Abstract
The cherry plum (Prunus cerasifera Ehrh.) is considered an invasive plant species in numerous areas of the temperate climate zones around the world. Although this exotic tree can naturalize in a wide spectrum of forest ecosystems, its invasion mechanisms remain totally unknown. This study is the first approach aiming to investigate potential drivers shaping the ecological success of P. cerasifera invasion, with an example of the temperate primeval forest as a model recipient ecosystem. Using generalized linear models, we suggest that the distance to the propagule source may shape considerably this exotic species occurrence in primeval forest’s understory, expressed by the presumably high role of birds and small mammals in short-, and large ungulates in long-distance seed dispersion. The probability of this invader occurrence decreased with decreasing functional and phylogenetic diversity of understory vegetation. This suggests the importance of habitat filtering generated in small tree-fall gaps in shaping the P. cerasifera success. Thus, interactions between natural disturbances, characteristics of recipient vegetation, as well as traits of various animal dispersers, may be identified as key drivers promoting the occurrence of P. cerasifera in the primeval forest ecosystem. However, further studies on the patterns of P. cerasifera invasion are needed to identify drivers promoting invasion, as well as the effects of this exotic plant on biodiversity and the functioning of ecosystems.
Similar content being viewed by others
Introduction
Understanding the mechanisms of biological invasions, with a special focus on their early stages, allows the formulation of effective strategies of alien species management, aiming the prevention of recipient ecosystems from further spread of invaders, thus minimizing the range of detrimental effects on biodiversity, trophic interactions, biogeochemical cycles, and overall ecosystem functioning (Blackburn et al. 2011; Aerts et al. 2017). Because economic and environmental costs of invasive species eradication, monitoring, and controlling of invasion progress increase rapidly, assessment of invasions risks in their early stages can be considered one of the most cost-efficient methods of alien species management (Basnou et al. 2015). Thus, the knowledge of the alien species invasion patterns is essential for the assessment of ecosystems’ invasibility and development of conservation priorities, of which accurate formulation is especially important for the maintenance of ecosystems identified as having the highest naturalness levels (Novoa et al. 2020; Sapsford et al. 2020).
Although primary forests are assumed to be of low invasibility and high invasion resistance (Cadenasso and Pickett 2001; Martin et al. 2009), they can often express even similar levels of alien plant invasions as secondary forests (Lapin et al. 2019). Due to the low contribution in the total forest cover of Europe (about 0.7%; Sabatini et al. 2018), and biases linked with the land-use history reconstruction, invasion mechanisms in primary forests are highly underexplored (von Holle et al. 2003; Levine et al. 2004; Wagner et al. 2017; Lapin et al. 2019; Nuñez et al. 2022). Moreover, the vast majority of previous studies have focused on high-intensity invasions, where exotic plants are well integrated with other ecosystem components (Elgersma and Ehrenfeld 2011), while less attention was paid to primeval forests (i.e. primary forests with the continuity of ecological processes not substantially transformed since deglaciation (Jaroszewicz et al. 2019) or forest ecosystems with early invasion stages (Lapin et al. 2019). A model ecosystem in Europe with an outstandingly high naturalness level is the Białowieża Forest (Faliński 1986; Sabatini et al. 2018). Despite the status of the most preserved forest ecosystem on the European lowland (Jaroszewicz et al. 2019) and relatively high invasion resistance, some exotic plants can successfully naturalize and spread in the Białowieża Forest (Faliński 1968, 1986; Mędrzycki and Pabjanek 2001; Łapok et al. 2018). One example of plant species belonging to this group is cherry plum Prunus cerasifera Ehrh. (= P. divaricata Ledeb., P. myrobalana (L.) Desf.).
Native geographical distribution of P. cerasifera comprises SE Europe, SW, and Central Asia (Kurtto 2009+, Popescu and Caudullo 2016, Weber 2017, POWO 2022), where it occurs in a wide elevation spectrum, up to 2200 m a.s.l. Cherry plum forms thickets developing at the forest edges, or grows in shrublands, and is an important component of shrub layers of numerous forest types. However, due to long-term and widespread cultivation as a fruit and decorative tree, the borders of its native range are obscured due to multiple escapes from cultivation and naturalisation events in newly colonised areas. For instance, POWO (2022) considers Ukraine and Romania to be within the native distribution of this species, whereas recent papers from these areas treat P. cerasifera as alien (Romania – Sîrbu and Oprea (2011), continental Ukraine – Burda and Koniakin (2019), Crimea –Yena (2012). In NW Europe P. cerasifera has been cultivated since 1592, where in general it is considered as invasive neophyte. In Italy and the Czech Republic, in turn, P. cerasifera has been cultivated before the year ~ 1500, where it is regarded as an invasive archaeophyte (Galasso et al. 2018; Pyšek et al. 2022). In Poland the species is considered as invasive neophyte, however precise time of its introduction is unknown (Tokarska-Guzik et al. 2012). Apart from some uncertainties in the assessment of P. cerasifera native range, it is considered as alien in numerous areas of the temperate climate zones around the world (Rejmánek and Richardson 2013), i.e.: NW Africa, W, and E North America, SW South America, New Zealand (Weber 2017; POWO 2022), and Europe (Popescu and Caudullo 2016; Dobrzycka-Krahel and Medina-Villar 2020). Similarly as regarded to the native range, P. cerasifera in the introduced range in Europe can naturalize in a wide spectrum of habitats, including rural and urban sites, post-agricultural wastelands, meadows, xerothermic grasslands and shrublands, Scots pine monocultures, secondary forests or even primary forests (Adamowski et al. 2002; Adamowski and Wołkowycki 2014). In North America, for instance, the species invades chaparral, roadsides, stream banks and canyons (Rohrer 2014). Despite relatively good recognisability by society and the status of alien, naturalized, or invasive species, P. cerasifera invasion mechanisms remain unknown (Dobrzycka-Krahel and Medina-Villar 2020).
This study is the first approach aiming to assess the P. cerasifera invasion mechanisms, with an example of the Białowieża Forest as a model recipient ecosystem representing the early stages of an invasion by this exotic plant. In this study we ask: (i) whether the ecological success of P. cerasifera in this primeval forest may depend on the distance to the propagule sources and (ii) whether the ecological success of this invasive plant may be influenced by understory vegetation characteristics of a recipient forest ecosystem
Methods
Study site
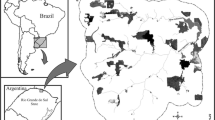
Białowieża Forest is the transboundary forest ecosystem, located in NE Poland and W Belarus. Its high naturalness degree is reflected by substantially high species richness and biodiversity, the presence of large old trees, high amounts of deadwood in various decomposition stages, high structural and spatial forest complexity and microhabitats heterogeneity, natural forest dynamics shaped by uncontrolled disturbances, and the continuity of ecological processes have not been substantially transformed over the last 12 000 years (Faliński 1986; Jaroszewicz et al. 2019). We conducted our study in the Strict Reserve of the Białowieża National Park (Fig. 1), which is the best-preserved part of this forest ecosystem, excluded from direct human impacts since 1921, thus playing the role of a unique living laboratory for ecological and evolutionary sciences (Jaroszewicz et al. 2019).
