Abstract
Small mammals in farmland hedgerows feed on soil surface invertebrates with non-native invasive species potentially affecting prey species and populations. We investigated terrestrial invertebrates using pitfall traps and leaf litter samples across four zones of invasion in Ireland: (1) native species only (wood mouse Apodemus sylvaticus and pygmy shrew Sorex minutus), (2) natives plus the non-native bank vole (Myodes glareolus), (3) natives plus the non-native greater white-toothed shrew (Crocidura russula) and (4) natives plus both. After accounting for regional and local environmental variation, small mammal invasion was associated with lower invertebrate species richness (9–39% lower than uninvaded zones), reduced abundance (18–56% lower), shorter arthropod body length (24–52% shorter) and lower arthropod biomass (63–89% lower). Negative effects were observed on a wide range of disparate functional groups spanning phytophagous, detritivorus, zoophagous and omnivorous taxa including: the Staphylinidae, Carabidae and Coleoptera larvae, Isopoda, Diplopoda, Diptera, Hymenoptera, Pulmonata and Acari. The greater white-toothed shrew had greater negative effects than the bank vole with presence of both having greater effects than either alone though the majority of their combined effects were largely attributable to the former. The bank vole may mitigate some of the negative effects of the greater white-toothed shrew on invertebrate abundance perhaps indicating of some form of trophic interaction. Predation of detritivores and large invertebrate predators may impact farmland ecosystem service delivery i.e. nutrient cycling and pest biocontrol. Hedgerow biodiversity loss may induce indirect trophic cascades negatively impacting other taxa including farmland birds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whilst there have been attempts to classify the diverse and predominantly negative impacts of invasive species, these are frequently underestimated (Epstein et al. 2019; Bellard et al. 2021). The direct effects of invasive species on native species and naïve ecosystems that are usually the focus of research, involve interactions between related or ecologically analogous species (Dick et al. 2017; Wauters et al. 2023). Indirect effects where species and processes unrelated to invasion are embroiled in ecological change brought about by non-native introductions, are less well documented (McGeoch et al. 2015). For example, introduced earthworms interact with native deer to alter the composition of understorey vegetation in North American forests (Fischielli et al. 2013). Such circumstances may result in a trophic cascade where many species and ecological interactions might be affected, triggering substantial loss of ecosystem services (Galiana et al. 2014; Walsh et al. 2016). The impacts of smaller alien invasive species are also more difficult to establish. St. Clair (2011), for example, observed: “Many reported impacts [of invasive rats and house mice on invertebrate communities on islands] are unquantified, come from uncontrolled and unreplicated designs, or rely on time-series with inadequate baseline data”.
The trophic model discriminating bottom-up and top-down effects, provides a template on which to investigate the impact of invasive species (Strong and Leroux 2014; David et al. 2017). However, this approach underestimates what might be happening where omnivorous invasive species do not fall into discrete, trophic categories (Pimm & Lawton 1978; Diehl 1993), multiple invasive species are involved (Jackson 2015), or where recipient ecosystems are rich in pathogens and parasites (Torchin and Mitchell 2004). Time from introduction of alien invasive species may also be important in the assessment of impact, not only in revealing effects but also in establishing whether immediate impacts persist, increase or decrease (Crystal-Ornelas and Lockwood 2020).
The impacts of commensal small mammals on islands are well documented in comparison to invasions by non-commensal species (Harris 2009). Invasions by the latter are less common and their impacts are less well known. Mus and Rattus species feature in 123 and 148 papers respectively, in 340 papers in ecological and related journals published over the last 20 years (Web of Science search terms: rat OR mouse AND island). Only 10 papers feature Apodemus spp., 9 Acomys spp. or other spiny mice and 6 Microtus spp. or other voles. Research on insular shrews features in approximately 25% of 800 papers in ecology and related journals over the last 20 years (Web of Science search terms: shrew AND island). Species interactions among indigenous non-commensal rodents are well studied revealing exploitative competition for food and interference competition (Ziv et al. 1993; Brown et al. 2000; Eccard and Ylonen 2003). Research on competition between shrew species, however, suggests that direct interspecific altercations are rare, and access of subordinate species to prey is blocked by auditory and olfactory cues (Dickman 1991).
Two invasive non-native small mammals occur in Ireland predominately inhabiting farmland hedgerows as their main habitat. The bank vole, Myodes glareolus, an omnivorous rodent, was introduced accidentally from Germany in the mid-1920s (Stuart et al. 2007). The greater white-toothed shrew, Crocidura russula, an insectivore, is a much more recent introduction mostly likely arriving around, or just before, 2000 (Tosh et al. 2008). The major part of the current range of the greater white-toothed shrew is contained within that of the bank vole, with only one known, small area supporting the former but not the latter invasive species (Fig. 1). Both species are expanding their ranges in all directions. The bank vole is expanding at 2–3 km per year (White et al. 2012) and the greater white toothed shrew at around 5–6 km per year (McDevitt et al. 2014). The isolated part of the greater white toothed shrew’s range and more recent records (see the National Biodiversity Data Centre: https://biodiversityireland.ie) suggest its invasion is less predictable involving jump dispersal (Suarez et al. 2001). Bank vole and greater white-toothed shrew partially replace an indigenous omnivorous rodent, the wood mouse, Apodemus sylvaticus, and completely replace the native pygmy shrew, Sorex minutus (Montgomery et al. 2012, 2015; McDevitt et al. 2014). All four species feed on small arthropods which are important components of ecological communities associated with farmland field margins and hedgerows (Montgomery et al. 2020). DNA metabarcoding suggests that there is a high level of overlap in the prey taxa of pygmy shrew and greater white-toothed shrew that could provide a basis for exploitation competition (Browett et al. 2023). Despite being considered granivores, small rodents eat considerable volumes of invertebrates, especially during summer months, possibly to replace scarce seeds or to promote reproduction (Hansson 1985; Von Blanckenhagen, et al. 2007; Yalden and Harris 2008). Hence, omnivorous rodents and insectivorous shrews may compete for trophic resources with negative effects on abundance and diversity of prey (Liesenjohann et al. 2011; Eckrich et al. 2018). Soricid shrews can also have a negative effect on body size of prey (Churchfield et al. 1991). Small mammals feeding on predatory arthropods, such as larger Coleoptera, may also disrupt species richness and interactions throughout the trophic web (Towns et al. 2009; St. Clair 2011; Strong and Leroux 2014). Opportunities to investigate the early stages of invasion, especially on islands, before the full potential range of an invader is occupied, offer rare insights into the mechanisms and impacts of an invasion including ranging behaviour at low densities, species interactions and effects on receiving ecosystems (MacKay et al. 2019).
