Abstract
It can be challenging to distinguish management impacts from other population drivers, including ‘natural’ processes and co-occurring threats. However, disentangling processes is important, particularly when management may have unintended consequences, such as mesopredator release. We explored the effects of long-term, broadscale poison-baiting programs on the distribution of red foxes Vulpes vulpes (targeted invasive predator), feral cats Felis catus (unmanaged invasive competitor) and two of their threatened native prey in two fire-affected regions of south-eastern Australia. We synthesised data from 3667 camera-trap deployments at 1232 sites (172,052 trap-nights), combining experimental manipulation of foxes and fire with space-for-time approaches. Fox control effectiveness—in terms of decreased probability of fox occurrence and increased probability of prey occurrence—depended on the duration and intensity of the poison-baiting program. The effects of fox control on prey occurrence also varied between the two native prey species: fox control was strongly beneficial to the long-nosed potoroo Potorous tridactylus but had no measurable effect on southern brown bandicoot Isoodon obesulus occurrence. Feral cat occupancy tended to be higher in landscapes with long-term fox control, although we found no effect of fox-bait density on fine-scale cat occurrence. Time since fire (0–80 years) was associated with the occurrence of each study species, but its association with invasive predators also differed among vegetation types. Invasive predators and altered fire regimes are key, often overlapping, biodiversity threats. Our work highlights the importance of fine-scale monitoring and consideration of multiple drivers in distribution models to develop effective, tailored conservation strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accurate and precise estimates of the effects of management are essential to inform conservation decision-making, ensure cost-effective allocation of resources and help identify potential unintended consequences of management interventions (Christie et al. 2020). However, reliable inference about the effects of landscape-scale management, including the cause of nil or perverse outcomes, is often difficult to achieve because target populations fluctuate naturally and are often subject to multiple co-occurring threats and management actions (Pressey et al. 2007; Sugihara et al. 2012). Separating management effects from other drivers is particularly difficult for species that occur patchily across broad distributions (Tulloch et al. 2016).
Invasive predator management is a prime example of these challenges. Predators can have devastating impacts on native biodiversity when introduced beyond their native range, and so invasive predators are often lethally controlled (Sih et al. 2010; Bellard et al. 2016; Doherty et al. 2016). Quantifying the degree of invasive predator suppression, the responses of their native prey and any unintended outcomes across gradients of predator control is key to designing cost-effective management programs (Baxter et al. 2008; Walsh et al. 2012; Cattarino et al. 2016). However, the ability of associated monitoring programs to detect these signals is often confounded by co-occurring threats, management actions and natural drivers. This is concerning because few benefits, or even negative effects, for native biodiversity are commonly observed following invasive predator control, particularly when multiple introduced species are present (Ballari et al. 2016).
There are several reasons why native prey species may not benefit from invasive predator control. Firstly, control efforts may not sufficiently reduce the density of invasive predators—predators can be resilient to low-effort culling (Lieury et al. 2015; Moseby et al. 2019). Secondly, invasive predator suppression may lead to the ‘release’ of a subordinate predator or competitor species (referred to as the ‘mesopredator release hypothesis’ Crooks & Soulé 1999), which could potentially worsen net outcomes for native species (Doherty and Ritchie 2017). Thirdly, predation by introduced species may not be the primary limit on native prey populations (Banks 1999). Hence, quantifying the degree of dominant predator suppression and testing the mesopredator release hypothesis (Crooks and Soulé 1999) are important steps toward understanding prey responses to lethal predator control and identifying the cause of a nil response, if observed (Salo et al. 2010).
The effects of invasive predator control on native prey may also be obscured or influenced by other concurrent processes. For example, fire is a key driver of species distributions in many terrestrial ecosystems (He et al. 2019). Fire can mediate predator–prey interactions (primarily through its effects on vegetation structure), and its effects may be long-lasting, non-linear and vary across environmental conditions (Monamy and Fox 2000; Haslem et al. 2011; Hradsky 2020). Fauna species’ responses to fire therefore often vary across heterogeneous landscapes (Nimmo et al. 2014; Swan et al. 2015). Likewise, other disturbance processes such as habitat fragmentation, as well as natural drivers such as vegetation type, terrain ruggedness and rainfall dynamics, play an important role in shaping the contemporary distribution of many predator and prey species (May and Norton 1996; Hale et al. 2016). Simultaneously accounting for the influence of these drivers is likely to improve our understanding of predator control outcomes, including potential release of other invasive mesopredators.
Here we investigated the effect of landscape-scale lethal management of introduced red foxes Vulpes vulpes (hereafter ‘foxes’) on the occupancy of foxes (the managed invasive species), feral cats Felis catus (an unmanaged invasive competitor; hereafter ‘cats’), and two threatened native prey species: the southern brown bandicoot Isoodon obesulus (hereafter ‘SBB’) and long-nosed potoroo Potorous tridactylus (hereafter ‘LNP’). Our study was conducted across two large, protected, fire-prone conservation regions of south-eastern Australia (the Glenelg region and Otway Ranges), and combined experimental and space-for-time approaches. We tested whether poison baiting was effective; that is, whether the density of poison fox-baits was negatively associated with the probability of fox occurrence and positively associated with SBB and LNP occurrence. In line with the mesopredator release hypothesis, we also tested whether bait density was positively associated with cat occurrence. Given that fox control has occurred over a longer period and baits are replaced more frequently in the Glenelg region than the Otway region, we predicted that these effects would be stronger in Glenelg. We examined these relationships while statistically accounting for the effects of other drivers, including time-since fire, distance from forest edge (a proxy for habitat fragmentation), vegetation type and topographic position. Our analysis allowed us to include non-linear effects and identify the relative importance of these different drivers for each species’ distribution.
Materials and methods
Study design
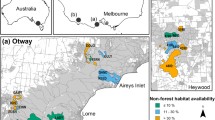
We compiled data from multiple camera-trap studies across two regions in south-west Victoria, Australia: the Glenelg region and Otway Ranges (Fig. 1). Introduced foxes and cats are the only functional medium-large mammalian terrestrial carnivores here: native dingoes Canis familiaris are long-absent throughout, while spotted-tailed quolls Dasyurus maculatus are long-absent in the Glenelg region and likely functionally extinct in the Otway Ranges (last confirmed sighting in 2014). Managers frequently implement prescribed fire across both regions, primarily to reduce fuel loads.
In broad sections of each region, government land managers conduct ongoing targeted lethal fox control for biodiversity conservation. Manufactured poison-baits (‘FoxOff’, Animal Control Technologies, Somerton) containing 3 mg of sodium fluoroacetate (compound 1080) are buried at a depth of 12–15 cm at 1-km intervals along accessible forest tracks and roads. Different road densities therefore result in spatially-variable densities of poison-baits.
In the Glenelg region, three distinct forest blocks have been subject to poison-baiting since October 2005, with baits replaced at fortnightly intervals. These forest blocks, along with three similar, unbaited forest blocks to the north have been simultaneously surveyed annually under the ‘Glenelg Ark’ fox control program since 2013, with 40 camera-trap sites per block (Robley et al. 2020). Here we used camera-trap data from 2013 to 2019. We also included a further 425 camera-trap deployments at unique locations from early 2018 (209 across two baited blocks, 206 across two unbaited blocks; Rees et al 2023a, 2023b). This totals 2,041 camera-trap deployments in the Glenelg region, collected in a control-impact experimental design (Supporting Information Table S1). Foxes had been continuously controlled for 8 - 14 years in the treatment landscapes at the time of these surveys.
In the Otway Ranges, fox-baiting commenced in small sections of the range in 2008. Large-scale systematic baiting began in 2016 - 2017 under the ‘Otway Ark’ program (Robley et al. 2019). For the first six weeks of the Otway Ark program, poison-baits were replaced weekly, this then changed to ongoing monthly bait-replacement. There was a pause in baiting for approximately six months during the second half of 2018. Fox control recommenced in late 2018 with four weeks of fortnightly bait-replacement, before returning to monthly bait-replacement. A large section of the Otway Ranges to the north-west remained unbaited throughout this time, as it was a known stronghold for native prey species, and so the need for fox control was not justified. This section is also monitored as part of the Otway Ark program, (Robley et al. 2019). The Otway Ark land-managers survey 372 camera-trap sites annually (sequentially across the region), with at least one ‘before’ baiting survey, totalling 1113 camera-trap deployments between 2016 and 2018 (Robley et al. 2019). We also included data from additional before-after control-impact surveys (one ‘before’ baiting survey and two ‘after’ bating surveys) in the western section of the Otway Ranges, conducted annually 2017–2019 (Rees et al. 2023a, 2023b). This added a further 195 sites and 524 camera-trap deployments (Supporting Information Table 1).