Our study was conducted in the oak-lime-hornbeam forest (Tilio-Carpinetum) of subcontinental character – a forest community predominating in the Polish part of the Białowieża Forest. The tree stand of Tilio-Carpinetum is composed of up to a three-layer tree canopy with a predominance of Carpinus betulus, Tilia cordata, Quercus robur, Acer platanoides, and Picea abies. Tilio-Carpinetum develops on various soils, including eutrophic brown soils, clay-illuvial soils, and stagnogleyic clay-illuvial soils. (Faliński 1986).
The earliest records of the cherry plum in the Białowieża Forest date back to the 1960s, when the cultivation of P. cerasifera in the Belarussian part of the Białowieża Forest was reported. In the next decades, through the usage of forest roads as migration corridors, the species started to spread into the Białowieża Forest, Naturalizing in ruderal sites or post-agricultural wastelands, and finally forest edges (Smirnov 1965; Łuczaj 1994; Adamowski et al. 1998, 2002). A recent survey of non-native flora conducted on areas located directly to the southern part of the Strict Reserve of the Białowieża National Park revealed a quick increase in the abundance of the spontaneous populations of P. cerasifera on abandoned farmlands, identifying them as one of the main propagule sources of this species in the Białowieża Forest (Adamowski 2016, unpubl.).
Data collection
Within the study site (located in the southern site of the Strict Reserve; ~0.47 km2), we used a random number generator to select 30 spatial units (50×50 m) for detail study, marked in the field using plastic tapes with alphanumeric codes. Within the center of each spatial unit, we regularly established four 5×5 m subplots, located each at 6.31 m from the unit center in four cardinal directions. Within each subplot, in July 2022 we recorded vascular plant species and their percentage cover in a non-transformed, nine-degree Braun-Blanquet scale (r – less than 0.01%, + – 0.1–1%, 1–1.1-3%, 2 m – 3.1-5%, 2a – 6–15%, 2b – 16–25%, 3–36–50%, 4–51–75%, and 5 – more than 75% cover). To estimate plant species cover at the plot level (n=30; 100 m2 each), we averaged cover of plant species within all four 5×5 m subplots in each spatial unit. We also counted all P. cerasifera juveniles, assuming that all of them were older than one year as in each specimen we found annual height increment marks. Because we could not destructively sample seedlings and because browsing obscures the height-age relationship for individual seedling, we did not estimate seedling age. For each plot (using the center of each spatial unit), we calculated distances from two propagule sources (Fig. 1): one inside the Strict Reserve (single fruiting tree with approximate age of ~15 years), and one outside (group of several fruiting trees with approximate age of ~40 years, nearest to study plots and connected with them via ungulates path). We used the former as a proxy for short-range distance spread, and the latter as a proxy for long-range distance dispersal of P. cerasifera.
Data preparation
We analyzed data using R software (R Core Team 2021). We obtained a phylogenetic tree for all species present in the study plots from the megatree included in the V.phylo.maker package. We obtained values of species functional traits from LEDA, BIEN, BiolFlor, and Pladias (Table 1). We choose functional traits describing the recipient communitys ability to inhibit or facilitate the presence of invasive plant species (Wang et al. 2019, 2021; Anibaba et al. 2023; Czortek 2023). We included cardinal traits representing main components of plant economic spectrum (Westoby 1998; Díaz et al. 2016): two traits representing the leaf economic spectrum: specific leaf area (SLA, cm2 g−1) and leaf dry matter content (LDMC, g g−1), and two traits representing plant size: plant height (H, m) and seed mass (SM, mg). These traits represent trade-offs in resources acquisition, as well as adaptations to competition and environmental stress. Traits describing reproduction biology: flowering beginning and duration (months), as well as pollination vectors (insects, wind, or self-pollination), expressed by binary variables, provide information about plants reproductive strategies, abilities to spread, as well as provide information on adaptations to disturbances. Lastly, we included lifeform, as a categorical trait expressing the strategy of persistence across winter and plant longevity, adopted as additional indicator of habitat stability, and Ellenbers Ecological Indicator Values (EIVs), expressing species ecological requirements. EIVs are expert-based indicators of species ecological requirements (Ellenberg and Leuschner 2010), assessed by vegetation ecologists and widely used to reconstruct species ecological niche when there are no instrumental measurements (e.g. Evangelista et al. 2016; Czortek et al. 2018; Ridding et al. 2020). Despite drawbacks resulting from their discontinuous and expert-based character (Schaffers and Sýkora 2000), these values were compared with instrumental measurements revealing their usefulness in indication of species requirements (e.g. Dzwonko 2001; Szymura et al. 2014).
Due to a lack of trait data completeness for some species (Table 1), we imputed missing values using the random forest-based imputation protocol (Penone et al. 2014) implemented in the missForest package. Prior to imputation we inspected structure of missing values to ensure that there are no patterns in traits completeness related to taxonomic structures. Trait completeness ranged from 70.4% (soil reaction EIV) to 100.0% (life form), and average completeness was high (92.3%), indicating that potential imputation error will have low influence on community-level variables. The imputation is based on the known trait values and phylogenetic eigenvectors (Diniz-Filho et al. 1998) obtained using the PVR package, and used in many trait-based studies (e.g. Pyšek et al. 2015; Paź-Dyderska et al. 2023). The first 15 phylogenetic eigenvectors covered 65.7% of the variation in phylogenetic distances among species. The normalized root mean squared error (a measure of the random forest-based imputation validity) of imputed traits for continuous variables was 0.71, representing good fitness of imputation.