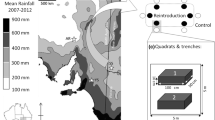
The ranges of four small mammals in Ireland: 1 Natives only (wood mouse and pygmy shrew), 2 plus the bank vole (+ BV), 3 plus the greater white-toothed shrew (+ GWTS) and 4 plus both (+ Both). The insert shows an example of the spatial location of five sites at which trapping occurred within each of which five pitfall traps were placed 10 m apart (n = 4 Zones × 5 Sites × 5 Pitfalls = 100)
We focus on farmland hedgerow small mammal communities subject to invasion by a non-native, non-commensal vole and a non-native shrew. Earlier work has demonstrated strong, mostly negative species interactions within and between rodents and shrew species (Montgomery et al. 2012, 2015). The present study aimed to investigate associations between two small mammal invasions (and their interaction) with invertebrate communities. Russell and Kaiser-Bunbury (2019) highlight the importance of interactions between invasive species and bottom-up rather than top-down effects on island ecosystems. The broad objectives of the present study were to compare species richness, abundance, body size and biomass of invertebrates, in four discrete zones created by the establishment and expansion of invasive species namely, the bank vole and the greater white toothed shrew in farmland hedgerows throughout Ireland. The four zones were: (1) native small mammals only; (2) natives plus the bank vole; 3) natives plus the greater white-toothed shrew; and, (4) natives plus both species (i.e. a fully factorial design).
Methods
Study sites and survey design
The rate of spread of the bank vole and the distance at which equilibrium abundance is attained from its point of origin suggests that at least 30 years are required for the full impact of invasion on native small mammals to be revealed (Montgomery et al. 2012; White et al. 2012). Although, dispersal of the greater white-toothed shrew is faster than that of the bank vole (McDevitt et al. 2014), it is less predictable with an increasing number of reports beyond its main range in Ireland (see the National Biodiversity Data Centre: https://biodiversityireland.ie). Long-term monitoring spanning a period before-and-after invasion would be impractical and would delay acquiring important information on which to base any intervention or management. However, sampling spatially provides an opportunity to compare invertebrate communities before-and-after invasion by one or more invading species. As outlined above, the concurrent invasions of Ireland by two invasive small mammals has created four zones (Fig. 1). Excluding offshore islands, the total contiguous extent of Ireland is 84,043 km2. Zone 1 (46,042 km2 or 55% of the island) is by far the oldest small mammal community as wood mouse and pygmy shrew have been present in Ireland for at least 8000 years (Montgomery et al. 2014). Zone 2 (27,893 km2 or 33% of the island) is an expanding area formed in the 1920s as the result of the introduction of the bank vole (Classens and Gorman 1965). Zone 3 (322 km2 or 0.4% of the island) was discovered in 2013 and is a limited area in central Ireland, suggesting greater white-toothed shrew is a recent introduction by jump dispersal from Zone 4 (9786 km2 or 12% of the island) which lies south of Zone 3, and was already a considerable area when discovered in 2007, suggesting that the bank vole and greater white-toothed shrew co-existed for at least 10 years before discovery (Tosh et al. 2008; McDevitt et al. 2014). The four zones vary not only in the age and composition of their small mammal fauna but also in parasite burden and behaviour of invaders (Perkins et al. 2017; Eccard et al. 2023). Thus, for reasons of comparability as well as logistical concerns, we concentrated sampling effort where small mammal communities were established for longest. In Zone 2, this was around the known point of introduction of the bank vole at Foynes, County Limerick (Stuart et al. 2007). The centroids (presumed approximate points of introduction) of the greater white-toothed shrew were used in Zone 3 (Mullingar, Westmeath; Montgomery, unpublished data) and Zone 4 (Dundrum, Tipperary; Montgomery et al. 2012). Samples were taken from just one habitat type: farmland hedgerows which supports all four species and is the major woody habitat for small mammals in Ireland (Montgomery et al. 2015) which is one of the least forested regions in Europe. Sampling sites were dominated by lowland pastoral agriculture (dairy and beef cattle grazing) separated by < 200 km (Fig. 1).
