and fish communities mostly composed of non-native species, as part of a process known as biotic homogenization (Rahel 2000, 2002). Although the number of studies showing the negative effects of invasive fish species has grown, the long-term impact of most introduced species has rarely been studied (García-Berthou et al. 2015). Analysing the effects of invasive species on native fauna can be challenging because baseline data on fish communities collected before the introduction takes place are almost always lacking. Moreover, the introduction of invasive species is rarely followed by a monitoring program to determine the impact on native fish communities (García-Berthou 2007; Clavero et al. 2013; Simberloff et al. 2013).
European catfish (Silurus glanis, L. 1758), also known as wels catfish or sheatfish, is one of the most successful species established outside its native range (Elvira and Almodóvar 2001; Copp et al. 2009). This species is native to Eastern Europe and Western Asia and it is among the largest European freshwater species, with a maximum record of 2.7 m in total length and 130 kg in body mass (Bouletreau and Santoul 2016) and individuals over 50 kg being regularly angled (Alp et al. 2011). The population growth of the European catfish and its impact on fish communities in areas outside its native range have been poorly studied, partly due to the lack of data before its introduction and the difficulty to catch this fish using traditional methods such as nets or electrofishing (Carol et al. 2007; Adrian-Kalchhauser and Burkhardt-Holm 2016; Cucherousset et al. 2018; Antognazza et al. 2022). This species is usually discovered years after its introduction when populations have already reached high densities, which hinders management plans or eradication attempts. The European catfish has been described as an opportunistic forager with great adaptability to new prey sources (Carol et al. 2009; Copp et al. 2009; Vejřík et al. 2017) and contrasting results on its impact following introduction outside its native range have been reported (Carol et al. 2009; Guillerault et al. 2015; Cucherousset et al. 2018). This paucity of information on the effects of the introduction of non-native fish top predators which are highly valued by anglers, has led to strong controversies between fishery managers and conservationists (Carpio et al. 2019), especially in ecosystems that are considered already degraded such as reservoirs. Thus, greater efforts should be made to monitor this species and develop alternative methods documenting its presence.
Fish populations in lakes and reservoirs are commonly monitored by the combined use of direct and indirect methods such as net sampling and hydroacoustics, respectively (Kubečka et al. 2009; Chinchorro-Franco 2012). The use of nets allows species community composition and their relative abundance and biomass to be determined. On the other hand, the hydroacoustic methods enable the estimation of total fish density and biomass, as well as the size structure of the whole fish community present in a water body. Moreover, hydroacoustic monitoring is a non-intrusive method that allows sampling of large areas in a relatively short time (Encina et al. 2001; Kubečka et al. 2009; Draštík et al. 2017). This method could be particularly advantageous to detect and monitor fish species such as European catfish since it can reach a much larger body size compared to the other fish species inhabiting the same system. In the present study, we monitored the fish community of the Torrejón reservoir, Tagus basin, central-western Spain, from 2010 to 2020. The reservoir has been systematically studied since 1981, being one of the longest uninterrupted series of fish assemblage monitoring in a reservoir in Spain. European catfish was recorded for the first time in the Tagus basin in 2001 (Doadrio 2001), while anglers started to report captures in 2010. Time series data allow, on the one hand, to evaluate the specific effect of the introduction of European catfish, separating it from other possible factors such as environmental degradation or the introduction of other exotic species; on the other hand, they enable to follow the spatio-temporal dynamics of the fish community after the introduction of European catfish, as the same sampling methodology was applied throughout the years. Thus, the main objectives of this study were: (i) to investigate the impact of European catfish on the fish community of the Torrejón reservoir, evidencing its effects on species abundance, especially on the native ones; (ii) to test the use of hydroacoustic methods as a tool to detect European catfish.
Materials and methods
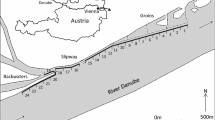
The Torrejón reservoir is located in the river Tagus, central-western Spain (Lat. 39º 53′ 50 N; Long. 5º 46′ 49 W) at around 240 m altitude and has a surface area of 980 ha and a maximum depth of 50 m (Fig. S1). The reservoir has a monomictic thermal cycle, with a mixing period in autumn-winter and stratification in spring-summer. Annual water temperatures range from 9.8 to 29.3 ºC (mean: 18.5 ºC) and the hypolimnion is characterized by marked anoxic conditions and high hydrogen sulfide concentrations. Two-thirds of the reservoir surface belong to the Monfragüe National Park, a protected area of relevance for the native terrestrial and aquatic fauna. The study was conducted in the Torrejón reservoir for eleven years, from 2010 to 2020. Two sampling campaigns were carried out each year: one during the stratification period (summer) and another one in the mixing period (winter), thus comprising a total of 22 sampling campaigns. The same sampling methodology was established in all campaigns, which included direct fishing sampling and indirect hydroacoustic sampling. We selected 10 fishing sampling sites distributed along the longitudinal axis of the reservoir; hydroacoustic surveys were carried out along 10 transects associated with the nets-sampling points. Based on the morphological and limnological characteristics of the reservoir, we identified 6 different study areas (Fig. S1, Table S1 in Supplementary Material). The Torrejón reservoir receives a discharge of hot water from the Arrocampo reservoir (located between areas A2 and A3, (Fig. S1) which is used as a cooling system for the Almaraz Nuclear Power Plant. Because of this, the annual average temperature in the first 8 m of the water column at the confluence point is 0,5 − 1,1 °C higher than other surrounding reservoirs and the area is marked by a strong thermocline. The warmer water plume normally extends 3 km upstream and 9 km downstream while the rest of the reservoir is not affected. It is worth mentioning that in the warm water discharge area from the Arrocampo reservoir, recreational fishing of European catfish is very popular among anglers who practice catch and release despite it being prohibited.