We decided to describe wide mechanisms shaping the composition of understory vegetation, the phylogenetic relatedness structure of species through phylogenetic diversity (Nicod and Gillet 2021), and community assembly processes through functional diversity (Czortek et al. 2021). As phylogenetic diversity allows a deep insight into species relatedness based on their phylogenesis and evolutionary trajectories, functional diversity allows assessments of the importance of main ecological mechanisms in shaping species composition (Czortek et al. 2021; Nicod and Gillet 2021). While low values of functional diversity metrics can be a reflection of a high role of environmental filters or interspecific competition, high values may illustrate the prominent role of niche partitioning in vegetation development (Carroll et al. 2011). We calculated functional richness (FRic), following Villéger et al. (2008), and functional dispersion (FDis) following Laliberté and Legendre (2010). Functional richness provides information on the quantity of plants realized niches, indicating the occupancy of the niche space by plants differing in life strategies (Villéger et al. 2008). Functional dispersion measures the mean distances of the species functional traits combinations to the centroid (center point) of the trait hypervolume (Hedberg et al. 2014). Despite limitations of the usage of functional richness in evaluation of invasion dynamics (Kuebbing et al. 2018), we decided to use both FRic and FDis of recipient vegetation as metrics of P. cerasifera success in the Strict Reserve. Both high occupancy of the niche space (high FRic) and high range of functional dissimilarities among species (high FDis) may suggest that the vast majority of environmental resources could be effectively utilised by functionally divergent plants, thus making the number of empty niches available to colonization by invasive plants low (e.g. Dyderski and Jagodziski 2018; Anibaba et al. 2023; Czortek 2023; Czortek et al. 2023). Due to the dependence of functional diversity indices on species richness, we calculated their standardized effect sizes (SESs), i.e. deviations of metric from the null model (Czortek et al. 2021). As null models, we used randomly assembled communities for 999 randomized community data matrices, based on the independent swap algorithm that maintains species occurrence frequency and sample species richness. We calculated SESs following the code presented by Czortek et al. (2021). This way, very high or very low values of SESs indicate that the observed value is higher or lower than expected by a chance, according to the null model for particular species richness. We calculated two phylogenetic diversity indices: Faiths phylogenetic diversity (PD; i.e. the sum of phylogenetic tree branch lengths, representing all species present in the community) and mean pairwise phylogenetic distance (MPD) between species within the community. We also standardized them using SESs, to exclude the effect of species richness. That way, negative values of PD and MPD indicate strong phylogenetic clustering, i.e. higher frequency of species representing particular clades than under random circumstances. We calculated PD and MPD using the PhyloMeasures package.
Data analysis
We used non-metric multidimensional scaling (NMDS) for the assessment of the understory vegetation and its main compositional gradients. We used the vegan package to conduct NMDS. For NMDS we used default settings for transformation and Bray-Curtis dissimilarity matrix and midpoints of percentage cover of species estimated using the Braun-Blanquet scale. We assessed correlations of the main gradients of species composition revealed by NMDS by passive fit of vegetation characteristics (i.e. CWMs of EIVs and plant functional traits, taxonomic, functional and phylogenetic diversity components) into the ordination space using the vegan::envfit() function. We assessed the goodness of this fit by a permutation test (n=999).
We assessed the relationship between P. cerasifera occurrence probability and propagule sources (one located inside and one located outside the Strict Reserve of the Białowieża National Park) or vegetation characteristics using generalized linear models assuming the binomial distribution of a dependent variable. Although we counted P. cerasifera natural regeneration, its distribution was uneven (only six plots with more than a single specimen and only one with more than three), and not allowed for using approaches typical of count data, e.g. Poisson or negative binomial models. We evaluated models using Akaikes Information Criterion, corrected for small sample size (AICc). For models based on the distance to propagule sources, we provided the AICc of models and the AICc of a null (intercept-only) model. For model accounting for vegetation characteristics, we first developed a full model, with all hypothesized characteristics (i.e. species richness, Shannons Index, MPD, PD, FRic, FDis, CMWs of functional traits: SLA, LDMC, H and SM, as well as CWMs of EIVs: light, moisture, soil reaction and soil fertility). Then, we excluded collinear variables based on the variance inflation factors, and we reduced the full model to minimize AICc, using the MuMIn::dredge() function. In such an approach we assumed models differing in AICc by <2 as equivalent, accounting for the most complex models within the set of models differing by AICc from minimum AICc value by 2 units. We visualized the result of the final model using marginal responses, i.e. predicted values assuming all other predictors at a constant (mean) level, using the ggeffects::ggpredict() function. This allowed us to account the effect sizes and biological importance of the patterns obtained instead of focus on the statistical significance of the results only.
Results
Spatial patterns of P. cerasifera occurrence
During the investigation, we recorded P. cerasifera juveniles presence in 15 (50%) of the study plots. Among them, in nine plots there were single specimens, in five plots we found two specimens and in one we found nine specimens (Fig. 1). We did not find any specimens taller than 1.5 m except a single plot with one 1.8 m non-fruiting tree and one 3.5 m fruiting tree. Analysis of relationships between distance to propagule sources and P. cerasifera occurrence probability revealed that models accounting for sources located inside (AICc=41.3) and outside the Strict Reserve (AICc=42.7) had higher AICc than the null model (AICc=43.7). The probability of P. cerasifera occurrence increased with increasing distance from the propagule source outside the Strict Reserve (from 0.20 to 650 m to 0.77 at 1300 m) while decreasing with increasing distance from the propagule source inside the Strict Reserve (from 0.86 in the study plot with propagule source to 0.19 at 480 m; Table 2; Fig. 2).
Relationships between P. cerasifera occurrence and understory characteristics
Analysis of understory vegetation species composition revealed two main gradients in the ordination space (Table 3; Fig. 3). The gradient along the NMDS1 axis ordered communities along light requirements, expressed by light EIV CWM. The gradient along the NMDS2 axis divided sites with high soil moisture, reaction, and fertility requirements, high SLA and height CWMs, as well as high phylogenetic diversity from sites with high functional and taxonomic diversity, as well as high seed mass CWM.
Result of non-metric multidimensional scaling (NMDS, stress=0.1382) ordination using study plots (dots, n=30), colored by presence/absence of P. cerasifera. Diamonds and lines indicate centroids and distance from centroids for plots with and without P. cerasifera. Black labels indicate passively fit of vegetation characteristics explaining dissimilarities in species composition amongst plots (for the goodness of fit see Table 3)
Direct analysis revealed that the final model of P. cerasifera presence comprised of species richness, Faiths PD, functional dispersion SES, and light EIV (AICc=41.3, full model AICc=50.5, null model AICc=43.7; Table 4; Fig. 4). The probability of P. cerasifera presence increased with increasing species richness: from 0.01 to 16 species to 0.98 at 60 species within the study plot, and with increasing light EIV: from 0.25 to 3.0 to 0.72 at 5.0. The probability of P. cerasifera occurrence decreased with increasing functional dispersion SES: from 0.67 at 2.0 to 0.25 at 2.5, and with increasing Faiths PD: from 0.88 at 2.5 to 0.19 at 2.0.