All camera-trap deployments consisted of a Reconyx (Holmen, Wisconsin) brand camera-trap (white or infrared flash), attached to a tree or a metal picket, facing a lure. The Glenelg Ark and Otway Ark fox monitoring programs positioned camera-traps at least 40 cm above the ground on a tree or a metal picket, angled downwards toward a lure approximately 1–1.5 m away (Robley et al. 2019, 2020). A GPS was used to ensure the camera-trap was placed in approximately the same site each year. The lure consisted of peanut butter, golden syrup and rolled oats mixed into a small ball, placed within a tea strainer or PVC pipe container and secured either to the ground, or 20–60 cm above ground on a wooden stake. The (Rees et al. 2023a) surveys across both regions positioned camera-traps lower on a tree (around 15–30 cm above the ground) angled only slightly downwards towards a tuna oil lure approximately 2–2.5 m away. Camera-traps were active for an average of 47 days (maximum 93 days), totalling 172,052 trap-nights. Camera-traps that were active for fewer than ten days were discarded from the dataset.
Study landscapes
In the Glenelg region (38\(^{\circ }\)05’54”S 141\(^{\circ }\)44’41”E), large patches of natural vegetation are fragmented, mostly by pastoral farming and residential properties (Fig. 1). Here, the primary vegetation communities are heathy woodland, lowland forest, herb-rich woodland and wet heathland (Department of Environment, Land, Water & Planning 2020a). The Glenelg region has an annual mean minimum temperature of 8 \(^{\circ }\)C in winter, and 20 \(^{\circ }\)C in summer, and a mean maximum temperature of 14 \(^{\circ }\)C in winter, and 22 \(^{\circ }\)C in summer (Bureau of Meteorology 2021, Portland Airport). The mean annual rainfall is 835 mm (Bureau of Meteorology 2021, Portland Airport). Terrain is gently undulating in the Glenelg region; study sites ranged from 12 to 180 m above sea level.
The Otway Ranges (38\(^{\circ }\)57’82”S 141\(^{\circ }\)68’41”E) is a largely continuous patch of natural vegetation with a strong east–west rainfall gradient (Fig. 1). A matrix of cool temperate rainforest and wet forest at high-altitudes in the south-west descend into a large heathland directly north, and into dry forests and then heathlands to the north-east. Annual rainfall averages 1955 mm in the southwest, dropping to 627 mm in the eastern Otways (Bureau of Meteorology 2021, Cape Otway). Mean minimum temperatures in the Otway Ranges are 8\(^{\circ }\) in winter and 13\(^{\circ }\) in summer, and mean maximum temperatures are 14 \(^{\circ }\)C in winter, and 21 \(^{\circ }\)C in summer (Bureau of Meteorology 2021, Cape Otway). Our study sites ranged from 23 to 617 m above sea level.
We identified the Ecological Vegetation Class group (standard units for vegetation classification in Victoria, Department of Environment, Land, Water & Planning 2020a - hereafter ‘vegetation type’) for each unique camera-trap site. There were eight vegetation types in total. However, we only surveyed 20 unique sites in rainforests, which are interspersed (primarily in low lying gullies) throughout wet and damp forests in the south-eastern Otway Ranges. Given the similarity, fine-scale interspersion of these vegetation types, and that both rarely or never experience fire, we merged them together (hereafter referred to as ‘wet forests’).
Occupancy-detection models
We first modelled species occupancy probabilities using occupancy-detection models (MacKenzie et al. 2002) implemented in a Bayesian framework using ‘stan’ (Carpenter et al. 2017) via the ‘ubms’ R-package (version 1.0.2, Kellner 2021). For each species, we fit a ‘stacked’ single-season model: we included a random intercept for each unique camera-site to account for repeat sampling (Kéry and Royle 2020). We defined survey occasions as 24-hour periods commencing at midday. We modelled the effect of fox control (categorical: baited or unbaited) on species occupancy and detectability, and included an interaction term with region to account for potentially different responses in the Glenelg and Otway regions. We also included the effect of vegetation type on occupancy (see Sect. 2.2), as well as lure type (peanut butter or tuna oil) on detectability. We fit models with uninformative priors, with four MCMC chains each with 10,000 iterations (including a 5000-iteration warm-up phase). To determine whether species’ detection probabilities were high enough to permit analysis with generalised additive models (which do not account for imperfect detection), we calculated cumulative detection probabilities for camera-trap survey lengths (Garrard et al. 2008) from one to 93 days (maximum survey duration).
Generalised additive models
The occupancy-detection models revealed high (> 75%) cumulative detection probabilities for each species in both baited and unbaited landscapes for each region, and for both camera-trap lure types, based on our average camera-trap survey effort, except for cats in the Glenelg region (Supporting Information Fig. S2; Fig. S3). We therefore went on to model species occurrence probabilities (using the raw presence-absence data) with binomial GAMs implemented in the ‘mgcv’ R-package (version 1.8-40, Wood 2017). The use of GAMS has the advantage of allowing modelling of non-parametric and nonlinear relationships between response (species occurrence) and explanatory variables in a computationally efficient framework. This is particularly important for estimating the responses to time since fire (in years; hereafter ‘TSF’) (Haslem et al. 2011).
The response variable in the GAMs was binary occupied-unoccupied for each species for the entire camera-trap deployment at a site in a year. To account for differences in survey duration at different sites and years, we specified a model offset for the log-transformed survey duration (number of days). We accounted for pseudoreplication from repeatedly surveying the same site in multiple years by including a random-intercept for each unique site. We used the double penalty model selection approach, which penalises model complexity both in terms of the model structure (which explanatory variables are included) and the shape (wiggliness) of the relationships between the response and explanatory variables (Marra and Wood 2011). We used the same model structure for each species, as detailed in the sections below. We assessed model fit and predictive performance measures (proportion of the null deviance explained, adjusted R-squared value and Akaike Information Criterion; hereafter ‘AIC’) score against a ‘null model’ for each species which only had a random intercept for each camera-trap site.
1080 poison-bait density
Poison-baits are deployed by land managers to suppress foxes with the aim of benefiting native prey. The degree of fox suppression is likely to be a function of the spatial arrangement of poison-baits (i.e., poison-bait density) relative to fox home-range size, as well as the frequency of bait replacement (Fleming 1996; Benshemesh et al. 2020). In the Otway and Glenelg regions, adult foxes travel an average maximum distance of 2.3 km from their home-range centre (Hradsky et al. 2017). Therefore, to examine the typical lethal control effort experienced by a fox in these landscapes, we summed the number of poison-bait stations within a 2.3 km radius around each camera-trap deployment. Bait densities ranged from 0 to 19 baits per 16.1 km2 circle (2.3 km radius), with a mean value of 10 and eight baits per circle in fox-baited landscapes in the Glenelg region and Otway Ranges, respectively. For ease of comparison with other studies, we converted these values to baits per square kilometre. We modelled a function of 1080 poison-bait density with separate responses for each region. Interpretation of these relationships should keep in mind that they are context-specific and conservative: they do not account for the fact that (i) in wet weather conditions, some poison-baits deteriorate and become unavailable to foxes and these baits do not get replaced, or (ii) there was an approximately six month pause in bait replacements in the Otway Ranges in 2018, or (iii) there are differences in baiting frequency between the regions.
Fire and vegetation type
We expected species occurrence to (i) differ across vegetation types, (ii) respond to TSF, and (iii) have variable responses to TSF in each vegetation type (as post-fire regeneration occurs at different speeds, Swan et al. 2015). We also expected species to respond to fire frequency, as this can have a strong effect on vegetation structure (Collins et al. 2012).
We derived fire frequency (the number of previous fires) and time since last fire (in years) for each camera trap deployment using coarse fire scar mapping provided by government managers, dating back to 1939 when large wildfires burnt both regions extensively. On average, sites for each camera-trap deployment had been burnt 1.5 times since 1939 at the time of camera deployment. The most frequently burnt sites (two deployments at different unique sites) had experienced eight fires; 397 camera-trap deployments (11%) had not burnt since 1939.