We used a total of 17 trammel nets during each survey, being the sampling unit a 12 × 2 m net, with a mesh size of 2.5 cm (inner panel) and 10 cm (outer panels) set for 24 h. One net was set on the surface and one on the bottom at each sampling site, except for shallow sites P1, P3, and P5, where nets were set only at the surface. All fish captured were identified at the species level and measured (total length Lt, in mm) and weighed (total weight W, in g) in situ. All native species were returned to the water while exotic ones were sacrificed, in compliance with the Spanish Law on exotic species (RD 11/2010, November 16th and RD 630/2013, August 2nd ). A 100 mg/l MS222 solution was used to euthanize fish. The qualitative composition of the ichthyofauna was determined for each area and for the entire reservoir, as well as the abundance and relative biomass of each species. Abundances and biomass were standardized as catch (CPUE) and biomass (BPUE) per unit effort. For the hydroacoustic surveys, we used a Simrad EK60 echosounder (Simrad Kongsberg Maritime AS, Horten, Norway) with a split-beam circular transducer operating at 200 kHz (ES200-7 C). The transducer was mounted on a stainless-steel frame fixed to the side of a boat. The sailing speed remained constant at around 8 km·h–1. The transducer was placed 1 m below the surface. The pulse duration was 0.128 ms, and the repetition rate was 10 pings per second. The acoustic unit was calibrated with a calibration copper sphere using the standard calibration method (Simrad 2004). Fish Target Strength (TS) data were geo-referenced in real time using a GPS, stored on a PC and later processed with the Sonar5 Prov.6.0.1 analysis software (Balk and Lindem 2011). Single echo detections (SED) were analysed 1 m away from the transducer for fish density (fish/ha) estimation; for fish biomass (kg/ha) estimation, TS-length and TS-weight conversion equations were incorporated into the program. In the case of the TS-length conversion, we used the Love equation (Love 1971) while for TS-weight conversion, we incorporated to the program the empirical length-weight equations obtained for each survey (Rodríguez-Sánchez et al. 2016a, b). Each transect was analysed entirely as well as separately in three different depth layers (< 5 m, 5–15 m and > 15 m). Fish density and biomass were estimated, and the associated TS distribution, differentiating individuals into 10 size ranges (Table S2).
We used generalized additive models (GAM) to explore temporal dynamics of fish CPUE, BPUE, density and biomass between 2010 and 2020. GAMs are a non-parametric technique that allows analysis of non-linear relationships over time series (Wood 2017; Sabel et al. 2020; Perales et al. 2021). Separate GAMs were established for total fish community, European catfish and the most abundant species such as Iberian barbel. All GAMs were built with a log link function and included a smooth function of the year and the categorical variables depth, season, and area. A Tweedie distribution was used since response variables had a high proportion of zeros and were non-negative (Dunn and Smyth 2005; Shono 2008). The Tweedie distributions are a subset of the Exponential Dispersion Model family and the power value of the model was reported to indicate if the data were closer to a gamma like (p = 1) or a Poisson-like (p = 2) distribution. The Restricted Maximum Likelihood (REML) was used as a smoothing parameter estimation method. Models were fit using the “mgcv” package and the gam.check function was used to validate each model and set the basis dimensions (k) for the smoother term (Wood 2017). The R statistical program version 4.2.2; (R Core Team 2022) was used for the analyses.
Results
The fish community of the reservoir was composed of 11 species, of which only 3 were native to the Iberian Peninsula: the Iberian barbel (Luciobarbus bocagei), Iberian long-snout barbel (Luciobarbus comizo), and Iberian straight-mouth nase (Pseudochondrostoma polylepis). The other 8 species were exotic: the common carp (Cyprinus carpio), gold fish (Carassius auratus), bleak (Alburnus alburnus), pumpkinseed sunfish (Lepomis gibbosus), largemouth bass (Micropterus salmoides), pike-perch (Sander lucioperca), catfish (Ameiurus melas), and European catfish (Silurus glanis). The pumpkinseed sunfish, largemouth bass, Iberian straight-mouth nase, catfish, and gold fish represented sporadic capture with CPUE values below 0.05 over the entire study period.