Invertebrate surveys
Invertebrate species richness and abundance was assessed using pitfall traps. Within each invasion zone, five sites were pre-selected along 15–20 km of minor, rural roads (classified as Local) close to the origin or centroid point. The roads used were usually 5–7 m wide with very low traffic levels and scattered development in the form of isolated farms and houses. Within each site, pitfall traps were set at five points with a 10 m interval between each (Fig. 1 insert). The total sample size was, therefore, n = 4 Zones × 5 Sites × 5 Pitfalls = 100 plots. Pitfall traps were set in roadside verges close to the base (< 1 m) of a farmland hedgerow (where the adjacent field was always pastoral grassland), for four weeks in July 2015 eliminating season as a potentially confounding factor. Each pitfall trap was a standard polyethylene container with a 10 cm opening, 20 cm depth and containing approximately 5 cm of isopropanol as a preservative. Each pitfall was covered with a square (15 cm) of plastic-coated wire mesh (2 cm gauge) to exclude small mammals, and a square (15 cm) of corrugated plastic set 5 cm above the open trap to prevent flooding with rainwater. After four weeks, the contents of pitfall traps were sieved in-situ and all specimens transferred to 70% ethanol. All samples were sorted and counted at species-level by the same author (WIM) following Kerney and Cameron (1979), Roberts (1995), Chinery (2009), Barnard (2011) and Brock (2014). Identifications to family-level were independently verified by a co-author (JO’N) using specialist taxonomic keys as required. Data were subsequently collapsed into major taxonomic groups following Tilling (1987). Individuals were further aggregated as arthropod and non-arthropod components. These were oven-dried at 60 °C for seven days before weighing for dry biomass.
Invertebrates caught and retained in pitfall fall traps over the duration of the month sampling period may swell due to absorption of the liquid medium distorting their body size. Thus, quadrat leaf litter sampling was used to investigate invertebrate body length (Southwood and Henderson 2009). Quadrats (0.25m2) were dropped blindly in undisturbed vegetation within 2 m of each pitfall trap on all transects (total n = 100 quadrats). All vegetation (< 30 cm) was clipped and bagged with soil surface leaf litter removed using a trowel. Quadrat samples were processed immediately by sifting in a white plastic tray (35 × 25 cm) with any live invertebrates extracted by hand, killed in 70% ethanol to immobilise them before measurement of body length using a calibrated binocular microscope (Leica Stereozoom S9i, Leica Microsystems, Switzerland). Some invertebrate taxa were excluded from analyses of body size including: slugs (Pulmonata) which retract their length upon manipulation, snails, aerial insects including the Hymenoptera and Diptera, and mites < 1 mm (Acari). Thus, body length was analysed for the most abundant arthropod taxa only: Carabidae, Staphylinidae, Diplopoda and Isopoda.
Regional and local environmental variation
Terrestrial invertebrate occurrence and abundance may be affected by landscape and habitat-scale variation in environmental parameters. Twelve environmental parameters were recorded at each sampling site. Climatic variables were downloaded from http://worldclim.org at 30 s (approximately 1 km) resolution and values extracted at each sampling site using ArcGIS 10.8 (ESRI, California, USA) including Bio1 annual mean temperature (which ranged from 9.0 to 11 °C) and Bio12 total annual precipitation (range 994–1282 mm/year). Surface living terrestrial invertebrates may also be affected by soil conditions. Top soil depth (range 25–50 mm) and pH, derived from 15 ml of soil mixed with 25 ml of deionised water using a laboratory pH meter (range 5.4–7.1), were recorded for each point using soil from the hole dug for each pitfall trap. Vegetation was characterised as percentage cover of grass (range 0–95%) and herbaceous plants (5–100%) using a 1 m2 quadrat and estimated by eye to the nearest 5%), plant species richness (number of species present; range 2–9 species), and vegetation height (m) taken as a mean of five measures at randomly selected points within each quadrat (range 0.1–0.7 m). Distance of the pitfall trap from the road (range 0.5–8.0 m), distance from hedge (0.8–3.0 m), and hedge cross-sectional area estimated as height multiplied by width (range 0.5–15 m2), were recorded. Number of years the hedgerow was last managed (cut) by saw or flailing, was estimated based on lateral growth (range 0–4 + years).
Statistical analyses
Principal components analysis
To capture and describe regional and local environmental gradients, the twelve parameters described above, were reduced using Principal Components Analysis (PCA) to orthogonal axes using varimax rotation (Table 1). Only axes with an eigenvalue of > 1 were retained for subsequent analysis.
Non-metric multidimensional scaling
Shifts in invertebrate community structure with small mammal invasion were tested using nonmetric multidimensional scaling (NMDS) ordination (Kenkel and Orlóci 1986) which related invertebrate taxon abundances as the main matrix (where rows were the 100 pitfall traps and columns where each invertebrate taxa and abundances were the cell values). The secondary matrix was the environmental PCA axes fitted as continuous variables and small mammal invasion zone fitted as a categorical grouping variable (Zones 1–4). Rare taxa which occurred in < 10% of pitfall plots were excluded as they were insufficiently numerous to generate meaningful correlations with either environmental data or invasion zone. The remaining species matrix was 4th-root (\(\sqrt[4]{}\)) transformed prior to analysis to downweight the influence of hyperabundant taxa (following Clarke and Warwick 2001) minimising distances between pitfall plots with the rarest and commonest taxa. Bray–Curtis was used as the distance measure with NMDS axes scores calculated by weighted averaging. Taxon correlations with axes were reported only if they had an r > 0.5. Samples within each Zone were enclosed using convex hulls to visually demonstrate the degree of overlap (similarity) in invertebrate communities between Zones.