We modelled an interaction between TSF and vegetation type (see Sect. 2.2) using a hierarchical model structure, which estimated an average TSF response for each species, along with separate responses to TSF in each vegetation type (model ‘GS’ detailed in Pedersen et al. 2019). This approach shares information on TSF responses and wiggliness across vegetation types, penalising functions which deviate strongly from the average response that are not substantially supported by the observation data. We initially fit a separate smooth for fire frequency; however, this had high concurvity with TSF (causing a lack of identifiability) and so we removed the fire frequency variable from all models.
Elevation, topographic ruggedness and wetness
Elevation is a strong driver of rainfall, moisture and temperature gradients in both regions, likely indirectly impacting the distribution of the study species through resource availability. High topographic complexity (i.e., ‘ruggedness’) can limit predator movement and predation rates, thereby benefiting prey (McKenzie et al. 2007; Hohnen et al. 2016; McDonald et al. 2017; Stobo-Wilson et al. 2020). Soil moisture (estimated by the topographic wetness index) impacts vegetation, as well as the availability of subterranean invertebrates and fungi - key food sources for LNPs and SBBs (Lobert 1990; Nuske et al. 2017).
We extracted the elevation above sea level (metres) of each site using a 10-m resolution digital elevation model (Department of Environment, Land, Water & Planning 2020b). We also used this elevation layer to calculate the median terrain ruggedness index (calculated as the difference in elevation between a central cell and eight adjacent cells, Riley, DeGloria, & Elliot 1999), taking the median value in a 30-m radius around each camera-trap site. The topographic wetness index estimates where water will accumulate by accounting for topographic influences on hydrological processes (Beven and Kirkby 1979). We also took the median topographic wetness index in a 30-m radius around each camera-trap site, derived from a 30-m resolution layer (Gallant and Austin 2012). We modelled the effect of elevation, as well as indices of topographic ruggedness and wetness (both derived from a digital elevation model) on species occurrences.
Proximity to forest edge
Invasive predators are well-documented to prefer edges between forest and cleared land as they facilitate efficient movement and hunting (e.g., McGregor et al. 2014; Hradsky et al. 2017; Nichols et al. 2019). We modelled the effect of the minimum distance from each camera-trap site to the nearest substantial area of non-native vegetation. We calculated this by inverting the extent of native vegetation (Department of Environment, Land, Water & Planning 2019) and removing cleared areas smaller than 30 ha, as per Geary et al. (2020).
Recent rainfall
Changes in short-term rainfall dynamics likely impact invasive predator and native prey species (Arthur et al. 2012; Wilson et al. 2012; Paull et al. 2013; Greenville et al. 2014). We therefore calculated the percentage difference in rainfall from the long-term median that had occurred prior to the start of each camera-trap deployment in a six, 12, 18 and 24 month period. We used rainfall data from the nearest weather station (n = 11, Bureau of Meteorology 2021) for each camera-trap. We modelled rainfall effects separately for each region. To identify the most appropriate rainfall window (6–24 months) for each species, we fit a separate model (including all other explanatory variables listed above) using each rainfall window and selected the top-ranked model using AIC scores (Burnham and Anderson 2004).
All analyses were conducted in R version 3.6.3 (R Core Team 2020), relying on the ‘dplyr’ R-package (Wickham et al. 2022) for data cleaning and ‘ggplot2’ (Wickham 2016) for visualisation.
Locations of our study regions in south-west Victoria, Australia. Camera-trap sites are denoted by white dots. The Glenelg region is to the west and Otway region to the east. Native vegetation is indicated by dark green, with hill shading. Map tiles by Stamen Design, under CC BY 3.0, map data by OpenStreetMap, under CC BY SA
Results
Red fox
Foxes were detected on 1453 of the 3667 camera-traps deployments (40% naïve occupancy rate; Supporting Information Table S3). The occupancy-detection model estimated that fox detectability was lower in landscapes with fox control; this effect was stronger in the Glenelg region than Otway Ranges (Supporting Information Fig. S3). Foxes were also more detectable on camera-traps with a peanut butter lure than a tuna oil lure (Supporting Information Fig. S2). Nonetheless, cumulative detection probabilities were universally high (>75%) for the average survey duration (47 days). In the baited sites (where fox detectability was lower relative to unbaited sites), accounting for imperfect detection made little difference to the average predicted site occupancy rate (0.23), relative to the naïve occurrence rate (0.20).
The occupancy-detection model estimated fox occupancy to be lower at sites in baited landscapes than unbaited landscapes; this effect was more than twice as strong in the Glenelg region than the Otway Ranges (Supporting Information Fig. S4). For example, in heathy woodlands of the Glenelg region, fox occupancy probability was 0.56 (95% CI 0.47-0.66) in unbaited landscapes and approximately three times lower in baited landscapes (0.19; 95% CI 0.13-0.26). In the heathy woodlands of the Otway Ranges, by comparison, fox occupancy was already relatively low without fox control (0.33; 95% CI 0.25-0.43) and was approximately 1.4 times lower with fox control (0.24; 95% CI 0.17-0.33). Foxes were ubiquitous across the study regions, but the probability of occupancy was nearly twice as high in dry forests, herb-rich woodlands and lowland forests, than heathlands, heathy woodlands and wet forests (Supporting Information Fig. S5).
The GAM showed that fox-bait density in the Glenelg region was the strongest driver of fox occurrence. Fox occurrence in the Glenelg region declined from a probability of 0.68 (95% CI 0.58-0.76) where fox-bait density was zero, to 0.04 (95% CI 0.02-0.11) where fox-bait density was highest (1.14 baits km-2; Fig. 2a). The effect of fox-bait density on fox occurrence in the Glenelg region was nonlinear: the probability of occurrence declined steeply between 0 and 0.3 baits km-2, there was little difference in fox occurrence across the range of 0.4–0.8 baits km-2, and greater suppression was again achieved at higher bait densities (Fig. 2a). Fox occurrence also declined with fox-bait density in the Otway Ranges, but this effect was linear, weaker and had higher uncertainty (Fig. 2a). In the Otway Ranges, fox occurrence declined from a probability of 0.40 (95% CI 0.32-0.48) where fox-bait density was zero, to 0.14 (95% CI 0.05-0.35) where fox-bait density was highest (1.1 baits km-2; Fig. 2a).
There was no average TSF response on fox occurrence. Fox occurrence declined linearly with TSF in dry forests and increased linearly with TSF in heathland, although there was considerable uncertainty in these estimates (Fig. 2g, h).
Fox occurrence declined linearly with increasing terrain ruggedness, and with distance to non-native vegetation for distances up to approximately 1.5–2.0 km (Fig. 2d, f). Elevation had a nonlinear and uncertain effect on fox occurrence, which was estimated to peak around 450 m above sea level (Fig. 2c). The effect of topographic wetness on fox occurrence was removed from the model, indicating that there was no support for this relationship (Fig. 2e). The fox GAM that considered rainfall deviation in the previous six months was ranked more highly than models with 18- (by only 1.4 AIC units), 12-, and 24-month periods (by at least 6.4 AIC units; Supporting Information Table S4); however, this effect was weak with relatively high uncertainty (Fig. 2c).
The top-ranked fox GAM had an adjusted R-square value of 0.27 and explained 26% of the null deviance. Relative to the null model (with only random intercept for site), the explanatory variables improved predictive performance considerably (242 AIC units lower), but slightly worsened the model fit (Supporting Information Table S5).
Feral cat
Cats were detected on 1010 camera-trap deployments (27.6%; Supporting Information Table S3). Cats were relatively poorly detected in the Glenelg region, where they had a 59% probability of detection if they were present for the average survey duration, compared to 83% in the Otway Ranges; Supporting Information Fig. S3). There was no strong difference in detectability between fox control treatment landscapes in either region (Supporting Information Fig. S3). Cats were more detectable on camera-traps lured with tuna oil relative to peanut butter: for a 47-day survey duration in the unbaited landscapes of the Glenelg region, cats had a detection probability of 57% with peanut butter lures and 70% for tuna oil lures (Supporting Information Fig. S2).