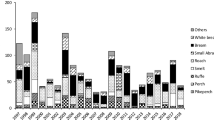
In general, there was a decrease in species CPUE from 2010 to 2020 (Fig. 1a). Total CPUE declined dramatically during the first study years, reaching the lowest value in 2016 and remaining around 1.5 the following years (Table S3). This decrease in total CPUE was significant over the study period (Fig. S2a, Table S4; p < 0.001). On the other hand, total BPUE also decreased during the first study years and reached the lowest value in 2016, but it remained above 3 BPUE towards the end of the study period (Fig. 1b). Total fish BPUE did not differ significantly over the study period (Fig. S2b, Table S4; p = 0.107). Total fish density estimated by hydroacoustic methods declined significantly between 2010 and 2020 (Fig. S2c, Table S4; p < 0.001). Fish density dropped from 2,400 fish/ha in 2010 to less than 300 fish/ha in 2020. Then, although fluctuating, it never reached again values above 500 fish/ha, hitting the lowest value in 2020 (Table S3). Total fish biomass followed a similar pattern to density and was significantly different over the study period (Fig. S2d, Table S4; p < 0.001).
As for the temporal pattern of each single species, we can observe a general decline in CPUE and BPUE of all fish species except for European catfish (Fig. 1). During the study period, a total of 36 European catfish was caught by the trammel nets. This species presented high individual body weight (10.4 ± 12.5 kg). Thus, although European catfish represented only 13.6% of total CPUE in 2020, it became the dominant species in terms of BPUE reaching 66% of the total biomass (Fig. 1a, b). This increase in European catfish CPUE and BPUE was significant during the study period (Fig. 2, Table S5; p < 0.001). On the other hand, the Iberian barbel showed a dramatic decrease. This species, which presented a consolidated and dominant population in the reservoir with over 74% of the total CPUE and BPUE in 2010, decreased significantly to only 32% of the total CPUE and 21% of the BPUE in 2020 (Fig. 2c, d, Table S5; p < 0.001). Thus, there was a clear reverse relationship between the increase in the European catfish captures and the decrease in the Iberian barbel population. Moreover, starting from 2016, there was a clear decrease in both CPUE (from 16 to 8% of the total catches) and BPUE (from 19 to 8%) of common carp and this decline was significant over the study period (Table S5; p < 0.001). In order to be able to monitor European catfish population with hydroacoustic methods, we analysed the size structure of the whole fish community. The species of the reservoir could be differentiated into three size groups: (1) a group of small fish with mean Total Length < 250 mm, mainly represented by bleak; (2) a very homogeneous group, with mean Total Length of 300–500 mm, including the Iberian barbel, Iberian long-snout barbel, and pike-perch; and (3) a group of large fish with mean Total Length > 800 mm, represented by European catfish (Fig. 3). In particular, the total length of European catfish captured ranged from 200 to 2000 mm, with mean size of 1.0 ± 0.4 m. After converting the total length of all the specimens captured throughout the study to their “acoustic size” (Table S2), it was observed that 71% of the captures of European catfish belonged to size range 10 (≥ 794 mm / ≥ −30 dB) and 6.5% to size 9 (≥ 631 mm / ≥ −32 dB), whereas none of the other species captured in the reservoir corresponded to such acoustic size range. This allowed using the hydroacoustic methods as a tool to study the European catfish population in the Torrejón reservoir quantitatively, at least for the adult individuals.
Mean total length for the fish species captured in the Torrejón reservoir during the study period. Dashed line corresponds to a total length of 631 mm (Target Strength − 32 db) and differentiates the size range of European catfish from the other fish species. Species with CPUE values lower than 0.05 over the study period were not displayed. Error bars represent standard deviation
While European catfish were caught for the first time in 2012, the hydroacoustic methods detected specimens of the size range corresponding to this species already in 2010, i.e., two years before. Both the density and biomass of the European catfish showed significant differences in time (Fig. 4, Table S6; p < 0.001). These two parameters decreased significantly in 2012, maintaining their values low until 2016; then, in 2017, high values were recorded again, followed by a decrease in 2018. Thus, there was a temporal evolution of the population that seemed to reflect a fluctuating dynamic, and which could be linked to predator-prey population cycles. The comparison of European catfish relative CPUE and density with the rest of the species (Fig. S3) effectively shows an interesting population dynamic that is similar to the stable limit cycles of the Lotka-Volterra predator-prey model (Begon et al. 2006).
Statistically significant differences were identified in the density of European catfish as a function of the reservoir’s area and limnological period (Fig. 5, Table S6). European catfish density from area A2 and A3 were more likely to be lower than density from area A1, A4, A5, and A6. Already from 2010, European catfish was detected from the tail area (A1) to the dam area (A5 and A6) of the reservoir. It is worth highlighting that the areas A2 and A3 influenced by the discharge of Arrocampo showed the lowest detection of European catfish (12 and 13 fish/ha, respectively). On the contrary, the areas A1 and A5 showed the highest density values (26 and 30 fish/ha, respectively). In respect of the limnological period, there was, in general, a greater detection of this species during winter (27 fish/ha) compared with summer (15 fish/ha). The results do not indicate any tendency regarding the bathymetric distribution of the species in the reservoir. For all the areas and throughout the limnological cycle, the density of European catfish detected in the shallowest stratum (< 5 m) was similar to that detected in the middle (5–15 m) and deep strata (> 15 m). The variation in European catfish biomass showed similar pattern to density (Table S6).