Marginal responses of P. cerasifera presence probability, estimated using a generalized linear model (Table 4)
Discussion
Spatial patterns of P. cerasifera occurrence
Our study revealed two contrasting patterns of P. cerasifera occurrence in the Strict Reserve, potentially shaped by the distance from two hypothesised propagule sources (i.e. one located inside and one located outside BNP). However, based on our observations we cannot accurately infer the significance of particular propagule sources for shaping the colonization abilities of this invasive tree. One plausible interpretation of our findings may be that the opposite impacts of two propagule sources on P. cerasifera success in the Strict Reserve are the effects of spatial arrangement of propagule sources (i.e. southeast of each other), thus making the importance of particular propagule source low. On the other hand, the observed patterns may suggest that different animal vectors could be responsible for short- and long-distance spread of P. cerasifera in the Strict Reserve. However, direct accounting for this aspect requires either molecular studies of P. cerasifera individuals (e.g. Pairon et al. 2006, Dering et al. 2018) or tracking of animal dispersers and patterns of seed predation (e.g. Myczko et al. 2014, Vergara-Tabares et al. 2015) to identify main vectors of seed dispersal. Although this makes our inference highly limited, the observed patterns can be used as inspiration for further research on P. cerasifera dispersion mechanisms in forest ecosystems.
The probability of P. cerasifera occurrence decreased significantly with increasing the distance from the propagule source inside the Strict Reserve, reaching about 30% at the distance of ~400 m from the source. This may hint at potentially important role of birds in shaping the short-distance dispersion of P. cerasifera seeds. The trends revealed by some earlier studies investigating the dispersion of species phylogenetically similar to P. cerasifera (e.g. Prunus genus or Rosaceae family) may support this idea. P. serotina, for instance, can disperse from a single propagule source up to 600 m, confirmed by microsatellite markers (Pairon et al. 2006), while Starfinger et al. (2003) reported unpublished data on P. serotina spread up to 900 m. Distance from the propagule source was found as an important driver of P. serotina natural regeneration (Jagodziski et al. 2019). Especially, with increasing distance to the nearest seed source, perching bird density, dropping density, and seedling density decrease (Deckers et al. 2008). However, most of the seeds fall beneath the parental tree canopy (Pairon et al. 2006). There are no direct studies on P. cerasifera ornitochory, except reported low frequency of its seeds (0.05%) in P. serotina-dominated seed banks under electricity pylons (Dylewski et al. 2017). Other vectors that may be of potentially similar importance for the short-distance dispersion of P. cerasifera seeds as birds may be small mammals, e.g. yellow-necked mouse (Apodemus flavicollis) or bank vole (Clethrionomys glareolus) (Selva et al. 2012). Regarding the propagule source outside the Strict Reserve, we revealed that the probability of P. cerasifera occurrence was higher further away from the propagule source. The probability of about 75% for P. cerasifera to be present at a distance~1.2 km may suggest presumably higher ecological importance of endozoochory by large ungulates. European roe deer (Cervus capreolus), red deer (Cervus elaphus), European bison (Bison bonasus) and wild boar (Sus scrofa) can potentially eat cherry plum fruits (see review in Delibes et al. 2019), and therefore may also transport the seeds over a long distance, as the daily movement distance of B. bonasus is on average 5.1 km, S. scrofa 6.8 km, while the daily range of C. elaphus, is 1.1, and S. scrofa is 1.3 km2 (Rouys 2003; Kamler et al. 2007; Podgórski et al. 2013).
The success of invasive P. cerasifera in a primeval forest may be influenced by the forest type growing in the surrounding of each of the propagule sources investigated. Surrounding the propagule source outside the Strict Reserve is dominated by ash-alder riparian forest. Episodic flooding, water stagnation-driven anoxia, and the high role of interspecific competition in shaping the understory vegetation may be the main filters limiting the P. cerasifera occurrence in this forest type, thus highlighting the potential role of long-distance seed dispersers in shaping this invaders success. Moreover, the importance of both short- and long-distance seed dispersion may be modified by habitat properties and understory vegetation characteristics of oak-lime-hornbeam forest.
Relationships between P. cerasifera occurrence and understory characteristics
Based on the indirect ordination approach we revealed that the probability of P. cerasifera presence was higher in plots characterized by both higher and lower taxonomic, functional, and phylogenetic diversity of understory. Similar patterns were revealed regarding the the community weighted means of plant functional traits and ecological requirements of understory species: P. cerasifera occurred more frequently when coexisted, for instance, with plants of both higher and lower canopy height and specific leaf area, as well as with light- and shade-tolerant species, or of different soil moisture and fertility requirements. On the one hand, this indicates the potentially high abilities of P. cerasifera to colonize a high variety of microhabitats developing in the primeval forest, occupied by native understory species differing strongly in ecological requirements, strategies of resources acquisition and utilization, as well as evolutionary history and phylogenetic relatedness structure. Such ability is typical of other invasive Prunus species, e.g. P. serotina (Starfinger et al. 2003; Dyderski and Jagodziski 2018). On the other hand, the occurrence of P. cerasifera in a wide spectrum of forest understory characteristics may be a visualization of its early invasion stages, suggesting that this exotic plant still did not colonize all available habitats. Moreover, all observed P. cerasifera individuals were older than one year, pointing out that they survived the critical stage of their growth, as the tree seedlings mortality is the highest in the first year after germination (Closset-Kopp et al. 2007; Dyderski and Jagodziski 2019). However, the ecological mechanisms shaping P. cerasifera seedlings survival remain unknown, so further studies are needed to address this issue.
We found that the probability of P. cerasifera occurrence increased if the total species richness and contribution of plant species of higher light demands were higher. This can correspond with the biotic acceptance theory, suggesting high resource availability is suitable for both alien and native species (Stohlgren et al. 2006). Knight et al. (2008) and Dyderski and Jagodziski (2018) revealed a positive relationship between understory species richness and invasive P. serotina natural regeneration density or biomass. The positive relationship of P. cerasifera occurrence probability with species richness and light EIV may hint at the significant role of small-area disturbances, caused by collapsing of single trees damaged by windthrows or killed by insect outbreaks, which could increase the success of P. cerasifera. A similar pattern has been described for P. serotina, taking advantage of disturbances in the forest canopy (Jagodziski et al. 2019). Following the gap dynamic model developed by Bobiec et al. (2000), the opening of the tree canopy increases the success of trees natural regeneration (Dyderski and Jagodziski 2018; De Lombaerde et al. 2020) and allows the occurrence of light-demanding plants. This group includes, inter alia, ruderal species or plants typical of semi-natural grasslands, which in the subsequent stages of the forest succession are replaced by late-successional, shade-tolerant species (Faliński 1986; Orczewska et al. 2019). Seeds of these early-successional plants occur in high numbers in soil seed banks of the mixed deciduous oak-lime-hornbeam forest of the Strict Reserve (Jankowska-Baszczuk et al. 1998). The creation of gaps may trigger their germination and growth rates under improved light conditions, thus increasing temporarily the total species richness and taxonomic diversity of the understory (Orczewska et al. 2019). In addition, the probability of P. cerasifera occurrence increased alongside decreasing functional dispersion SES, expressing the positive role of habitat filters and lowered competition in shaping this invaders colonization abilities, generated by pioneer microhabitat conditions developing in gaps. Moreover, the probability of P. cerasifera occurrence was lower if Faiths phylogenetic diversity of co-existing vegetation was higher, indicating that P. cerasifera could co-occur more frequently with plant species similar in regards to their evolutionary history. On the one hand, the low heterogeneity of phylogenetic relatedness structure may be an expression of the development of evolutionary adaptations to occur in pioneer gap conditions, illustrated in high similarities of niches realized by these species (low values of functional dispersion SES).