The occupancy-detection models estimated that cat occupancy in the Glenelg region was higher in landscapes with fox control (e.g., 0.25 in heathy woodlands; 95% CI 0.16-0.35 in heathy woodlands) than those without fox control (0.12 in heathy woodlands; 95% CI 0.07-0.19). In contrast, there was no association between cat occupancy and baiting in the Otway Ranges (Supporting Information Fig. S4). Cat occupancy was most strongly driven by vegetation type: it was highest in the wet forest, followed by heathland, swampy scrub, herb-rich woodland and dry forest, and very low in lowland forest and heathy woodland (Supporting Information Fig. S5).
The cat GAM showed no effect of fox-bait density, elevation or topographic wetness on cat occurrence (Fig. 3a, c, e). Cats responded to TSF differently across each vegetation type, with the average TSF response removed from the model (Fig. 3g, h). Cat occurrence probability increased with terrain ruggedness, and declined with distance from the nearest area of non-native vegetation, although uncertainty was high (Fig. 3d, f). The different rainfall deviation windows were indistinguishable based on AIC scores; but in all cases except six months, the rainfall variable was removed from the model. The model that considered rainfall deviations in the previous six months was marginally top-ranked (by 0.7 AIC units) and estimated that cat occurrence slightly increased as rainfall increased relative to the long-term average (Fig. 3c).
The top-ranked cat GAM had an adjusted R-square value of 0.24 and explained 24% of the null deviance. Relative to the null model, the explanatory variables improved predictive performance considerably (168 AIC units lower), but only slightly improved the model fit (less than 1% increase in null deviance explained and 0.02 for the R-squared value; Supporting Information Table S5).
Southern brown bandicoot
We detected SBBs on 394 of the 3,667 camera-traps (10.7%; Supporting Information Table S3). SBBs were highly detectable, with a greater than 95% detection probability reached after 31 and 43 survey days in the Glenelg and Otway Ranges, respectively (Supporting Information Fig. S4). Baiting was associated with a decrease in SBB detectability in Glenelg but an increase in detectability in the Otways (Supporting Information Fig. S3). SBBs were slightly more detectable on camera-traps lured with peanut butter relative to tuna oil. However, neither baiting nor lure type had a meaningful effect on the culmulative detection probability across an average 47-day survey period (all probabilities greater than 95%; Supporting Information Fig. S2).
There was no discernible effect of fox control on SBB occupancy in either region (Supporting Information Fig. S4). SBBs were most likely to occupy heathy woodlands (Supporting Information Fig. S5) and they were largely absent from wet forests (Supporting Information Table S3). The few SBB detections in wet forest occurred at sites adjacent to other vegetation types (SBBs are largely replaced by long-nosed bandicoots Perameles nasuta in wet forest; M. Rees, unpublished data).
SBB occurrence probability was very low. There was some indication SBB occurrence increased slightly with fox-bait density in the Glenelg region, peaked at around 150 m above sea level and 2 km from the nearest forest edge, and declined with increasing terrain ruggedness and TSF; however, these explanatory variables had high uncertainty relative to the strength of the effects (Fig. 4). There was no evidence that rainfall affected SBB occurrence – the top-ranked models in terms of AIC scores had the effects of rainfall completely removed (Supporting Information Table S4).
The top-ranked GAMs for SBBs had an adjusted R-square value of 0.31 and explained 41% of the null deviance. Relative to the null model, the explanatory variables improved predictive performance considerably (211 AIC units lower) and slightly improved the model fit by 4% of null deviance explained and 0.05 for the R-squared value (Supporting Information Table S5).
Long-nosed potoroo
We detected LNPs on 331 camera-trap deployments (9%; Supporting Information Table S3). LNPs were the most detectable of our study species, with culmulative detection probabilities for the average 47-day survey period exceeding 95% for all baiting treatments, regions and lure types. LNP daily detectability in the Glenelg region was twice as high in landscapes with fox control relative to those without (Supporting Information Fig. S3). LNPs were slightly more detectable on camera-traps lured with peanut butter relative to tuna oil (Supporting Information Fig. S2).
Occupancy of LNPs was highest in heathlands (Supporting Information, Fig. S5). The GAM showed that the probability of LNP occurrence improved from 0.05 (95% CI 0.02-0.11) to 0.33 (95% CI 0.16-0.55) across the fox-bait density gradient in the Glenelg region (Fig. 5a). In contrast, LNP occupancy showed no relationship with fox-bait density in the Otway region (Fig. 5a). LNP occurrence probability increased linearly with elevation (Fig. 5c) and peaked in the mid-range of topographic wetness (Fig. 5e). LNP occurrence was low initially after fire, but peaked around 20 years and remained steady in the years afterwards, although there was considerable uncertainty (Fig. 5e). There were no discernible differences in LNP responses to TSF across the vegetation types (Fig. 5f). The rainfall term was removed for the top-ranked model (at least 3.5 AIC units higher than models that included it).
The top-ranked model had an adjusted R-square value of 0.47 and explained 53% of the null deviance. Relative to the null model, the explanatory variables improved predictive performance (94 AIC units lower) and slightly improved the model fit by 3% of null deviance explained and 0.02 for the R-squared value (Supporting Information Table S5).
Discussion
We found that consistent, long-term and intense lethal control can reduce a widespread invasive dominant predator (fox) to a near-zero occurrence probability and—importantly—increase the occurrence probability of a threatened prey species (LNP) more than 6-fold. However, prey responses to predator suppression are not universal (Sinclair et al. 1998; Duncan et al. 2020). Despite Robley et al. (2014) finding that SBB site occupancy increased in the early stages of the Glenelg Ark fox baiting program, we found little evidence that SBB occurrence increased with fox control in either region. This may have been because feral cat occurrence was slightly higher in the landscapes with fox control relative to those without in the Glenelg region, potentially signalling mesopredator release. In the Otway region (where fox control recently commenced and baiting was less frequent), foxes were suppressed to a lesser extent (as expected), and neither threatened prey species nor cats showed signs of improvement. Our study reinforces that lethal invasive predator control can be a highly effective conservation strategy, but only for some species and when sustained continuously over the long-term.
The Glenelg Ark program has continuously controlled foxes across approximately 100,000 ha of public land since 2005 (Robley et al. 2014) and is one of the few fox control programs in Australia to demonstrate a sustained reduction in fox occupancy (see also Stobo-Wilson et al. 2020). Our study provides empirical evidence that the effectiveness of fox control from poison-baiting programs depends on the density of poison-baits deployed (Fig. 2a). This has only previously been inferred by comparing fox baiting programs across different regions (where fox ecology, environmental conditions, and study designs, such as aerial and pulse-baiting, differ). The densities of poison-baits in our study regions (maximum 1.14 baits km-2) were far below the recommended 5–10 baits km-2 (mostly derived from studies in arid and semi-arid regions with different baiting regimes, Saunders & McLeod 2007), but nonetheless were effective at suppressing fox occurrence in the Glenelg region (Supporting Information Fig. S4). Increasing bait density to at least 0.3 baits km-2 was particularly effective at controlling foxes in the Glenelg region, reducing fox occurrence by up to four-fold compared to unbaited regions; at higher bait densities, increasing poison-bait densities continued to increase suppression of foxes, but with a weaker rate of return (Fig. 2a). Increased bait caching at high bait densities may explain why fox suppression tapered off, which is likely to result in a sublethal dose when eventually consumed and potential bait aversion (Saunders and McLeod 2007). Nonetheless, benefits to threatened native prey is the best metric of fox control effectiveness. The probability of LNP occurrence increased linearly with poison-bait density in the Glenelg region (Fig. 5a), confirming that high fox control effort leads to improved conservation outcomes.