Discussion
In this study we showed that the introduction of the European catfish has resulted in a substantial decline in fish abundance within the Torrejón reservoir. Furthermore, it has caused notable disruptions to the fish community, leading to shifts in species composition and alterations in biomass distribution. The illegal introduction of exotic species into this reservoir has been a usual practice over the years of monitoring. In 2020, there was a total of eight exotic species in the reservoir and only two native ones. Before the European catfish was introduced, the fish community in the reservoir was relatively stable, with the Iberian barbel and common carp dominating in terms of abundance and biomass and coexisting with previously introduced exotic predator species such as the largemouth bass and pikeperch. However, the arrival of the European catfish marked a significant shift in the fish community, leading to a sharp decline in overall fish populations. Since 2016, the fish populations have remained consistently low, failing to recover.
The Iberian barbel was the only endemic species that had an abundant and well-consolidated population, with a large-size structure in the reservoir. Regarding the other two native species, the Iberian nase exhibited a minimal population at the beginning of this study, with 2011 marking the last recorded year for this species. Similarly, the Iberian long-snout barbel presented low captures, albeit constant during the study period. Currently, the Iberian barbel is considered as a vulnerable species in Spain (Freyhof and Kottelat 2008; Doadrio et al. 2011), and the introduction of the European catfish makes the survival of this species extremely difficult, exerting an added pressure on its already vulnerable populations.
The European catfish is recognized as one of the largest freshwater fish species globally, ranking among the top 20(Stone 2007) and has been described as an opportunistic omnivorous predator with acute predatory senses, positioning it as an apex predator (Stolyarov 1985; Vejřík et al. 2017; Ferreira et al. 2019). Its remarkable dietary plasticity, combined with its predatory abilities (Carol et al. 2009; Copp et al. 2009), confers this species a significant role in the fluctuations of fish populations within the ecosystems it colonizes. This poses a substantial risk to any potential prey species residing in the aquatic environment (Castaldelli et al. 2013; Haubrock et al. 2020) and particularly to native fish populations that are not adapted to heavy predation, such as in the Iberian Peninsula with a significant presence of endemic species with restricted distributions (Doadrio 2001; Elvira and Almodóvar 2001; Encina et al. 2006; Ribeiro et al. 2009). Likewise, our results suggest that the predation by the European catfish could be the leading drive of the decrease of the fish populations in the reservoir, as demonstrated by the population dynamics presented by this species compared with the other fish (all of which are in fact potential preys), and it fits a typical fluctuating predator-prey dynamic (Begon et al. 2006). Other authors have also highlighted the indirect consequences of the European catfish introduction, particularly the increased pressure on introducing additional exotic species (Elvira and Almodóvar 2001; García-Berthou et al. 2015) The piscivorous nature of the European catfish generates a need for suitable prey species to support its population. These species, such as the bleak (Alburnus alburnus), are usually introduced to compensate for the high predator pressure on the fish fauna, especially on the native species (Vinyoles et al. 2007; Almeida et al. 2014), and to maintain the population stock of both predators and their prey in a certain balance. As it can be observed in Torrejón, the European catfish appeared almost at the same time as the bleak in the study, being an exotic species that had not been caught in the reservoir before the year 2009.
The hydroacoustic methods used in this study do not allow for remote species identification. However, the early large size reached by European catfish allowed differentiating the adult specimens of this species from the rest of the fish present in the reservoir. In this study, an acoustic size of over − 30 dB was selected, thereby ensuring that 100% of the detected specimens of such size corresponded to adult European catfish individuals. This allowed estimating the density and biomass of adult European catfish specimens in the reservoir, as well as analysing its spatio-temporal distribution. This is of great relevance given the difficulty to catch European catfish with standard fish sampling methods and thus evaluate and monitor this species, leading to underestimation of its populations (Benejam et al. 2007; Carol et al. 2007). The hydroacoustic results confirmed those obtained by fishing, showing the increase in density and biomass of European catfish over the study period. In fact, in the last study year it represented the species with the highest percentage of contribution to the fish biomass. Considering the relatively low fishing effort with respect to the large size of the reservoir, along with the estimated population density ranging between 3 fish/ha and 48 fish/ha during the study period and with a mean of 13 fish/ha for the 11 years analysed, the capture results confirm that this species is not only well-established in the reservoir, but also abundant. It is important to note that due to methodological limitations of hydroacoustic sampling of small European catfish individuals, the density and biomass of the whole population in the reservoir is likely to be higher.