Generalised linear mixed models
To explicitly test the impact of each invasive small mammal species, five separate Generalised Linear Mixed Models (GLMMs) were created for each of the following dependent variables: (1) invertebrate taxon richness, (2) invertebrate total abundance, (3) arthropod body length, (4) arthropod biomass and (5) NMDS1 scores. All models had an identical structure: each of the four environmental PCA axes were fitted as covariates to explicitly account for environmental variation while bank vole (BV) presence/absence (0/1) and greater white-toothed shrew (GWTS) presence/absence (0/1) where fitted as binary two-level fixed factors, and their interaction (BV*GWTS). Site was fitted as a Random Factor to account for spatial variation between clusters of sampling locations while Site(Plot) was fitted as a nested Random Factor to account for similarities in variation between sampling locations within clusters of sites. Invertebrate taxon richness and abundance models were fitted assuming a Poisson distribution and log-link function as is conceptually appropriate for count data. Models for arthropod body length, biomass and NMDS1 scores were fitted assuming a Gaussian distribution and identity-link function as is appropriate for normally distributed data. Model residuals were checked for normality using Shapiro–Wilk tests demonstrating the data conformed to the fitted distributions. All variables were standardised prior to analysis to have a mean (x̄) = 0 and a standard deviation (σ) = 1 such that the magnitude of all regression coefficients were directly comparable. The effects of each small mammal, and their interaction, were plotted using the back-transformed estimated marginal (predicted) mean values were Random Factors were fitted using original values and all PCA covariates were fitted at their mean value (0). This approach retained variation between Sites, and, between Plots within Sites but neutralised local environmental variation. Comparisons between non-invaded and invaded zones were expressed as percentage differences except for NMDS1 scores which included negative values (no percentage change value could be calculated).
All analyses were conducted using SPSS v25 (IBM Corp 2017) and all plots were drawn using Sigmaplot v14 (Systat, USA).
Results
Twenty-seven invertebrate taxa, comprising 610 species and 15,336 individuals, were captured in pitfall traps (Supplementary Information Table S1). Invertebrate communities were structured unevenly with some hyperabundant taxa occurring in virtually all samples (e.g. Pulmonata (slugs), Collembola, Diptera, Carabidae and Acari) whilst others were rare occurring singly or as a few individuals (e.g. Pseudoscorpionida, Lepidoptera, Psocoptera, Thysanura, Neuroptera and Thysanoptera).
Principal components analysis
PCA reduced twelve environmental parameters to four orthogonal axes with eigenvalues > 1 captured 65% of variation (Table 1). PC1 was positively correlated with pitfall trap distance from the road, adjacent hedgerow size and management i.e. time from last cut. PC2 was positively correlated with herb cover and distance from the hedge base and negatively correlated with grass cover. PC3 was positively correlated with vegetation height, soil pH and mean annual temperature. PC4 was positively correlated with annual precipitation. These represented environmental gradients along which invertebrate communities could conceivably respond.
Non-metric multidimensional scaling
Ordination of invertebrate taxon abundance captured 81.2% of the total variation where NMDS1 accounted for 62.9% and was positively correlated with ten taxa/groups: the Staphylinidae, Pulmonata (slugs), Carabidae, Diptera, Diplopoda, Isopoda, Acari, Coleoptera larvae, Hymenoptera and Pulmonata (snails). NMDS2 accounted for 18.3% of variation and was positively correlated with the Oligochaete only (Fig. 2). None of the environmental PCA axes were significantly associated with either NMDS axis.
Nonmetric multidimensional scaling (NMDS) biplot of invertebrate taxon abundance illustrating convex hulls and weighted centroids (numbered) within Zones of small mammal invasion. Rare taxa occurring at < 10% of plots were excluded and the species matrix was 4th-root transformed to downweight hyperabundant taxa (see Table S1). Taxa correlated with each axis (r > 0.5) are listed. Axes were rotated to yield positive correlations for ease of interpretation. No environmental PCA vectors are plotted as none were significantly correlated with either NMDS axis
The NMDS biplot suggested a shift from highest NMDS1 scores (i.e. highest abundance of all ten correlated taxa) in Zone 1 to lowest NMDS1 scores (i.e. lowest abundance of all ten correlated taxa) in Zone 3 suggesting the addition of the greater white-toothed shrew had greatest negative effects evident across numerous major taxa spanning phytophagous, detritivorus, zoophagous and omnivorous groups. Convex hulls overlapped least between Zone 1 and 3 again suggesting least similarity between the invertebrate community between native small mammals and areas invaded by the greater white-toothed shrew. The convex hulls and centroids for Zone 2 (addition of the bank vole) and Zone 4 (both species) fell between those of Zones 1 (natives only) and Zone 3 (addition of the greater white-toothed shrew) suggesting intermediate effects. Convex hulls almost completely overlapped and their centroid locations were highly similar on NDMS2 suggesting small mammal invasion had little effect on the Oligochaeta.
Generalised linear mixed models
Invertebrate taxon richness was significantly positively correlated with environmental PC1 (Table 2a) i.e. infrequently managed, mature hedges further from the road had higher invertebrate taxon richness than frequently managed, smaller hedges closer to the road. None of the other environmental principal components were associated with any other measure of the invertebrate community (Table 2).
After fitting environmental PCA axis scores as covariates, bank vole invasion was associated with 9% lower invertebrate taxon richness, 18% lower abundance, 24% shorter arthropod body length, 63% lower arthropod biomass and lower NMDS1 scores (Fig. 3). Whilst values were lower, the bank vole (BV) did not have a significant effect at a 95% level except on arthropod biomass (Table 2). In contrast, greater white-toothed shrew invasion was universally associated with significant (p < 0.05) negative impacts across all measures (Table 2) with 33% lower invertebrate taxon richness, 46% lower abundance, 35% shorter arthropod body length, 89% lower arthropod biomass and significantly lower NMDS1 scores (Fig. 3). Invasion by both non-native small mammals resulted in equal or usually greater negative impacts than either species alone (Fig. 3). Presence of both species was associated with 39% lower invertebrate taxon richness, 56% lower abundance, 52% shorter arthropod body length, 89% lower arthropod biomass and lower NMDS1 scores than in Zone 1 with native species only (Fig. 3). Invertebrate taxon richness and arthropod body length were unaffected by the interaction between the bank vole and greater white-toothed shrew (Table 2a and c) indicating that the addition of the latter to the bank vole (Zone 4) compared to bank vole only (Zone 2) had similar negative effects to those when it was added to natives species only (Zone 3 compared to Zone 1) i.e. the regression coefficients (slopes of the lines) did not differ. In these cases, the bank vole had a negative though non-significant effect, the greater white-toothed shrew had a larger significant negative effect and the effect of both species was similar to or greater than the greater white-toothed shrew (Fig. 3c and i). In contrast, invertebrate abundance, arthropod biomass and NMDS1 scores were significantly affected by the interaction of both species (Table 2b, d and e). In these cases, the effect of adding the greater white-toothed shrew to the bank vole had no additional additive effect to adding the greater white-toothed shrew alone (the regression coefficients between Zones 1–3 and 2–4 were different). This suggests the bank vole may weaken the effect of the greater white-toothed shrew on overall invertebrate abundance, arthropod biomass and the abundance of most taxa associated with NMDS1 perhaps indicating a complex trophic interaction?