We slightly underestimated the effect of bait density across both regions because the models assumed all bait-stations were constantly active, despite some bait-replacements being missed due to wet weather events or more pressing management concerns (namely wildfire). Our estimates of species’ responses to fox-baiting can therefore be considered conservative, given we modelled a more consistent control effort than actually happened. We more strongly underestimated the effect of bait density in the Otway Ranges because we also did not account for a near six-month pause in bait replacement in 2018. We also expect fox-baits to be less effective in the Otway Ranges than the Glenelg region due to the higher rainfall which more quickly degrades the poison (Saunders et al. 2000; Gentle et al. 2007). Additionally, fox occupancy was already much lower in the Otway Ranges prior to fox control than in the unbaited landscapes of the Glenelg region, likely because the wetter conditions and more intact forest patches in the Otways provide less suitable fox habitat. Nonetheless, fox occupancy in the Otway Ranges was still negatively associated with fox-bait density (Fig. 2a), suggesting that intense and sustained fox control is likely to be effective in that region. Future research will benefit from accounting for the role of prey availability and environmental conditions (e.g. the effects of soil wetness on bait degradation) on baiting effectiveness (Saunders and McLeod 2007; Carter and Luck 2013), as well as baiting frequency and interference with baits by non-target species (Fairbridge et al. 2000; Glen and Dickman 2003; Marlow et al. 2015).
Evidence that fox control caused mesopredator release of feral cats in terms of occupancy was mixed. Cat occupancy was higher in sites with fox control in the Glenelg region, but not the Otway Ranges (occupancy-detection model; Supporting Information Fig. S4). However, assuming perfect detection, cat occurrence did not change across gradients of poison-bait density in either region (GAM; Fig. 3a). Cat detectability in the Glenelg region was particularly low, although there was no effect of fox-baiting on cat detectability (Supporting Information Fig. S3a). This result could signal that cats respond to fox suppression at the landscape level rather than at finer spatial scales. In addition, potential changes in population density and behaviour following mesopredator release (Brashares et al. 2010), such as cats reducing their ranging behaviour following fox control, as found by Molsher et al. (2017) and Rees et al. (2023c), could skew inference around occupancy estimates (McCarthy et al. 2013; Neilson et al. 2018; Broadley et al. 2019). Cats had weak associations with most explanatory variables, the poorest detection rates and worst model fits of our study species, further highlighting the challenges of monitoring this elusive generalist predator (Fisher et al. 2015; Stokeld et al. 2015; Rees et al. 2019; Algar et al. 2020).
In the Glenelg region, LNP—but not SBB—occupancy improved with fox control (Supporting Information Fig. S4b). Using increases in prey occupancy to measure the effectiveness of predator control rests on the assumption that there is suitable habitat for prey to expand into. While SBBs had a narrower distribution relative to LNPs across our study regions (largely absent from wet forests), there were 34 heathy woodland sites in the Glenelg region where they were never observed (from 196 camera-trap deployments), suggesting there may have been suitable habitat for them to colonise. However, vegetation type classes such as “heathy woodland” are coarse, model-generated categoriesy; there may have been an environmental variable which precluded SBB presence at these 34 sites, such as local habitat structure (Swan et al. 2015), although Smith (2013) found no association between habitat structure and SBB occupancy in the 240 Glenelg Ark monitoring sites. Alternatively, the different prey responses in our study could reflect the relative vulnerability of these species to fox and cat predation: LNPs (and other small macropods) appear more strongly limited by fox predation, whereas SBBs tend to be more closely associated with cat populations (Arthur et al. 2012; Hunter et al. 2018) and so may have experienced negative consequences from higher cat occupancy and density (Rees et al. 2023c) in Glenelg landscapes with fox control. This would also help explain why SBB occupancy was highest in heathy woodlands, where cat occupancy was lowest (Supporting Information Fig. S4c). Formally testing whether invasive predator occupancy impacts the probability of prey occupancy using multispecies models (Rota et al. 2016) is a priority for future research.
In the Otway Ranges, fox control did not improve SBB or LNP occupancy (Supporting Information Fig. S4; Fig. 4a; Fig. 5a). This is unsurprising given fox suppression in the Otway Ranges was weak, likely because fox control had only recently commenced and bait replacement was relatively inconsistent. Despite the high fecundity of these prey species, two years was may have been insufficient time to measure an effect of fox control on prey occupancy. Additionally, we averaged fox control effects over a 0–2 year post-baiting time period in the Otway Ranges; our findings concur with those of Robley et al. 2019, who estimated annual occupancy probabilities using a more traditional BACI analysis with one of the three datasets we used. The ongoing broadscale monitoring of the Otway Ark fox control program and other local initiatives will better elucidate occupancy trends over time.
While there is now considerable research which has demonstrated that invasive predator impacts are heightened in recently burnt areas (Meek and Saunders 2000; Green and Sanecki 2006; McGregor et al. 2014, 2016; Leahy et al. 2016; Hradsky et al. 2017, 2017), there is a comparatively poor understanding of how long-term fire patterns impact invasive predators (reviewed in Hradsky 2020). Similar to many previous studies, we found no average response to TSF for foxes or cats, however, both predators had varying responses to TSF across vegetation types (Figs. 2g, h; 3g, h). This is the first evidence of this kind for predators (albeit uncertainty around these relationships was high). Studies often merge similar vegetation types due to some groups having small sample sizes; however, we found no clear way of grouping vegetation types that was relevant to multiple species. Our hierarchical specification of the TSF and vegetation type interaction was powerful in this regard, as it allowed separate responses for each vegetation type, while sharing information across vegetation types, and provided confidence that there were data to back-up differently shaped responses given the penalisation to the average response (Pedersen et al. 2019).
Fire can have long-term impacts on the occurrence of small-medium sized native mammals (Claridge and Barry 2000; Monamy and Fox 2000; Arthur et al. 2012). Previously, Smith (2013) found extinction probabilities for both LNPs and SBBs were high for up to 18-months post-fire in the Glenelg region. Here we found that LNP occurrence was low immediately post-fire and peaked 20 years post-fire (Fig. 5g). However, seemingly contrary to Smith (2013), SBB occurrence declined with increasing TSF in our study (Fig. 4g). Given the uncertainty around our estimates and importance for managers implementing prescribed fire, further research is required to clarify and understand the mechanisms behind these species' responses to fire.
Accounting for other drivers of species in models that estimate responses to management is critical, but too rarely undertaken. For example, unexpected declines and local extinctions of small-medium sized mammals following 40 years of fox control have occurred in south-west Western Australia, and are posited to be the result of a mesopredator release of cats (Wayne et al. 2017), but the Intergovernmental Panel on Climate Change has identified this region as a ‘drying hotspot’ (Kala et al. 2021) and there are strong concerns around the intensity of prescribed fire operations (e.g., Bennett & Edwards 2021), offering alternative or contributing explanations for native mammal declines. Mammal communities have also collapsed following long-term fox-control in Booderee National Park (Lindenmayer et al. 2018), but a severe wildlife burnt through approximately half the region in the same year fox control commenced (leaving greater than 73% of park burnt within the last decade, Foster et al. 2017). Similarly, recent fire events have been skewed towards the baited landscapes of the Glenelg region since fox control began, with the majority of long-unburnt vegetation occurring in unbaited landscapes (Supporting Information Figure S1). We were partially able to compensate for this confounding by including an additional 424 sites in the Glenelg region, as well as the Otway Ranges datasets—providing a wider range of fire history patterns in each vegetation type with and without fox control (Supporting Information Figure S1). Our study demonstrates the value of bringing together multiple smaller datasets to compare management effectiveness.
Fox control is a major expenditure of Australian conservation and agricultural programs (approximately AUD 16 million annually, McLeod & Norris 2004). It is critical to ensure cost-effectiveness from both a monetary and ethical standpoint. Here we demonstrate a clear relationship between control effort and effectiveness in terms of fox suppression. However, the evidence for benefits to native prey species was mixed. Native prey species may not benefit from invasive predator control if the suppression of one invasive species leads to mesopredator release of other predators, or if prey are constrained by other factors such as lack of suitable habitat. Many species that are vulnerable to fox predation are also sensitive to fire-induced changes in habitat structure (Woinarski et al. 2015), and so integrating conservation strategies to consider habitat and invasive predator management in concert is a priority. Our work highlights the importance of fine-scale monitoring, considering multiple drivers and tailoring conservation strategies to local contexts.
Data availability
Data and code available from the Dryad Digital Repository https://doi.org/10.5061/dryad.msbcc2g4p (Rees et al. 2023c).