Based on the hydroacoustic results, the species was active throughout the entire year in Torrejón, with significantly higher density detected in winter. In its native range and in other cold areas where the species has been introduced, S. glanis shows a marked seasonal behaviour, with a clear tendency to hibernation or lethargy in winter, whereas in spring and summer it increases its activity. This seasonal pattern seems to be clearly marked by environmental variables such as temperature, water flow and oxygen concentration (Slavík et al. 2007; Daněk et al. 2014, 2016; Alp 2017; Capra et al. 2018) It is important to take into account that this species lives in waters whose temperature range varies between below 0 ºC and over 30 ºC, with a physiological optimum range of 22–27 ºC (Copp et al. 2009; Lindell 2021). Kuzishchin et al. (2018) stated that, with a water temperature of over 2.5 °ºC in the coldest climates, the spring activity already begins, and with temperatures of 7–12 ºC, the European catfish is already clearly active. Thus, in warmer climates such as the Iberian Peninsula, this species could be relatively active throughout the entire year (Bergé 2012; Capra et al. 2014).
Surprisingly, a lower density of specimens was detected in summer compared to winter; furthermore, a smaller number of individuals was captured in summer than in winter throughout the study period (8.5 CPUE vs. 11.25 CPUE). These results could be associated with a change in the preferences of spatial occupancy of the species in the reservoir, for example, towards shallow coves or tributaries. S. glanis reproduces between May and August, for which it could move to tributaries (low-order streams are used mostly for breeding) and shallow areas, where they usually gather and build their nests for spawning (Wolter and Bischoff 2001; Alp et al. 2004; Gago et al. 2016; Antognazza et al. 2022). Other studies have also reported the appearance of large groups of European catfish in shallow areas and tributaries, associated with the breeding area of other fish species, which thus constitute an abundant and easily accessible resource. Kuzishchin et al. 2018 pointed out the case of European catfish located at depths of only 40–50 cm, where they keep their mouth open for feeding. Boulêtreau et al. (2011) also reported unexpected colossal aggregations of European catfish, but not associated with reproduction or foraging behaviour. Nonetheless, the Technical Service of the National Park of Monfragüe has reported that the European catfish tends to gather in the tributaries of the Torrejón reservoir during the summer season (personal communications) and has utilized this phenomenon to occasionally remove some individuals from the reservoir by electric fishing.
Regarding the spatial distribution, it is worth mentioning that area A3, which receives warm water directly from the Arrocampo reservoir, showed a low density of European catfish both in winter and summer. However, considering that the average annual temperature in this area is within the thermal tolerance range of the species, we can exclude the effect of higher water temperatures on fish density. A possible cause of the lower density of European catfish could be the negative effect of the turbulence of water discharge from the Arrocampo reservoir in this area. The area A2 also shows low densities of European catfish and presents high water temperatures. However, in a similar way to the area A3, the summer temperature is well below the species thermal tolerance limit. This area is also subject to great turbulence and presents a large proportion of shallow areas (< 8 m) which could have determined a general lower detectability of fish by vertical acoustic methods.
Regarding the depth distribution, the preferred habitat for this species has been described as slow-moving lotic or lentic waters of considerable depth. However, European catfish is also able to take advantage of different habitats (Cucherousset et al. 2018; Ferreira 2019). The results of the bathymetric analysis show that there are no significant differences in the density of European catfish among the three depth strata The presence of this species in shallow habitats can be attributed to three factors. Firstly, these habitats provide a great availability of prey that are both more abundant and easier for the European catfish to capture. Secondly, the higher water temperature in these areas allows for optimal energy expenditure. Lastly, the presence of large tree roots and sunken trees, remnants of a submerged forest from before the reservoir’s construction, serves as both hunting grounds and resting places for the European catfish. The results also show the great tolerance of S. glanis to low oxygen concentration (even anoxia in deeper layers). These conditions are typical of the hypolimnion of Torrejón reservoir throughout the entire stratification period (from May to October), affecting the whole water column from 10 m in depth (data obtained from the technical-limnological monitoring of the reservoir by the Almaraz Nuclear Power Plant). Nevertheless, in the stratum of 15 m, the density of European catfish detected was not significantly different from that of the epilimnetic strata. Thus, these results corroborate the high tolerance of this species to low oxygen concentration (Copp et al. 2009).
Based on our study, it is evident that S. glanis (European catfish) has successfully established in the Torrejón reservoir. Several factors have contributed to its rapid establishment. Firstly, the species demonstrates remarkable plasticity, particularly in relation to its trophic spectrum, enabling it to adapt and exploit new resources and habitats. Additionally, the European catfish benefits from the elevated temperatures present in the reservoir which allows the species to remain active and thrive throughout the year. Its establishment in the reservoir has led to a decrease in the fish density that this reservoir presented before its appearance, with the greatest impact on the autochthonous population of the Iberian barbel. S. glanis has a relevant impact in the ecosystems where it is introduced, even in greatly modified ecosystems such as reservoirs. These environments provide the ideal place for its establishment and growth, with a direct impact on the native populations, and which, in the case of many species (e.g., the Iberian barbel), constitute the stock of reproductive individuals that, in turn, maintain the population in the upstream sections of the basins in which they are located. It is fundamental to monitor the evolution of the species, in terms of both its population growth and distribution. In this sense, the hydroacoustic methodology implemented in the study was proven to be an ideal tool for the management of the species, as it allows detecting it, analysing its distribution patterns, and quantitatively estimating both its abundance and biomass. Thus, the results obtained in this study represent an important contribution to demonstrate the effect of the introduction of the European catfish on autochthonous fish populations, as well as for the implementation of new methodological tools for the management of this invasive species in freshwater ecosystems.