GLMM predicted mean ± 95%CIs showing effects of the bank vole (left column), b greater white-toothed shrew (middle column) and their interaction (right column) on a–c invertebrate taxon richness, d–f abundance, g–i arthropod body length, j–l arthropod biomass and m–o NMDS1 scores. Predicted values account for spatial autocorrelation i.e. Site + Site(Plot) were fitted as Random Factors and local environmental variation i.e. PCA axes scores were fitted at their mean value. Percentage differences are shown at the top right except for NMDS1 scores which had negative values
Discussion
After explicitly accounting for regional and local environmental and habitat variation statistically, there were negative associations of soil surface invertebrate taxon richness, abundance, arthropod body length and biomass with the presence of one or both invasive small mammals in farmland hedgerows throughout Ireland. The effects of more than one invader were not necessarily additive but driven primarily by the presence of the greater white-toothed shrew. Whilst causation cannot be demonstrated here, the fact that highly significant differences remain after our best attempts to account for regional and local environmental and habitat variation suggests that invasive small mammals may have a substantial negative impact on soil surface invertebrates with effects observed across multiple taxa, functional groups and feeding guilds. Invasive small mammal predation on detritivorus and predatory invertebrates may, therefore, interfere with farmland ecosystem service delivery including nutrient cycling and natural pest biocontrol.
Bottom-up trophic cascades have been observed when grazing rodents of various sizes are excluded from grassland, altering vegetation and, thus, invertebrate communities (Vandegehuchte et al. 2017). Exclusion experiments also demonstrate that predatory shrews have a greater top-down effect on invertebrate prey than omnivorous rodents with effects varying between prey taxa (Churchfield et al. 1991). Leaf litter food webs on New Zealand offshore islands invaded by rats, Rattus norvegicus, are smaller and less complex than on rat-free islands (Thoresen et al. 2017). Norbury et al. (2023) found negative relationships between abundance of invasive house mice, Mus musculus, and arthropods in a conservation areas consisting of grassland and shrubland suggesting that impact of invasive small mammals on invertebrates may be of general importance. Moreover, it has been suggested that it may take considerable time for affected invertebrate communities to recover following eradication of the rats from islands suggesting long-term, legacy effects (Towns et al. 2009). These studies underscore the ecological importance of the present study whereby small mammal presence or absence may lead to sustained disruption of diverse ecological processes and farmland ecosystem delivery by terrestrial invertebrates.
In this study, the presence of alien invasive species was associated with reduced soil surface invertebrate species richness, abundance, body size and arthropod biomass across many functional groups spanning all feeding guilds. These effects could not be accounted for by spatial variation, or variation in local environmental conditions including habitat, and related factors, but varied with the presence or absence of each and both invasive species. Bank vole invasion was associated with a consistently negative, though non-significant, shifts in the invertebrate community composition and reduced species richness, abundance, body size and arthropod biomass. Greater white-toothed shrew invasion was associated with significant negative change in invertebrate communities with substantially reduced species richness, abundance, body size and arthropod biomass. Where both invaders were established, the effects were seemingly additive with most combined effects largely attributable to the greater white-toothed shrew.
In this study, invaded invertebrate communities notably lacked large-bodied taxa, e.g. predatory ground beetles such as Carabus spp. (known colloquially as ‘clocks’), with impacted communities dominated by smaller size classes. Invertebrate predator and prey size are correlated with larger predators taking a wider range of prey sizes (Cohen et al. 1993). Body size of predators and prey is also more similar in higher than lower trophic levels (Reide et al. 2011). Thus, overlap in diet may explain replacement of the native pygmy shrew by the larger invasive greater white-toothed shrew (Browett et al. 2023). The latter authors suggest greater white-toothed shrews are not dependent on large invertebrates and may shift their diet to smaller invertebrates as large taxa are lost. The impact of the greater white toothed shrew was evident in Zone 3, the smallest zone in terms of extent, where it was first recorded in 2013 such that the process whereby larger arthropods are predated becoming locally extinct must be rapid. This differential predation drives major species loss and population declines likely cascading from the largest to smallest invertebrate taxa in terms of body size. Predation-induced downsizing of invertebrate communities in response to invasion by the greater-white toothed shrew may benefit invading bank voles by reducing the abundance of larger, predator and detritivore arthropods. This in turn leads to more, smaller prey which bank voles can consume. Eccard et al. (2023) suggest that bank voles are a “timid” invader that outcompetes wood mice through greater efficiency in exploiting common resources. The effects described here are not fully consistent with ‘invasional meltdown’ (Montgomery et al. 2012) but interspecific interaction effects may still be important with the presence of a second invader potentially counteracting some of the impact of the first invader. Dietary and trophic niche studies would quantify prey selectivity and the consumptive effect of both invaders alone and in combination, for example, with regards to which invading species arrived first. Reduction in invertebrate size may result in increased exploitation competition between invading and native insectivores. Initially, greater white toothed shrew feed on larger arthropods which are not accessible to pygmy shrews. Once these larger arthropods are depleted greater white toothed shrews may switch to smaller prey on which pygmy shrews are dependent driving the loss of the native species.