References
Algar D, Johnston M, Tiller C, Onus M, Fletcher J, Desmond G, Hamilton N, Speldewinde P (2020) Feral cat eradication on Dirk Hartog Island, Western Australia. Biol Invasions 22:1037–1054. https://doi.org/10.1007/s10530-019-02154-y
Arthur AD, Catling PC, Reid A (2012) Relative influence of habitat structure, species interactions and rainfall on the post-fire population dynamics of ground-dwelling vertebrates. Austral Ecol 37:958–970. https://doi.org/10.1111/j.1442-9993.2011.02355.x
Ballari SA, Kuebbing SE, Nuñez MA (2016) Potential problems of removing one invasive species at a time: A meta-analysis of the interactions between invasive vertebrates and unexpected effects of removal programs. Peer J 4:e2029. https://doi.org/10.7717/peerj.2029
Banks PB (1999) Predation by introduced foxes on native bush rats in Australia: do foxes take the doomed surplus? J Appl Ecol 36:1063–1071. https://doi.org/10.1046/j.1365-2664.1999.00463.x
Baxter PW, Sabo JL, Wilcox C, McCarthy MA, Possingham HP (2008) Cost-effective suppression and eradication of invasive predators. Conserv Biol 22:89–98. https://doi.org/10.1111/j.1523-1739.2007.00850.x
Bellard C, Genovesi P, Jeschke J (2016) Global patterns in threats to vertebrates by biological invasions. Proc Royal Soc B Biol Sci 283:20152454. https://doi.org/10.1098/rspb.2015.2454
Bennett M, Edwards T (2021) Prescribed burn devastates one of WA’s last two endangered numbat habitats, https://www.abc.net.au/news/2021-05-02/prescribed-burn-decimates-numbat-habitat-wa/100110960
Benshemesh J, Southwell D, Barker R, McCarthy M (2020) Citizen scientists reveal nationwide trends and drivers in the breeding activity of a threatened bird, the malleefowl (Leipoa ocellata). Biol Conserv 246:108573. https://doi.org/10.1016/j.biocon.2020.108573
Beven KJ, Kirkby MJ (1979) A physically based, variable contributing area model of basin hydrology. Hydrol Sci J 24:43–69. https://doi.org/10.1080/02626667909491834
Brashares JS, Prugh LR, Stoner CJ, Epps CW (2010) Ecological and conservation implications of mesopredator release. Trophic cascades: Predators, prey, and the changing dynamics of nature, pp 221–240. Island Press
Broadley K, Burton AC, Avgar T, Boutin S (2019) Density-dependent space use affects interpretation of camera trap detection rates. Ecol Evol 9:14031–14041. https://doi.org/10.1002/ece3.5840
Bureau of Meteorology (2021) Climate data online, http://www.bom.gov.au/climate/data/
Burnham KP, Anderson DR (2004) Multimodel inference: Understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304. https://doi.org/10.1177/0049124104268644
Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A (2017) Stan: A probabilistic programming language. J Stat Softw 76:1–32. https://doi.org/10.18637/jss.v076.i01
Carter A, Luck GW (2013) Fox baiting in agricultural landscapes: Preliminary findings on the importance of bait-site selection. Wildl Res 40:184–195. https://doi.org/10.1071/WR12169
Cattarino L, Hermoso V, Bradford LW, Carwardine J, Wilson KA, Kennard MJ, Linke S (2016) Accounting for continuous species’ responses to management effort enhances cost-effectiveness of conservation decisions. Biol Conserv 197:116–123. https://doi.org/10.1016/j.biocon.2016.02.030
Christie AP, Amano T, Martin PA, Petrovan SO, Shackelford GE, Simmons BI, Smith RK, Williams DR, Wordley CF, Sutherland WJ (2020) Poor availability of context-specific evidence hampers decision-making in conservation. Biol Conserv 248:108666. https://doi.org/10.1016/j.biocon.2020.108666
Claridge AW, Barry SC (2000) Factors influencing the distribution of medium-sized ground-dwelling mammals in southeastern mainland Australia. Austral Ecol 25:676–688. https://doi.org/10.1111/j.1442-9993.2000.tb00074.x
Collins L, Bradstock RA, Tasker EM, Whelan RJ (2012) Can gullies preserve complex forest structure in frequently burnt landscapes? Biol Conserv 153:177–186. https://doi.org/10.1016/j.biocon.2012.04.021
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566
Department of Environment, Land, Water & Planning (2019) Native vegetation - modelled 2005 ecological vegetation classes (with Bioregional Conservation Status), http://services.land.vic.gov.au/catalogue/metadata?anzlicId=ANZVI0803003495 &publicId=guest &extractionProviderId=1
Department of Environment, Land, Water & Planning (2020a) Bioregions and EVC Benchmarks, https://www.environment.vic.gov.au/biodiversity/bioregions-and-evc-benchmarks
Department of Environment, Land, Water & Planning (2020b) Vicmap Elevation DEM 10m, https://www.land.vic.gov.au/maps-and-spatial/spatial-data/vicmap-catalogue/vicmap-elevation
Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR (2016) Invasive predators and global biodiversity loss. Proc Natl Acad Sci 113:11261–11265. https://doi.org/10.1073/pnas.1602480113
Doherty TS, Ritchie EG (2017) Stop jumping the gun: A call for evidence-based invasive predator management. Conserv Lett 10:15–22. https://doi.org/10.1111/conl.12251
Duncan RP, Dexter N, Wayne A, Hone J (2020) Eruptive dynamics are common in managed mammal populations. Ecology 101:e03175. https://doi.org/10.1002/ecy.3175
Fairbridge D, Fisher P, Busana F, Pontin K, Edwards A (2000) Observations of the behaviour of free living bush rat, Rattus fuscipes and southern brown bandicoot, Isoodon obesulus at buried bait stations. Aust Mammal 22:125–127. https://doi.org/10.1071/AM00125
Fisher P, Algar D, Murphy E, Johnston M, Eason C (2015) How does cat behaviour influence the development and implementation of monitoring techniques and lethal control methods for feral cats? Appl Anim Behav Sci 173:88–96
Fleming PJ (1996) Ground-placed baits for the control of wild dogs: Evaluation of a replacement-baiting strategy in north-eastern New South Wales. Wildl Res 23:729–740. https://doi.org/10.1071/WR9960729
Foster C, Barton P, Robinson N, MacGregor C, Lindenmayer DB (2017) Effects of a large wildfire on vegetation structure in a variable fire mosaic. Ecol Appl 27:2369–2381. https://doi.org/10.1002/eap.1614
Gallant J, Austin J (2012) Topographic wetness index derived from 1” SRTM DEM-H. V2. 11 CSIRO. Data Collection. https://data.csiro.au/collection/csiro:5588
Garrard GE, Bekkessy SA, McCarthy MA, Wintle BA (2008) When have we looked hard enough? A novel method for setting minimum survey effort protocols for flora surveys. Aust Ecol 33:986–998. https://doi.org/10.1111/j.1442-9993.2008.01869.x
Geary WL, Hradsky BA, Robley A, Wintle BA (2020) Predators, fire or resources: What drives the distribution of herbivores in fragmented mesic forests? Aust Ecol 45:329–339. https://doi.org/10.1111/aec.12861
Gentle M, Saunders G, Dickman C (2007) Persistence of sodium monofluoroacetate (1080) in fox baits and implications for fox management in south-eastern Australia. Wildl Res 34:325–333. https://doi.org/10.1071/WR06163
Glen AS, Dickman CR (2003) Monitoring bait removal in vertebrate pest control: A comparison using track identification and remote photography. Wildl Res 30:29–33. https://doi.org/10.1071/WR01059
Green K, Sanecki G (2006) Immediate and short-term responses of bird and mammal assemblages to a subalpine wildfire in the snowy mountains, Australia. Aust Ecol 31:673–681. https://doi.org/10.1111/j.1442-9993.2006.01629.x
Greenville AC, Wardle GM, Tamayo B, Dickman CR (2014) Bottom-up and top-down processes interact to modify intraguild interactions in resource-pulse environments. Oecologia 175:1349–1358. https://doi.org/10.1007/s00442-014-2977-8
Hale S, Nimmo DG, Cooke R, Holland G, James S, Stevens M, De Bondi N, Woods R, Castle M, Campbell K, Senior K, Cassidy S, Duffy R, Holmes B, White JG (2016) Fire and climatic extremes shape mammal distributions in a fire-prone landscape. Divers Distrib 22:1127–1138. https://doi.org/10.1111/ddi.12471