Data Availability
The data will be provided upon request on authors.
References
Adrian-Kalchhauser I, Burkhardt-Holm P (2016) An eDNA assay to monitor a globally invasive fish species from flowing freshwater. PLoS ONE 11:e0147558. https://doi.org/10.1371/journal.pone.0147558
Almeida D, Stefanoudis PV, Fletcher DH et al (2014) Population traits of invasive bleak Alburnus alburnus between different habitats in Iberian fresh waters. Limnologica 46:70–76. https://doi.org/10.1016/j.limno.2013.12.003
Alp A (2017) Diet shift and prey selection of the native European catfish, Silurus glanis, in a Turkish reservoir. J Limnol Freshw Fish Res 3:15–15. https://doi.org/10.17216/limnofish.288217
Alp A, Kara C, Büyükçapar HM (2004) Reproductive biology in a native European catfish, Silurus glanis L., 1758, population in menzelet reservoir. Turk J Vet Anim Sci 28:613–622
Alp A, Kara C, Üçkardeş F et al (2011) Age and growth of the European catfish (Silurus glanis) in a Turkish reservoir and comparison with introduced populations. Rev Fish Biol Fish 21:283–294. https://doi.org/10.1007/s11160-010-9168-4
Antognazza CM, Costantini T, Campagnolo M, Zaccara S (2022) One year monitoring of ecological interaction of Silurus glanis in a novel invaded oligotrophic deep lake (Lake Maggiore). Water (Switzerland) 14:1–9. https://doi.org/10.3390/w14010105
Balk H, Lindem T (2011) Sonar4 and Sonar5-Pro post processing systems, operator manual version, Lindem Data Acquis Humleveien 4b 870
Begon M, Townsend CR, Harper JL (2006) Ecology from individuals to ecosystems, 4th edn. Blackwell Publishing, Oxford
Benejam L, Carol J, Benito J, García-Berthou E (2007) On the spread of the European catfish (Silurus glanis) in the Iberian Peninsula: first record in the Llobregat river basin. Limnetica 26:169–171. https://doi.org/10.23818/limn.26.14
Bergé J (2012) Apport de la télémétrie acoustique pour la compréhension de l’utilisation dynamique des habitats par les poissons dans un grand fleuve aménagé, le Rhône
Bouletreau S, Santoul F (2016) The end of the mythical giant catfish. Ecosphere. https://doi.org/10.1002/ecs2.1606
Boulêtreau S, Cucherousset J, Villéger S et al (2011) Colossal aggregations of giant alien freshwater fish as a potential biogeochemical hotspot. PLoS ONE 6:4–7. https://doi.org/10.1371/journal.pone.0025732
Capra H, Pella H, Ovidio M (2014) Movements of endemic and exotic fish in a large river ecosystem (Rhône. France), 10 edn. Int Symp Ecohydraulics
Capra H, Pella H, Ovidio M (2018) Individual movements, home ranges and habitat use by native rheophilic cyprinids and non-native catfish in a large regulated river. Fish Manag Ecol 25:136–149. https://doi.org/10.1111/fme.12272
Carol J, Zamora L, García-Berthou E (2007) Preliminary telemetry data on the movement patterns and habitat use of European catfish (Silurus glanis) in a reservoir of the River Ebro, Spain. Ecol Freshw Fish 16:450–456. https://doi.org/10.1111/j.1600-0633.2007.00225.x
Carol J, Benejam L, Benito J, García-Berthou E (2009) Growth and diet of European catfish (Silurus glanis) in early and late invasion stages. Fundam Appl Limnol 174:317–328. https://doi.org/10.1127/1863-9135/2009/0174-0317
Carpio AJ, De Miguel RJ, Oteros J et al (2019) Angling as a source of non-native freshwater fish: a European review. Biol Invasions 21:3233–3248. https://doi.org/10.1007/s10530-019-02042-5
Castaldelli G, Pluchinotta A, Milardi M et al (2013) Introduction of exotic fish species and decline of native species in the lower Po basin, north-eastern Italy. Aquat Conserv Mar Freshw Ecosyst 23:405–417. https://doi.org/10.1002/aqc.2345
Clavero M, Hermoso V, Aparicio E, Godinho FN (2013) Biodiversity in heavily modified waterbodies: native and introduced fish in Iberian reservoirs. Freshw Biol 58:1190–1201. https://doi.org/10.1111/fwb.12120
Copp GH, Robert Britton J, Cucherousset J et al (2009) Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish Fish 10:252–282. https://doi.org/10.1111/j.1467-2979.2008.00321.x
Cucherousset J, Horky P, Slavík O et al (2018) Ecology, behaviour and management of the European catfish. Rev Fish Biol Fish 28:177–190. https://doi.org/10.1007/s11160-017-9507-9
Daněk T, Kalous L, Petrtýl M, Horký P (2014) Move or die: change in European catfish (Silurus glanis L.) behaviour caused by oxygen deficiency. Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2014020