The removal of large-bodied invertebrate predators, such as ground beetles, is likely to result in further effects at the soil surface and belowground. Such changes, thus, may affect other ecosystem components of hedgerows numerically and functionally, such as insectivorous farmland birds especially during the breeding season. Grames et al. (2023) in a review and meta-analysis of studies from around the world, have shown that insect food is generally a limiting factor for breeding songbirds affecting both chick body condition and reproductive success. Small mammal invasion of Ireland has affected the diet of birds of prey, principally barn owls, Tyto alba, and kestrels, Falco tinnunculus (Smiddy 2017, 2018). The impacts of invasive small mammals in Ireland and the potential for a trophic cascade are likely to be enacted in a moving trophic wave, spreading out from the south-west from the sites of first introduction and expanding north-east with the entire island likely colonised by 2100 (White et al. 2012; McDevitt et al. 2014). Thus, farmland invertebrate communities, and the ecosystem services they provide (see Lacher et al. 2019), may be threatened throughout the ever-diminishing remainder of Zone 1. The greater white-toothed shrew has recently been recorded in Great Britain (Bond et al. 2023) where it may also have negative effects, although these are likely to differ to those observed in Ireland due to the higher species richness of native small mammals communities in Great Britain.
Ireland is one of the least forested regions in Europe (DAFM 2019), with pastoral (dairy, beef and sheep) farmland covering ca. 85% of the island (see EEA 2020). Consequently, farmland hedges represent the majority of woody biomass throughout Ireland and, thus, are one of the most important habitats for maintaining agrobiodiversity. Significant reduction in species richness, abundance and biomass in native invertebrate communities represent a significant and substantial threat to Ireland’s biodiversity, unique island biogeography and, potentially, agricultural productivity. Russell and Kaiser-Bunbury (2019) highlight the importance of interactions between invasive species and bottom-up effects. They prioritize removal of introduced animal and plant species from islands. This may not always be possible or practicable due to presence of similar, perhaps vulnerable, native species, area invaded and diversity of the island, cost and contrary public opinion. Maintenance, restoration and expansion of native woody semi-natural habitats (for example, deciduous woodland, scrub and heath) may provide refuge for native small mammal and invertebrate communities. Wood mice persist in deciduous woodland and coniferous plantations which are largely avoided by bank voles (Montgomery et al. 2015). The focus of the present study and the distribution of small mammals in Ireland is along field boundary hedges and road verges. There is 413,000 km of hedgerow throughout Ireland, constituting the most important terrestrial agroecosystem (Montgomery et al. 2020). Management of farmland hedges and road verges may mitigate some of the effects of invasive species (Montgomery et al. 2012) by promoting structurally diverse, high volume and dense hedges, wide field margins and road verges connecting remaining patches of semi-natural habitats that support native small mammals and invertebrate communities. Here, invertebrate taxon richness was associated with infrequently managed, mature hedges further from the road. Management prescriptions promoting longer rotation cutting allowing hedges to attain larger size where there is no risk to road safety could substantially improve farmland hedgerows as a habitat for small mammals. Invasive bank vole and greater white-toothed shrew populations will dominate field margins and hedgerows throughout pastoral farmland in Ireland, but it is possible that changes to land cover and favourable habitat could create habitats in which native species may persist. This study flags the importance of early information gathering during non-native species invasions with systematic, intensive studies of possible, as well as probable, impacts on recipient ecosystems. Without such data, impact of invasive species will be underestimated locally, regionally and globally, as a major driver of biodiversity loss in its own right as well as interacting with agriculture and climate change.
Data availability
The datasets generated during and analysed during the current study are available in the Zenodo Data Repository at https://doi.org/10.5281/zenodo.8297888.
References
Barnard PC (2011) The royal entomological society book of British insects. Wiley and Blackwell, Oxford
Bellard C, Bernery C, Leclerc C (2021) Looming extinctions due to invasive species: irreversible loss of ecological strategy and evolutionary history. Global Change Biol 27:4967–4979. https://doi.org/10.1111/gcb.15771
Bond IF, Gilford E, McDevitt AD, Young MA, Coomber FG (2023) First records of the greater white-toothed shrew Crocidura russula from Great Britain. Mammal Commun 8:24–28
Brock PD (2014) A comprehensive guide to insects of Britain and Ireland. Pisces Publications, Berkshire UK
Browett SS, Synnott R, O’Meara DB, Antwis RE, Browett SS, Bown KJ, McDevitt AD (2023) Resource competition drives an invasion-replacement event among shrew species on an island. J Animal Ecol 92(3):698–709
Brown JH, Fox BJ, Kelt DA (2000) Assembly rules: desert rodent communities are structured at scales from local to continental. Am Nat 156(3):314–321. https://doi.org/10.1086/303385