Haslem A, Kelly LT, Nimmo DG, Watson SJ, Kenny SA, Taylor RS, Avitabile SC, Callister KE, Spence-Bailey LM, Clarke MF, Bennett AF (2011) Habitat or fuel? Implications of long-term, post-fire dynamics for the development of key resources for fauna and fire. J Appl Ecol 48:247–256. https://doi.org/10.1111/j.1365-2664.2010.01906.x
He T, Lamont BB, Pausas JG (2019) Fire as a key driver of earth’s biodiversity. Biol Rev 94:1983–2010. https://doi.org/10.1111/brv.12544
Hohnen R, Tuft K, McGregor HW, Legge S, Radford IJ, Johnson CN (2016) Occupancy of the invasive feral cat varies with habitat complexity. PLoS One 11:e0152520. https://doi.org/10.1371/journal.pone.0152520
Hradsky BA (2020) Conserving Australia’s threatened native mammals in predator-invaded, fire-prone landscapes. Wildl Res 47:1–15. https://doi.org/10.1071/WR19027
Hradsky BA, Mildwaters C, Ritchie EG, Christie F, Di Stefano J (2017) Responses of invasive predators and native prey to a prescribed forest fire. J Mammal 98:835–847. https://doi.org/10.1093/jmammal/gyx010
Hradsky BA, Robley A, Alexander R, Ritchie EG, York A, Di Stefano J (2017) Human-modified habitats facilitate forest-dwelling populations of an invasive predator, Vulpes vulpes. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-12464-7
Hunter DO, Lagisz M, Leo V, Nakagawa S, Letnic M (2018) Not all predators are equal: A continent-scale analysis of the effects of predator control on Australian mammals. Mamm Rev 48:108–122. https://doi.org/10.1111/mam.12115
Kala J, Robson B, Fontaine J, Beatty S, Wernberg T (2021) Drying land and heating seas: Why nature in Australia’s southwest is on the climate frontline. https://theconversation.com/drying-land-and-heating-seas-why-nature-in-australias-southwest-is-on-the-climate-frontline-170377
Kellner K (2021) ubms: bayesian models for data from unmarked animals using ’Stan’.https://kenkellner.com/ubms/
Kéry M, Royle JA (2020) Applied hierarchical modeling in ecology: analysis of distribution, abundance and species richness in R and Bugs. Volume 2: Dynamic and Advanced Models. Academic Press, Cambridge
Leahy L, Legge SM, Tuft K, McGregor HW, Barmuta LA, Jones ME, Johnson CN (2016) Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildl Res 42:705–716. https://doi.org/10.1071/WR15011
Lieury N, Ruette S, Devillard S, Albaret M, Drouyer F, Baudoux B, Millon A (2015) Compensatory immigration challenges predator control: An experimental evidence-based approach improves management. J Wildl Manag 79:425–434. https://doi.org/10.1002/jwmg.850
Lindenmayer DB, Wood J, MacGregor C, Foster C, Scheele B, Tulloch A, Barton P, Banks S, Robinson N, Dexter N, O’Loughlin LS, Legge S (2018) Conservation conundrums and the challenges of managing unexplained declines of multiple species. Biol Conserv 221:279–292. https://doi.org/10.1016/j.biocon.2018.03.007
Lobert B (1990) Home range and activity period of the southern brown bandicoot (Isoodon obesulus) in a Victorian heathland. Bandicoots and Bilbies, pp 319–325
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Andrew Royle J, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. https://doi.org/10.1890/0012-9658(2002)083%5B2248:ESORWD%5D2.0.CO;2
Marlow NJ, Thomas ND, Williams AA, Macmahon B, Lawson J, Hitchen Y, Angus J, Berry O (2015) Lethal 1080 baiting continues to reduce european red fox (Vulpes vulpes) abundance after more than 25 years of continuous use in south-west Western Australia. Ecol Manag Restor 16:131–141. https://doi.org/10.1111/emr.12162
Marra G, Wood SN (2011) Practical variable selection for generalized additive models. Comput Stat Data Anal 55:2372–2387. https://doi.org/10.1016/j.csda.2011.02.004
May SA, Norton T (1996) Influence of fragmentation and disturbance on the potential impact of feral predators on native fauna in Australian forest ecosystems. Wildl Res 23:387–400. https://doi.org/10.1071/WR9960387
McCarthy MA, Moore JL, Morris WK, Parris KM, Garrard GE, Vesk PA, Rumpff L, Giljohann KM, Camac JS, Bau SS (2013) The influence of abundance on detectability. Oikos 122:717–726. https://doi.org/10.1111/j.1600-0706.2012.20781.x
McDonald PJ, Nano CEM, Ward SJ, Stewart A, Pavey CR, Luck GW, Dickman CR (2017) Habitat as a mediator of mesopredator-driven mammal extinction. Conserv Biol 31:1183–1191. https://doi.org/10.1111/cobi.12905
McGregor HW, Legge S, Jones ME, Johnson CN (2014) Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PloS one 9:e109097. https://doi.org/10.1371/journal.pone.0109097
McGregor HW, Legge S, Jones ME, Johnson CN (2016) Extraterritorial hunting expeditions to intense fire scars by feral cats. Sci Rep 6:1–7. https://doi.org/10.1038/srep22559
McKenzie N, Burbidge A, Baynes A, Brereton R, Dickman C, Gordon G, Gibson L, Menkhorst P, Robinson A, Williams M et al (2007) Analysis of factors implicated in the recent decline of Australia’s mammal fauna. J Biogeogr 34:597–611. https://doi.org/10.1111/j.1365-2699.2006.01639.x
McLeod R, Norris A (2004) Counting the cost: impact of invasive animals in Australia, 2004. In: Cooperative research centre for pest animal control canberra
Meek PD, Saunders G (2000) Home range and movement of foxes (Vulpes vulpes) in coastal New South Wales, Australia. Wildl Res 27:663–668. https://doi.org/10.1071/WR98030
Molsher R, Newsome AE, Newsome TM, Dickman CR (2017) Mesopredator management: Effects of red fox control on the abundance, diet and use of space by feral cats. PLoS One 12:e0168460. https://doi.org/10.1371/journal.pone.0168460
Monamy V, Fox BJ (2000) Small mammal succession is determined by vegetation density rather than time elapsed since disturbance. Aust Ecol 25:580–587. https://doi.org/10.1111/j.1442-9993.2000.tb00063.x
Moseby KE, Letnic M, Blumstein DT, West R (2019) Understanding predator densities for successful co-existence of alien predators and threatened prey. Aust Ecol 44:409–419. https://doi.org/10.1111/aec.12697
Neilson EW, Avgar T, Burton AC, Broadley K, Boutin S (2018) Animal movement affects interpretation of occupancy models from camera-trap surveys of unmarked animals. Ecosphere 9:e02092. https://doi.org/10.1002/ecs2.2092
Nichols M, Ross J, Glen AS, Paterson AM (2019) An evaluation of systematic versus strategically-placed camera traps for monitoring feral cats in New Zealand. Animals 9:687. https://doi.org/10.3390/ani9090687
Nimmo DG, Kelly LT, Farnsworth LM, Watson SJ, Bennett AF (2014) Why do some species have geographically varying responses to fire history? Ecography 37:805–813. https://doi.org/10.1111/ecog.00684
Nuske S, Vernes K, May T, Claridge A, Congdon B, Krockenberger A, Abell S (2017) Redundancy among mammalian fungal dispersers and the importance of declining specialists. Fungal Ecol 27:1–13. https://doi.org/10.1016/j.funeco.2017.02.005
Paull DJ, Mills DJ, Claridge AW (2013) Fragmentation of the southern brown bandicoot (Isoodon obesulus): Unraveling past climate change from vegetation clearing. Int J Ecol 2013. https://doi.org/10.1155/2013/536524
Pedersen EJ, Miller DL, Simpson GL, Ross N (2019) Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 7:e6876. https://doi.org/10.7717/peerj.6876
Pressey RL, Cabeza M, Watts ME, Cowling RM, Wilson KA (2007) Conservation planning in a changing world. Trends Ecol Evol 22:583–592. https://doi.org/10.1016/j.tree.2007.10.001
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rees MW, Pascoe JH, Wintle BA, Le Pla M, Birnbaum EK, Hradsky BA (2019) Unexpectedly high densities of feral cats in a rugged temperate forest. Biol Conserv 239:108287. https://doi.org/10.1016/j.biocon.2019.108287
Rees MW, Pascoe JH, Le Pla M, Robley A, Birnbaum EK, Wintle BA, Hradsky BA (2023a) Mesopredator release among invasive predators: Controlling red foxes can increase feral cat density and alter their behaviour. J Appl Ecol 60:1100–1114. https://doi.org/10.1111/1365-2664.14402