High phylogenetic clustering may also visualize similar trajectories of the evolution of seed dispersal strategies (Jaroszewicz et al. 2023), with endozoochory identified as having one of the highest importance for understory species dispersal both in managed (Eycott et al. 2007) and primeval forest ecosystems (Heinken et al. 2002; Jaroszewicz et al. 2013). Kuijper et al. (2009) revealed that the visitation frequency of all five wild ungulates (i.e. B. bonasus, C. capreolus, C. elaphus, A. alces and S. scrofa) considered in their experiment was almost twice higher in forest gaps than in a closed forest. Improved light availability may increase food quality, driven by the higher photosynthesis abilities of graminoids and forbs growing in forest gaps, reaching there higher richness and biomass (Modry et al. 2004). Moreover, Jaroszewicz et al. (2013) in their study on endozoochory in the Białowieża Forest demonstrated that the same ungulate species as investigated by Kuijper et al. (2009) were of the unique and high importance of seed dispersal of numerous species representing a wide habitat spectrum. Thus, forest gaps may be identified not only as substantial places for grazing and browsing, but as important sites where the probability for P. cerasifera seed dispersion may be high. Apart from feeding activities, ungulates spend there much time walking (i.e. almost 43% in the case of C. elaphus or above 50% regarding B. bonasus) or rooting (i.e. about 89% of the time spent by S. scrofa) (Kuijper et al. 2009). Therefore, the development of trampling- or rooting-created microhabitats with disturbed and lowered vegetation cover (expressed by high habitat filtering and low competition), may increase substantially the success of species of higher light demands, as well as of similar functions and phylogenesis. These plants may germinate from seed banks (Sondej and Kwiatkowska-Faliska 2017 or colonize these microhabitats through endozoochory (Jaroszewicz et al. 2013; Delibes et al. 2019)as may occur in case of P. cerasifera spread.
Conclusions
Our study suggests that both short- and long-distance spread of P. cerasifera to a large extent can increase potentially the success of this invasive plant species in the primeval forest ecosystem. Interactions between natural disturbances, characteristics of recipient vegetation, as well as dispersal abilities may be identified as other presumably important driver promoting the occurrence of P. cerasifera in the Białowieża Forest. A high tendency of P. cerasifera to occur in a wide spectrum of forest understory characteristics may hint at a high adaptive potential to invade and spread onto new habitats in the primeval forest. Our study is the first insight to illustrate the invasion mechanisms of P. cerasifera under conditions of the primeval forest ecosystem, however indicating that most of the mechanisms of this plant species invasion are not known. Therefore, further studies on the patterns of P. cerasifera invasion in different types of recipient ecosystems are needed to identify drivers promoting invasion, as well as the effects of this exotic plant on biodiversity and the functioning of ecosystems. This knowledge would be crucial in developing early-warning systems, which may not only minimize the risk of further invasion, but reduce the range of effects on indigenous ecosystems functioning, and potential costs of P. cerasifera invasion management both in managed and protected forests. Monitoring of potential P. cerasifera spread should focus on recently-disturbed sites near propagule sources. Our insights suggest that promoting high canopy closure can be helpful in preventing spread of the studied species.
Data availability
Raw data used for the conductance of this study are stored in the figshare under the following link: https://figshare.com/s/8567ceacbde96e091195. The DOI number (https://doi.org/10.6084/m9.figshare.22146680) related for these data will be active after the potential acceptance of our article for publication in the Biological Invasions.
Change history
28 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10530-023-03219-9
References
Adamowski W, Wołkowycki D (2014) Flora roślin Naczyniowych. In: Wołkowycki D (ed) Przyroda okolic wsi Haćki na Równinie Bielskiej. Fundacja “Zielone Płuca Polski", Białystok, pp 71–98
Adamowski W, Mędrzycki P, Łuczaj Ł (1998) The penetration of alien woody species into the plant communities of the Białowieża Forest: the role of biological properties and human activities. Phytocoenosis 10 (N.S). Suppl Cartogr Geobot 9:211–228
Adamowski W, Dvorak L, Ramanjuk I (2002) Atlas of alien woody species of the Białowieża Primaeval Forest. Phytocoenosis (N.S.) 14. Suppl Cartogr Geobot 14:1–303
Aerts R, Ewald M, Nicolas M, Piat J, Skowronek S et al (2017) Invasion by the alien tree Prunus serotina alters ecosystem functions in a temperate deciduous forest. Front Plant Sci 8:179. https://doi.org/10.2289/fpls.2017.00179
Anibaba QA, Dyderski MK, Woźniak G, Jagodziński AM (2023) Native plant community characteristics explain alien species success in post-industrial vegetation. NeoBiota 85:1–22. https://doi.org/10.3897/neobiota.85.97269
Basnou C, Iguzquiza J, Pino J (2015) Examining the role of landscape structure and dynamics in alien plant invasion from urban Mediterranean coastal habitats. Landsc Urban Plan 136:156–164. https://doi.org/10.1016/j.landurbplan.2014.12.001
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP et al (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339. https://doi.org/10.1016/j.tree.2011.03.023
Bobiec A, van der Burgt H, Meijer K, Zuyderduyn C, Haga J, Vlaanderen B (2000) Rich deciduous forests in Białowieża as a dynamic mosaic of developmental phases: premises for nature conservation and restoration management. For Ecol Manag 130:159–175. https://doi.org/10.1016/S0378-1127(99)00181-4