Chinery M (2009) British Insects: a photographic guide to every common species. Collins, London
Churchfield S, Hollier J, Brown VK (1991) The effects of small mammal predators on grassland invertebrates, investigated by field exclosure experiment. Oikos 60:283–290. https://doi.org/10.2307/3545069
Claassens A, O’Gorman F (1965) The Bank Vole, Clethrionomys glareolus Schreber: a mammal new to Ireland. Nature 205:923–924
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. Plymouth, PRIMER-E
Cohen JE, Pimm SL, Yodzis P, Saldana J (1993) Body sizes of animal predators and animal prey in food webs. J Anim Ecol 62:67–78. https://doi.org/10.2307/5483
Corp IBM (2017) IBM SPSS statistics for windows, version 25.0. IBM Corp, Armonk, NY
Crystal-Ornelas R, Lockwood JL (2020) Cumulative meta-analysis identifies declining but negative impacts of invasive species on richness after 20 yr. Ecology 101(8):e03082
DAFM (2019) Forest Statistics—Ireland 2019, Dublin, Department of Agriculture, Food, and the Marine, p 9–41
David PE, Thebault O, Anneville P-F, Duyck E, Chapuis N, Loeuille N (2017) Impacts of invasive species on food webs: a review of empirical data. Adv Ecol Res 56:1–60
Dick JT, Laverty C, Lennon JJ, Barrios-O’Neill D, Mensink PJ, Robert Britton J, Caffrey JM (2017) Invader relative impact potential: a new metric to understand and predict the ecological impacts of existing, emerging and future invasive alien species. J Appl Ecol 54(4):1259–1267
Dickman CR (1991) Mechanisms of competition among insectivorous mammals. Oecologia 85:464–471. https://doi.org/10.1007/BF00323757
Diehl S (1993) Relative consumer sizes and the strengths of direct and indirect interactions in omnivorous feeding relationships. Oikos 68:151–157. https://doi.org/10.2307/3545321
Eccard JA, Ylönen H (2003) Interspecific competition in small rodents: from populations to individuals. Evol Ecol 17:423–440
Eccard JA, Mazza V, Holland C, Stuart P (2023) The timid invasion: behavioural adjustments and range expansion in a non-native rodent. Proc R Soc B 290(2003):20230823
Eckrich CA, Flaherty EA, Ben-David M (2018) Functional and numerical responses of shrews to competition vary with mouse density. PLoS ONE 13:e0189471. https://doi.org/10.1371/journal.pone.0189471
EEA (European Environment Agency) (2020); https://land.copernicus.eu/pan-european/corine-land-cover/clc2018
Epstein G, Foggo A, Smale DA (2019) Inconspicuous impacts: Widespread marine invader causes subtle but significant changes in native macroalgal assemblages. Ecosphere 10(7):e02814. https://doi.org/10.1002/ecs2.2814
Fisichelli NA, Frelich LE, Reich PB, Eisenhauer N (2013) Linking direct and indirect pathways mediating earth-worms, deer, and understory composition in Great Lakes forests. Biol Invasions 15:1057–1066
Galiana N, Lurgi M, Montoya JM, Lopez BC (2014) Invasions cause biodiversity loss and community simplification in vertebrate food webs. Oikos 123:721–728. https://doi.org/10.1111/j.1600-0706.2013.00859.x
Grames EM, Montgomery GA, Youngflesh C, Tingley MW, Elphick CS (2023) The effect of insect food availability on songbird reproductive success and chick body condition: evidence from a systematic review and meta-analysis. Ecol Lett 26(4):658
Hansson L (1985) The food of bank voles. wood mice and yellow-necked mice. Symp Zool Soc Lond 55:141–168
Harris DB (2009) Review of negative effects of introduced rodents on small mammals on islands. Biol Invasions 11:1611–1630
Jackson MC (2015) Interactions among multiple invasive animals. Ecology 96(8):2035–2041
Kenkel NC, Orlóci L (1986) Applying metric and nonmetric multidimensional scaling to ecological studies: some new results. Ecology 67:919–928
Kerney MP, Cameron RAD (1979) A field guide to the land snails of Britain and North–west Europe. Collins, London
Lacher TE, Davidson AD, Fleming TH, Gomez-Ruiz EP, McCracken GF, Owen-Smith N, Peres CA, Vander Wall SB (2019) The functional roles of mammals in ecosystems. J Mammal 100:942–964. https://doi.org/10.1093/jmammal/gyy183
Liesenjohann M, Liesenjohann T, Trebaticka L, Haapakoski M, Sundell J, Ylonen H, Eccard JA (2011) From interference to predation: type and effects of direct interspecific interactions of small mammals. Behav Ecol Sociobiol 65:2079–2089. https://doi.org/10.1007/s00265-011-1217-z
MacKay JWB, Russell JC, Clout MN, Murphy EC, Hauber ME (2019) See how they run: increased ranging behavior counters potential Allee effects in experimentally introduced house mice on an island. Biol Invasions 21:1669–1681
Yalden DW, Harris S (eds.) (2008) Mammals of the British Isles: Handbook, 4th edition. Mammal Society
McDevitt AD, Montgomery WI, Tosh DG, Lusby J, Reid N, White TA, McDevitt CD, O’Halloran J, Searle JB, Yearsley JM (2014) Invading and expanding: range dynamics and ecological consequences of the greater white-toothed shrew (Crocidura russula) invasion in Ireland. PLoS ONE 9:e100403. https://doi.org/10.1371/journal.pone.0100403
McGeoch MA, Lythe MJ, Henriksen MV, McGrannachan CM (2015) Environmental impact classification for alien insects: a review of mechanisms and their biodiversity outcomes. Curr Opin Insect Sci 12:46–53
Montgomery WI, Lundy MG, Reid N (2012) “Invasional meltdown”: evidence for unexpected consequences and cumulative impacts of multispecies invasions. Biol Invasions 14:1111–1125
Montgomery WI, Provan J, McCabe AM, Yalden DW (2014) Origin of British and Irish mammals: disparate post-glacial colonisation and species introductions. Quat Sci Rev 98:144–165
Montgomery WI, Montgomery SSJ, Reid N (2015) Invasive alien species disrupt spatial and temporal ecology and threaten extinction in an insular, small mammal community. Biol Invasions 17:179–189