Rees MW, Pascoe JH, Le Pla M, Robley A, Birnbaum EK, Wintle BA, Hradsky BA (2023b) Data from: Mesopredator release among invasive predators: controlling red foxes can increase feral cat density and alter their behaviour. https://doi.org/10.5061/dryad.69p8cz95w
Rees MW, Wintle BA, Robley A, Pascoe JH, Le Pla M, Birnbaum EK, Hradsky BA (2023c). Data from: Fox control and fire influence the occurrence of invasive predators and threatened native prey. https://doi.org/10.5061/dryad.msbcc2g4p
Riley SJ, DeGloria SD, Elliot R (1999) Index that quantifies topographic heterogeneity. Intermt J Sci 5:23–27
Robley A, Gormley AM, Forsyth DM, Triggs B (2014) Long-term and large-scale control of the introduced red fox increases native mammal occupancy in Australian forests. Biol Conserv 180:262–269. https://doi.org/10.1016/j.biocon.2014.10.017
Robley A, Moloney P, Parks Victoria West Coast District Team (2019) The Otway Ark: Response of predators and native species 2016–2018. arthur rylah institute for environmental research technical report series No. 299. Department of Environment, Land, Water; Planning, Heidelberg, Victoria
Robley A, Moloney P, Stringer L, Donald S (2020) Glenelg Ark 2005–2019: Long-term predator and native mammal response to predator control. Arthur Rylah Institute for environmental research technical report series no. 318. In: Department of Environment, Land, Water; Planning, Heidelberg, Victoria
Rota CT, Ferreira MA, Kays RW, Forrester TD, Kalies EL, McShea WJ, Parsons AW, Millspaugh JJ (2016) A multispecies occupancy model for two or more interacting species. Methods Ecol Evol 7:1164–1173. https://doi.org/10.1111/2041-210X.12587
Salo P, Banks PB, Dickman CR, Korpimäki E (2010) Predator manipulation experiments: Impacts on populations of terrestrial vertebrate prey. Ecol Monogr 80:531–546. https://doi.org/10.1890/09-1260.1
Saunders G, McLeod L (2007) Improving Fox Management Strategies in Australia. Bureau of Rural Sciences, Canberra, Australia
Saunders G, McLeod S, Kay B (2000) Degradation of sodium monofluoroacetate (1080) in buried fox baits. Wildl Res 27:129–135. https://doi.org/10.1071/WR99031
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. https://doi.org/10.1111/j.1600-0706.2009.18039.x
Sinclair ARE, Pech RP, Dickman CR, Hik D, Mahon P, Newsome AE (1998) Predicting effects of predation on conservation of endangered prey. Conserv Biol 12:564–575. https://doi.org/10.1111/j.1523-1739.1998.97030.x
Smith JK (2013) Fire, foxes and foliage: Conservation management of the southern brown bandicoot and long-nosed otoroo. PhD thesis, In: Department of Zoology; University of Melbourne. http://hdl.handle.net/11343/38628
Stobo-Wilson AM, Brandle R, Johnson CN, Jones ME (2020) Management of invasive mesopredators in the Flinders Ranges, South Australia: Effectiveness and implications. Wildl Res 47:720–730. https://doi.org/10.1071/WR19237
Stobo-Wilson AM, Stokeld D, Einoder LD, Davies HF, Fisher A, Hill BM, Mahney T, Murphy BP, Stevens A, Woinarski JCZ, Rangers B, Rangers W, Gillespie GR (2020) Habitat structural complexity explains patterns of feral cat and dingo occurrence in monsoonal Australia. Divers Distrib 26:832–842. https://doi.org/10.1111/ddi.13065
Stokeld D, Frank AS, Hill B, Choy JL, Mahney T, Stevens A, Young S, Rangers D, Rangers W, Gillespie GR (2015) Multiple cameras required to reliably detect feral cats in northern Australian tropical savanna: an evaluation of sampling design when using camera traps. Wildl Res 42:642–649. https://doi.org/10.1071/WR15083
Sugihara G, May R, Ye H, Hsieh C, Deyle E, Fogarty M, Munch S (2012) Detecting causality in complex ecosystems. Science 338:496–500. https://doi.org/10.1126/science.1227079
Swan M, Christie F, Sitters H, York A, Di Stefano J (2015) Predicting faunal fire responses in heterogeneous landscapes: The role of habitat structure. Ecol Appl 25:2293–2305. https://doi.org/10.1890/14-1533.1
Tulloch AIT, Mortelliti A, Kay GM, Florance D, Lindenmayer D (2016) Using empirical models of species colonization under multiple threatening processes to identify complementary threat-mitigation strategies. Conserv Biol 30:867–882. https://doi.org/10.1111/cobi.12672
Walsh JC, Wilson KA, Benshemesh J, Possingham HP (2012) Unexpected outcomes of invasive predator control: The importance of evaluating conservation management actions. Anim Conserv 15:319–328. https://doi.org/10.1111/j.1469-1795.2012.00537.x
Wayne AF, Maxwell MA, Ward CG, Wayne JC, Vellios CV, Wilson IJ (2017) Recoveries and cascading declines of native mammals associated with control of an introduced predator. J Mamm 98:489–501. https://doi.org/10.1093/jmammal/gyw237
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New York. https://ggplot2.tidyverse.org
Wickham H, François R, Henry L, Müller K (2022) Dplyr: A grammar of data manipulation. https://CRAN.R-project.org/package=dplyr
Wilson BA, Valentine LE, Reaveley A, Isaac J, Wolfe KM (2012) Terrestrial mammals of the Gnangara Groundwater System, Western Australia: History, status, and the possible impacts of a drying climate. Aust Mamm 34:202–216. https://doi.org/10.1071/AM11040
Woinarski JCZ, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proc Natl Acad Sci 112:4531–4540. https://doi.org/10.1073/pnas.1417301112
Wood SN (2017) Generalized Additive Models: An Introduction with R. CRC Press, Florida
Acknowledgements
We acknowledge and pay respects to the Gunditjmara and Eastern Maar peoples on whose traditional lands this study took place. We thank the Department of Environment, Land, Water, and Planning (DELWP) Heywood team and Parks Victoria West Coast District Team for sharing camera-trapping datasets. The M.W.R PhD surveys were conducted with the assistance of many fieldwork volunteers, under the University of Melbourne Animal Ethics Committee approval 1714119.5 and Victorian Government Department of Energy, Environment and Climate Action Research Permit 10008273. This study was generously supported by the Conservation Ecology Centre, the Victorian Government Department of Energy, Environment and Climate Action, Parks Victoria, Holsworth Wildlife Research Endowment – Equity Trustees Charitable Foundation and Ecological Society of Australia, Australia Government through the National Environmental Science Program’s Threatened Species Recovery Hub, and Australian Research Council Linkage Project LP170101134. M.W.R also received funding from an Australian Government Research Training Program Scholarship and a Victorian Government DELWP top-up scholarship.
Funding
Open access funding provided by CSIRO Library Services. This study was generously supported by the Conservation Ecology Centre, the Victorian Government DELWP, Parks Victoria, Holsworth Wildlife Research Endowment – Equity Trustees Charitable Foundation and Ecological Society of Australia, the Australia Government through the National Environmental Science Program’s Threatened Species Recovery Hub, and Australian Research Council Linkage Project LP170101134. M.W.R also received funding from an Australian Government Research Training Program Scholarship and a Victorian Government DELWP top-up scholarship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and analysis were performed by MR. MR, AR, JP, MP, EB and BH contributed to the data collection. The first draft of the manuscript was written by MR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rees, M.W., Wintle, B.A., Robley, A. et al. Fox control and fire influence the occurrence of invasive predators and threatened native prey. Biol Invasions 26, 685–703 (2024). https://doi.org/10.1007/s10530-023-03200-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03200-6