Daněk T, Horký P, Kalous L et al (2016) Seasonal changes in diel activity of juvenile European catfish Silurus glanis (Linnaeus, 1758) in Byšická Lake, Central Bohemia. J Appl Ichthyol 32:1093–1098. https://doi.org/10.1111/jai.13146
de Chinchorro-Franco AJS (2012) Desenvolvimento do método hidroacústico como ferramenta de gestão piscícola e da qualidade ecológica em albufeiras: Aplicação à Albufeira do Maranhão Adolfo José De Sá. Chichorro Franco Dissertação para obtenção do Grau de Mestre em Gestão e Conservação
Doadrio I (2001) Atlas y libro rojo de Los Peces continentales de España, MNCN-CSIC 364, Madrid
Doadrio I, Perea S, Garzón-Heydt P, González JL (2011) Ictiofauna continental española. Bases para su seguimiento
Doherty TS, Glen AS, Nimmo DG et al (2016) Invasive predators and global biodiversity loss. Proc Natl Acad Sci USA 113:11261–11265. https://doi.org/10.1073/pnas.1602480113
Draštík V, Godlewska M, Balk H et al (2017) Fish hydroacoustic survey standardization: a step forward based on comparisons of methods and systems from vertical surveys of a large deep lake. Limnol Oceanogr Methods 15:836–846. https://doi.org/10.1002/lom3.10202
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc 81:163–182. https://doi.org/10.1017/S1464793105006950
Dunn PK, Smyth GK (2005) Series evaluation of Tweedie exponential dispersion model densities. Stat Comput 15:267–280. https://doi.org/10.1007/s11222-005-4070-y
Elvira B, Almodóvar A (2001) Freshwater fish introductions in Spain: facts and figures at the beginning of the 21st century. J Fish Biol 59:323–331. https://doi.org/10.1006/jfbi.2001.1753
Encina L, Rodríguez-Ruiz A, Granado-Lorencio C, Escot-Muñoz C (2001) Gestión y evaluación de embalses. Universidad de Sevilla, Estudio de las Poblaciones de Peces
Encina L, Rodriguez A, Granado-Lorencio C (2006) The Iberian ichthyofauna: ecological contributions. Limnetica 25:349–368. https://doi.org/10.23818/limn.25.24
Ferreira MAMF (2019) European catfish (Silurus glanis) movements and diet ecology in a newly established population in the Tagus drainage. 1–66
Ferreira M, Gago J, Ribeiro F (2019) Diet of European catfish in a newly invaded region. Fishes 4:1–11. https://doi.org/10.3390/fishes4040058
Freyhof J, Kottelat M (2008) Luciobarbus bocagei. The IUCN Red List of Threatened Species 2008: e.T2584A9458518. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T2584A9458518.en
Gago J, Anastácio P, Gkenas C et al (2016) Spatial distribution patterns of the non-native European catfish, Silurus glanis, from multiple online sources – a case study for the River Tagus (Iberian Peninsula). Fish Manag Ecol 23:503–509. https://doi.org/10.1111/fme.12189
García-Berthou E (2007) The characteristics of invasive fishes: what has been learned so far? J Fish Biol 71:33–55. https://doi.org/10.1111/j.1095-8649.2007.01668.x
García-Berthou E, Almeida D, Benejam L et al (2015) Impacto ecológico De Los peces continentales introducidos en la penísula ibérica. Ecosistemas 24:36–42. https://doi.org/10.7818/ECOS.2015.24-1.07
Guillerault N, Delmotte S, Boulêtreau S et al (2015) Does the non-native European catfish Silurus glanis threaten French river fish populations? Freshw Biol 60:922–928. https://doi.org/10.1111/fwb.12545
Haubrock PJ, Azzini M, Balzani P et al (2020) When alien catfish meet—resource overlap between the north American Ictalurus punctatus and immature European Silurus glanis in the Arno River (Italy). Ecol Freshw Fish 29:4–17. https://doi.org/10.1111/eff.12481
Kubečka J, Hohausová E, Matěna J et al (2009) The true picture of a lake or reservoir fish stock: a review of needs and progress. Fish Res 96:1–5. https://doi.org/10.1016/j.fishres.2008.09.021
Kuzishchin KV, Gruzdeva MA, Pavlov DS (2018) Traits of Biology of European Wels Catfish Silurus glanis from the Volga–Ahtuba Water System, the Lower Volga. J Ichthyol 58:833–844. https://doi.org/10.1134/S0032945218060103
Lindell N (2021) Habitat preference and activity pattern of wels catfish (Silurus glanis) at its Northernmost Distribution Area (PhD Thesis)
Love RH (1971) Dorsal‐Aspect Target Strength of an Individual Fish. J Acoust Soc Am 49:816–823. https://doi.org/10.1121/1.1912422
Perales KM, Hansen GJA, Hein CL et al (2021) Spatial and temporal patterns in native and invasive crayfishes during a 19-year whole-lake invasive crayfish removal experiment. Freshw Biol 66:2105–2117. https://doi.org/10.1111/fwb.13818