Montgomery WI, Caruso T, Reid N (2020) Hedgerows as ecosystems: service delivery, management, and restoration. Ann Rev Ecol Evol Syst 51:81–102
Norbury G, Wilson DJ, Clarke D, Hayman E, Smith J, Howard S (2023) Density-impact functions for invasive house mouse (Mus musculus) effects on indigenous lizards and invertebrates. Biol Invasions 25:801–815
Perkins SE, White TA, Pascoe EL, Gillingham EL (2017) Parasite community dynamics in an invasive vole–From focal introduction to wave front. Int J Parasitol Parasit Wildl 6(3):412–419
Pimm SL, Lawton JH (1978) On feeding on more than one trophic level. Nature 275:542–544. https://doi.org/10.1038/275542a0
Reide JO, Brose U, Ebenman B, Jacob U, Thompson R, Townsend CR, Jonsson T (2011) Stepping in Elton’s footprints: a general scaling model for body masses and trophic levels across ecosystems. Ecol Lett 14:169–178. https://doi.org/10.1111/j.1461-0248.2010.01568.x
Roberts MJ (1995) Spiders of Britain and northern Europe. Harper Collins, London
Russell JC, Kaiser-Bunbury CN (2019) Consequences of multispecies introductions on island ecosystems. Annu Rev Ecol Evol Syst 50:169–190
Smiddy P (2017) Diet of the common kestrel Falco tinnunculus in East Cork and West Waterford: an insight into the dynamics of invasive mammal species. Biol Environ Proc R Irish Acad 117B:131–138. https://doi.org/10.3318/BIOE.2017.16
Smiddy P (2018) Dominance of invasive small mammals in the diet of the barn owl Tyto alba in county Co. Cork, Ireland. Biol Environ Proc R Irish Acad 118B(1):49–53. https://doi.org/10.3318/BIOE.2018.04
Southwood TRE, Henderson PA (2009) Ecological methods. Wiley
St. Clair JJH, Poncet S, Sheehan DK, Szekely T, Hilton GM (2011) Responses of an island endemic invertebrate to rodent invasion and eradication. Animal Conserv 14:66–73. https://doi.org/10.1111/j.1469-1795.2010.00391.x
Strong JS, Leroux SJ (2014) Impact of non-native terrestrial mammals on the structure of the terrestrial mammal food web of Newfoundland, Canada. PLoS ONE 9(8):e106264. https://doi.org/10.1371/journal.pone.01062640
Stuart P, Mirimin L, Cross TF, Sleeman DP, Buckley NJ, Telfer S, Birtles RJ, Kotli P, Searle JB (2007) The origin of Irish bank voles Clethrionomys glareolus assessed by mitochondrial DNA analysis. Irish Nat J 28:440–446
Suarez AV, Holway DA, Case TJ (2001) Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proc Natl Acad Sci USA 98(3):1095–1100
Thoresen JJ, Towns D, Leuzinger S, Durrett M, Mulder CPH, Wardle DA (2017) Invasive rodents have multiple indirect effects on seabird island invertebrate food web structure. Ecol Appl 27:1190–1198. https://doi.org/10.1002/eap.1513
Tilling SM (1987) A key to the major groups of the British terrestrial invertebrates. Field Stud Council 6(4):695–766
Torchin ME, Mitchell CE (2004) Parasites, pathogens, and invasions.by plants and animals. Front Ecol Environ 2:183–190
Tosh DG, Lusby J, Montgomery WI, O’Halloran J (2008) First record of greater white-toothed shrew Crocidura russula in Ireland. Mammal Rev 38:321–326
Towns DR, Wardle DA, Mulder CPH, Yeates GW, Fitzgerald BM, Parrish GR, Bellingham PJ, Bonner KI (2009) Predation of seabirds by invasive rats: multiple indirect consequences for invertebrate communities. Oikos 118:420–430. https://doi.org/10.1111/j.1600-0706.2008.17186.x
Vandegehuchte ML, Schutz M, de Schaetzen F, Risch AC (2017) Mammal-induced trophic cascades in invertebrate food webs are modulated by grazing intensity in subalpine grassland. J Anim Ecol 86:1434–1446. https://doi.org/10.1111/1365-2656.12744
Von Blanckenhagen F, Eccard JA, Ylonen H (2007) Animal protein as a reproductive constraint in spring reproduction of the bank vole. Ecoscience 14:323–329. https://doi.org/10.2980/1195-6860(2007)14[323:APAARC]2.0.CO;2
Walsh JR, Carpenter SR, Zanden MJV (2016) Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc Natl Acad Sci USA 113:4081–4085
Wauters LA, Lurz PW, Santicchia F, Romeo C, Ferrari N, Martinoli A, Gurnell J (2023) Interactions between native and invasive species: a systematic review of the red squirrel-gray squirrel paradigm. Front Ecol Evol 11:1083008
White TA, Lundy MG, Montgomery WI, Montgomery S, Perkins SE, Lawton C, Meehan JM, Hayden TJ, Heckel G, Reid N, Searle JB (2012) Range expansion in an invasive small mammal: influence of life-history and habitat quality. Biol Invasions 14:2203–2215
Ziv Y, Abramsky Z, Kotler BP, Subach A (1993) Interference competition and temporal and habitat partitioning in two gerbil species. Oikos 66(2):246. https://doi.org/10.2307/3544810
Acknowledgements
We are grateful to the landowners and farmers of Ireland for access to their land, and the kindness of strangers. No live mammals were trapped or handled during the course of this study. No external funds were involved in support of this study. We are grateful to anonymous reviewers and editorial staff for their insightful comments.
Author information
Authors and Affiliations
Contributions
WIM and NR designed the study. WIM and SSJM conducted the fieldwork. WIM and JO’N conducted the laboratory work. NR analysed the data. WIM and NR wrote the draft manuscript. All authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montgomery, W.I., Montgomery, S.S.J., O’Neill, J. et al. Invasion of farmland hedgerows by non-native small mammals is associated with lower soil surface invertebrate diversity, abundance, body size and biomass. Biol Invasions 26, 671–684 (2024). https://doi.org/10.1007/s10530-023-03199-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03199-w