Burda RI, Koniakin SN (2019) The non-native woody species of the flora of Ukraine: introduction, naturalization and invasion. Biosyst Divers 27:276–290. https://doi.org/10.15421/011937
Cadenasso ML, Pickett STA (2001) Effect of edge structure on the flux of species into forest interiors. Conserv Biol 15:91–97. https://doi.org/10.1111/j.1523-1739.2001.99309.x
Carroll IT, Cardinale BJ, Nisbet RM (2011) Niche and fitness differences relate the maintenance of diversity to ecosystem function. Ecology 92:1157–1165. https://doi.org/10.2307/23213778
Closset-Kopp D, Chabrerie O, Valentin B, Delachapelle H, Decocq G (2007) When Oskar meets Alice: does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? For Ecol Manag 247:120–130. https://doi.org/10.1016/j.foreco.2007.04.023
Czortek P (2023) Landscape-driven effects on taxonomic, functional, and phylogenetic diversity of vegetation developing on sand-gravel pits of early successional stages. Ecol Inf 76:102123. https://doi.org/10.1016/j.ecoinf.2023.102123
Czortek P, Kapfer J, Delimat A, Eycott AE, Grytnes J-A, Orczewska A, Ratyńska H, Zięba A, Jaroszewicz B (2018) Plant species composition shifts in the Tatra Mts as a response to environmental change: a resurvey study after 90 years. Folia Geobot 53:333–348. https://doi.org/10.1007/s12224-018-9312-9
Czortek P, Orczewska A, Dyderski MK (2021) Niche differentiation, competition or habitat filtering? Mechanisms explaining co-occurrence of plant species on wet meadows of high conservation value. J Veg Sci 32:e12983. https://doi.org/10.1111/jvs.12983
Czortek P, Królak E, Borkowska L, Bielecka A (2023) Effects of surrounding landscape on the performance of Solidago Canadensis L. and plant functional diversity on heavily invaded post-agricultural wastelands. Biol Invasions 25:2477–2494. https://doi.org/10.1007/s10530-023-03050-2
De Lombaerde E, Blondeel H, Baeten L, Landuyt D, Perring MP et al (2020) Light, temperature and understorey cover predominantly affect early life stages of tree seedlings in a multifactorial mesocosm experiment. For Ecol Manag 461:117907. https://doi.org/10.1016/j.foreco.2020.117907
Deckers B, Verheyen K, Vanhellemont M, Maddens E, Muys B, Hermy M (2008) Impact of avian frugivores on dispersal and recruitment of the invasive Prunus serotina in an agricultural landscape. Biol Invasions 10:717–727. https://doi.org/10.1007/s10530-007-9164-3
Delibes M, Castañeda I, Fedriani JM (2019) Spitting seeds from the cud: a review of an endozoochory exclusive to ruminants. Front Ecol Evol 7:265. https://doi.org/10.3389/fevo.2019.00265
Dering M, Sękiewicz K, Iszkuło G, Chojnacka A, Tomaszewski D, Pers-Kamczyc E, Karolewski P (2018) Spatial genetic structure and clonality of Prunus serotina Ehrh. During invasive spread into scots pine forests. Silva Fenn 52:9987. https://doi.org/10.14214/sf.9987
Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S et al (2016) The global spectrum of plant form and function. Nature 529:167–171. https://doi.org/10.1038/nature16489
Diniz-Filho JAF, de Sant’Ana CER, Bini LM (1998) An eigenvector method for estimating phylogenetic inertia. Evolution 52:1247–1262. https://doi.org/10.1111/j.1558-5646.1998.tb02006.x
Dobrzycka-Krahel A, Medina-Villar S (2020) Alien species of Mediterranean origin in the Baltic Sea Region: current state and risk assessment. Environ Rev 28:339–356. https://doi.org/10.1139/er-2019-0074
Dyderski MK, Jagodziński AM (2018) Drivers of invasive tree and shrub natural regeneration in temperate forests. Biol Invasions 20:2363–2379. https://doi.org/10.1007/s10530-018-1706-3
Dyderski MK, Jagodziński AM (2019) Seedling survival of Prunus serotina Ehrh., Quercus rubra L. and Robinia pseudoacacia L. in temperate forests of Western Poland. Forest Ecol Manag 450:117498. https://doi.org/10.1016/j.foreco.2019.117498
Dylewski Ł, Kurek P, Wiatrowska B, Jerzak L, Tryjanowski P (2017) Man-made perching sites – electricity pylons accelerate fleshy-fruited plants succession in farmlands. Flora 231:51–56. https://doi.org/10.1016/j.flora2017.04.004
Dzwonko Z (2001) Assessment of light and soil conditions in ancient and recent woodlands by Ellenberg indicator values. J Appl Ecol 38:942–951. https://doi.org/10.1046/j.1365-2664.2001.00649.x
Elgersma KJ, Ehrenfeld JG (2011) Linear and non-linear impacts of a non-native plant invasion on soil microbial community structure and function. Biol Invasions 13(3):757–768. https://doi.org/10.1007/s10530-010-9866-9
Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. UTB, Stuttgart
Evangelista A, Frate L, Carranza ML, Attorre F, Pelino G, Stanisci A (2016) Changes in composition, ecology and structure of high-mountain vegetation: a re-visitation study over 42 years. AoB Plants. https://doi.org/10.1093/aobpla/plw004. (AoB PLANTS:plw004)
Eycott AE, Watkinson AR, Hemami MR, Dolman PM (2007) The dispersal of vascular plants in a forest mosaic by a guild of mammalian herbivores. Oecologia 154:107–118. https://doi.org/10.1007/s00442-007-0812-1
Faliński JB (1986) Vegetation dynamics in temperate lowland primeval forest. Ecological studies in Białowieża forest. Geobotany 8:1–357
Faliński JB (1998) Invasive alien plants and vegetation dynamics. In: Starfinger U, Edwards K, Kowarik I, Williamson M (eds) Plant invasions: ecological mechanisms and human responses. Backhyus Publishers, Leiden, pp 3–21
Galasso G, Conti F, Peruzzi L, Ardenghi NMG, Banfi E et al (2018) An updated checklist of the vascular flora alien to Italy. Plant Biosyst 152(3):556–592. https://doi.org/10.1080/11263504.2018.1441197
Hedberg P, Kozub Ł, Kotowski W (2014) Functional diversity analysis helps to identify filters affecting community assembly after Fen restoration by top-soil removal and hay transfer. J Nat Conserv 22:50–58. https://doi.org/10.1016/j.jnc.2013.08.004
Heinken T, Hanspach H, Raudnitschka D, Schaumann F (2002) Dispersal of vascular plants by four species of wild mammals in a deciduous forest. NE Ger Phytocoenologia 32(4):627–643. https://doi.org/10.1127/0340-269X/2002/0032-0627