R Core Team (2022) R: a language and environment for statistical computing
Rahel FJ (2000) Homogenization of fish faunas across the United States. Science 288:854–856. https://doi.org/10.1126/science.288.5467.854
Rahel FJ (2002) Homogenization of freshwater faunas. Annu Rev Ecol Syst 33:291–315
Ribeiro F, Collares-Pereira MJ, Moyle PB (2009) Non-native fish in the fresh waters of Portugal, Azores and Madeira Islands: a growing threat to aquatic biodiversity. Fish Manag Ecol 16:255–264. https://doi.org/10.1111/j.1365-2400.2009.00659.x
Rodríguez-Sánchez V, Encina-Encina L, Rodríguez-Ruiz A et al (2016) Horizontal target strength of Cyprinus carpio using 200 kHz and 430 kHz split-beam systems. Fish Res 174:136–142. https://doi.org/10.1016/j.fishres.2015.09.011
Sabel M, Eckmann R, Jeppesen E et al (2020) Long-term changes in littoral fish community structure and resilience of total catch to re-oligotrophication in a large, peri-alpine European lake. Freshw Biol 65:1325–1336. https://doi.org/10.1111/fwb.13501
Shono H (2008) Application of the Tweedie distribution to zero-catch data in CPUE analysis. Fish Res 93:154–162
Simberloff D, Martin J-L, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66. https://doi.org/10.1016/j.tree.2012.07.013ï
Simrad AS (2004) Operator manual, Simrad EK60 Scientific Echosounder application
Slavík O, Horký P, Bartoš L et al (2007) Diurnal and seasonal behaviour of adult and juvenile European catfish as determined by radio-telemetry in the River Berounka, Czech Republic. J Fish Biol 71:101–114. https://doi.org/10.1111/j.1095-8649.2007.01471.x
Stolyarov IA (1985) Dietary features of catfish, Silurus glanis, and pike-perch, Stizostedion lucioperca in Kizlyarsk Bay, northern Caspian Sea. J Ichthyol 25:140–145
Stone R (2007) The last of the leviathans. Sci (80-) 316:1684–1688
Vejřík L, Vejříková I, Blabolil P et al (2017) European catfish (Silurus glanis) as a freshwater apex predator drives ecosystem via its diet adaptability. Sci Rep 7:1–15. https://doi.org/10.1038/s41598-017-16169-9
Vinyoles D, Robalo J, de Sostoa A et al (2007) Spread of the alien blake Alburnus alburnus (Linnaeus 1758)(Actinopterygii, Cyprinidae) in the Iberian Peninsula: the role of reservoirs. Graellsia 63:101–110
Wolter C, Bischoff A (2001) Seasonal changes of fish diversity in the main channel of the large lowland river oder. River Res Appl 17:595–608. https://doi.org/10.1002/rrr.645
Wood SN (2017) Generalized additive models: an introduction with R. CRC Press, Boca Raton
Acknowledgements
The authors thank the Almaraz Power Plant (CNA), especially the P.R. and Environmental Department, for their financial and logistical support, as well as their involvement in the ecological and ichthyofauna studies in Torrejón reservoir. Thanks to Nicolás Guillén, Responsible of the P.R. and Environmental Department of CNA and Jordi Noguero Ribes, Director of Limnological Area and Water Quality of AECOM. Finally, thanks to the reviewers of the original manuscript for their valuable suggestions.
Funding
Funding for open access publishing: Universidad de Sevilla/CBUA. This study has been funded by the Almaraz Nuclear Power Plant (CNA). All the authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
EL: writing first draft, conceptualization, data analysis, data collection, review; RRA: conceptualization, data collection, review; OC: data collection, writing, editing, review; CJR: data collection, editing, review; dMI: data analysis, editing, review; GLC: conceptualization, editing, review. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
This study has been funded by the Almaraz Nuclear Power Plant (CNA). All the authors have no relevant financial or non-financial interests to disclose. The study has followed the ethical guidelines for research involving fish. All exotic species were sacrificed in compliance with the Spanish Law on Exotic species, while native species were released.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Encina, L., Rodríguez-Ruiz, A., Orduna, C. et al. Impact of invasive European catfish (Silurus glanis) on the fish community of Torrejón reservoir (Central Spain) during a 11-year monitoring study. Biol Invasions 26, 745–756 (2024). https://doi.org/10.1007/s10530-023-03204-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03204-2