Jagodziński AM, Dyderski MK, Horodecki P, Knight KS, Rawlik K, Szmyt J (2019) Light and propagule pressure affect invasion intensity of Prunus serotina in a 14-tree species forest common garden experiment. NeoBiota 46:1–21. https://doi.org/10.3897/neobiota.46.30413
Jankowska-Błaszczuk M, Kwiatkowska AJ, Panufnik D, Tanner E (1998) The size and diversity of the soil seed banks and the light requirements of the species in sunny and shady natural communities of the Białowieża Primeval Forest. Plant Ecol 136:105–118
Jaroszewicz B, Pirożnikow E, Sondej I (2013) Endozoochory by the guild of ungulates in Europe’s primeval forest. For Ecol Manag 305:21–28. https://doi.org/10.1016/j.foreco.2013.05.004
Jaroszewicz B, Cholewińska O, Gutowski JM, Samojlik T, Zimny M, Latałowa M (2019) Białowieża Forest – a relic of the high naturalness of European forests. Forests 10(10):849. https://doi.org/10.3390/f10100849
Jaroszewicz B, Coissac E, Taberlet P, Czajkowska M, Świsłocka M, Kowalczyk R, Ratkiewicz M (2023) Is endozoochoric seed dispersal by large herbivores an evolutionary adaptation? Revisiting the Janzen’s ‘foliage is the fruit.’ Acta Oecol 118:103888. https://doi.org/10.1016/j.actao.2022.103888
Kamler JF, Jedrzejewska B, Jedrzejewski W (2007) Factors affecting daily ranges of red deer Cervus elaphus in Bialowieza Primeval Forest, Poland. Acta Ther 52:113–118. https://doi.org/10.1007/BF03194206
Knight KS, Oleksyn J, Jagodziński AM, Reich PB, Kasprowicz M (2008) Overstorey tree species regulate colonization by native and exotic plants: a source of positive relationships between understorey diversity and invasibility. Divers Distrib 14:666–675. https://doi.org/10.1111/j.1472-4642.2008.00468.x
Kuebbing SE, Maynard DS, Bardford MA (2018) Linking functional diversity and ecosystem processes: a framework for using functional diversity metrics to predict the ecosystem impact of functionally unique species. J Ecol 106:687–698. https://doi.org/10.1111/1365-2745.12835
Kuijper DPJ, Cromsigt JPGM, Churski M, Adam B, Jędrzejewska B, Jędrzejewski W (2009) Do ungulates preferentially feed in forest gaps in European temperate forest? For Ecol Manag 258:1528–1535. https://doi.org/10.1016/j.foreco.2009.07.010
Kurtto A (2009) Rosaceae (pro parte majore). In: Euro + Med Plantbase-the information resource for Euro-Mediterranean plant diversity
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. https://doi.org/10.1890/08-2244.1
Lapin K, Oettel J, Steiner H, Langmaier M, Sustic D, Starlinger F, Kindermann G, Frank G (2019) Invasive alien plant species in unmanaged forest reserves, Austria. NeoBiota 48:71–96. https://doi.org/10.3897/neobiota.48.34741
Levine JM, Alder PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989. https://doi.org/10.1111/j.1461-0248.2004.00657.x
Łapok R, Borkowska L, Lembicz M, Jensen K, Kasprzykowski Z (2018) A narrow-gauge railway in the Białowieża Primeval Forest as a corridor for non-native species migration. Flora 240:40–47. https://doi.org/10.1016/j.flora.2018.01.002
Łuczaj Ł (1994) Development of forest edge scrub communities in the Białowieża Forest in north-eastern Poland. Fragm Flor Geobot 39(2):589–604
Martin PH, Canham CD, Marks PL (2009) Why forests appear resistant to exotic plant invasions: intentional introductions, stand dynamics, and the role of shade tolerance. Front Ecol Environ 7:142–149. https://doi.org/10.1890/070096
Mędrzycki P, Pabjanek P (2001) Linking land use and invading species features: a case study of Acer negundo in Białowieża village (NE Poland). In: Brundu G et al (eds) Plant invasions: Species Ecology and Ecosystem Management. Backhuys Publishers, Leiden, pp 123–132
Modry M, Hubeny D, Rejsek K (2004) Differential response of naturally regenerated European shade tolerant tree species to soil type and light availability. For Ecol Manag 188:185–195. https://doi.org/10.1016/j.foreco.2003.07.029
Myczko Ł, Dylewski Ł, Zduniak P, Sparks TH, Tryjanowski P (2014) Predation and dispersal of acorns by European Jay (Garrulus glandarius) differs between a native (Pedunculate Oak Quercus robur) and an introduced oak species (Northern Red Oak Quercus rubra) in Europe. For Ecol Manag 331:35–39. https://doi.org/10.1016/j.foreco.2014.07.027
Nicod C, Gillet F (2021) Recent changes in mountain hay meadows of high conservation value in eastern France. Appl Veg Sci 24:e12573. https://doi.org/10.1111/avsc.12573
Novoa A, Richardson DM, Pyšek P, Meyerson LA, Bacher S et al (2020) Invasion syndromes: a systematic approach for predicting biological invasions and facilitating effective management. Biol Invasions 22:1801–1820. https://doi.org/10.1007/s10530-020-02220-w
Nuñez M, Chiuffo MC, Seebens H, Kuebbing S, McCary M, Lieurance D, Zhang B, Simberloff D, Meyerson LA (2022) Two decades of data reveal that biological Invasions needs to increase participation beyond North America, Europe, and Australasia. Biol Invasions 24:333–340. https://doi.org/10.1007/s10530-021-02666-6
Orczewska A, Czortek P, Jaroszewicz B (2019) The impact of salvage logging on herb layer species composition and plant community recovery in Białowieża Forest. Biodivers Conserv 28:3407–3428. https://doi.org/10.1007/s10531-019-01795-8
Pairon M, Jonard M, Jacquemart A-L (2006) Modelling seed dispersal of black cherry, an invasive forest tree: how microsatellites may help? Can J For Res 36:1385–1394. https://doi.org/10.1139/x06-018
Paź-Dyderska S, Jagodziński AM (2023) In search of a perfect trait set: A workflow presentation based on the conservation status assessment of Poland’s dendroflora. Ecol Evol 13:e9979. https://doi.org/10.1002/ece3.9979
Penone C, Davidson AD, Shoemaker KT, Di Marco M, Rondinini C, Brooks TM, Young BE, Graham CH, Costa GC (2014) Imputation of missing data in life-history trait datasets: which approach performs the best? Methods Ecol Evol 5:961–970. https://doi.org/10.1111/2041-210X.12232
Podgórski T, Baś G, Jędrzejewska B, Sönnichsen L, Śnieżko S, Jędrzejewski W, Okarma H (2013) Spatiotemporal behavioral plasticity of wild boar (Sus scrofa) under contrasting conditions of human pressure: primeval forest and metropolitan area. J Mamm 94:109–119. https://doi.org/10.1644/12-MAMM-A-038.1
Popescu I, Caudullo G (2016). In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) Prunus cerasifera in Europe: distribution, habitat, usage and threats. European Atlas of Forest Tree Species, Luxembourg, p 142
POWO (2022) Plants of the world online. https://powo.science.kew.org/
Pyšek P, Manceur AM, Alba C, McGregor KF, Pergl J et al (2015) Naturalization of central European plants in North America: species traits, habitats, propagule pressure, residence time. Ecology 96:762–774. https://doi.org/10.1890/14-1005.1
Pyšek P, Sádlo J, Chrtek J, Chytrý M, Kaplan Z et al (2022) Catalogue of alien plants of the Czech Republic (3rd edition): species richness, status, distributions, habitats, regional invasion levels, introduction pathways and impacts. Preslia 94:447–577. https://doi.org/10.23855/preslia.2022.447
R Core Team (2021) R: a language and environment for statistical computing
Rejmánek M, Richardson DM (2013) Trees and shrubs as invasive alien species – 2013 update of the global database. Divers Distrib 19:1093–1094. https://doi.org/10.1111/ddi.12075
Ridding LE, Bullock JM, Pescott OL, Hawes P, Walls R, Pereira MG, Thacker SA, Keenan PO, Dragosits U, Pywell RF (2020) Long-term change in calcareous grassland vegetation and drivers over three time periods between 1970 and 2016. Plant Ecol 221:377–394. https://doi.org/10.1007/s11258-020-01016-1
Rohrer JR (2014) Prunus Linnaeus. (eds) Flora of North America North of Mexico, vol 9. Oxford University Press, New York, pp 352–383. Flora of North America Editorial Committee
Rouys S (2003) Winter movements of European bison in the Białowieża Forest, Poland. Mamm Biol 68:122–125. https://doi.org/10.1078/1616-5047-00072
Sabatini FM, Burrascano S, Keeton WS, Levers C, Lindner M et al (2018) Where are Europe’s last primary forests? Divers Distrib 24(10):1426–1439. https://doi.org/10.1111/ddi.12778
Sapsford SJ, Brandt AJ, Davis KT, Peralta G, Dickie IA et al (2020) Towards a framework for understanding the context-dependence of impacts of non-native tree species. Funct Ecol 34:944–955. https://doi.org/10.1111/1365-2435.13544
Schaffers AP, Sýkora KV (2000) Reliability of Ellenberg indicator values for moisture, nitrogen and soil reaction: a comparison with field measurements. J Veg Sci 11:225–244. https://doi.org/10.2307/3236802
Selva N, Hobson KA, Cortés-Avizanda A, Zalewski A, Donázar JA (2012) Mast pulses shape trophic interactions between fluctuating rodent populations in a primeval forest. PLoS ONE 7:e51267. https://doi.org/10.1371/journal.pone.0051267
Sîrbu C, Oprea A (2011) Plante adventive în flora României. Editura “Ion Ionescu de la Brad", Iasi
Smirnov NS (1965) Ekzoty Belovežskoj Pušči i ejo okresnostej. Mscr., pp 127
Sondej I, Kwiatkowska-Falińska AJ (2017) Effects of wild boar (Sus scrofa L.) rooting on seedling emergence in Białowieża Forest. Pol J Ecol 65:380–389. https://doi.org/10.3161/15052249PJE2017.65.4.007
Starfinger U, Kowarik I, Rode M, Schepker H (2003) From desirable ornamental plant to pest to accepted addition to the flora? – the perception of an alien tree species through the centuries. Biol Invasions 5:323–335. https://doi.org/10.1023/B:BINV.0000005573.14800.07
Stohlgren TJ, Jarnevich C, Chong GW, Evangelista PH, Pyšek P, Kaplan Z, Richardson DM (2006) Scale and plant invasions: a theory of biotic acceptance. Preslia 78:405–426
Szymura TH, Szymura M, Macioł A (2014) Bioindication with Ellenberg’s indicator values: a comparison with measured parameters in Central European oak forests. Ecol Ind 46:495–503. https://doi.org/10.1016/j.ecolind.2014.07.013
Tokarska-Guzik B, Dajdok Z, Zając M, Zając A, Urbisz A, Danielewicz W, Hołdyński C (2012) Rośliny Obcego Pochodzenia w Polsce Ze szczególnym uwzględnieniem gatunków inwazyjnych. Generalna Dyrekcja Ochrony Srodowiska, Warszawa
Vergara-Tabares DL, Badini J, Peluc SI (2015) Fruiting phenology as a triggering attribute of invasion process: do invasive species take advantage of seed dispersal service provided by native birds? Biol Invasions 18:677–687. https://doi.org/10.1007/s10530-015-1039-4
Villéger S, Mason NW, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301
Holle B, Delacourt HR, Simberloff D (2003) The importance of biological inertia in plant community resistance to invasion. J Veg Sci 14:425–432. https://doi.org/10.1111/j.1654-1103.2003.tb02168.x
Wagner V, Chytrý M, Jiménez-Alfaro B, Pergl J, Hennekens S et al (2017) Alien plant invasions in European woodlands. Divers Distrib 23:969–981. https://doi.org/10.1111/ddi.12592
Wang C, Wu B, Jiang K, Zhou J, Du D (2019) Canada goldenrod invasion affect taxonomic and functional diversity of plant communities in heterogeneous landscapes in urban ecosystems in East China. Urban Urban Green 38:145–156. https://doi.org/10.1016/j.ufug.2018.12.006
Wang C, Cheng H, Wei M, Wang S, Wu B, Du D (2021) Plant height and leaf size: which one is more important in affecting the successful invasion of Solidago Canadensis and Conyza canadensis in urban ecosystems? Urban For Urban Green 59:127033. https://doi.org/10.1016/j.ufug.2021.127033
Weber E (2017) Invasive plant species of the world: a reference guide to environmental weeds. CABI, Wallingford
Westoby M (1998) A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199:213–227. https://doi.org/10.1023/A:1004327224729
Yena AV (2012) Spontaneous flora of the Crimean Peninsula. N. Orianda, Simferopol
Funding
The study was partially supported by statutory research of the Institute of Dendrology Polish Academy of Sciences and support from the Foundation for Polish Science (FNP) from the START scholarship.
Author information
Authors and Affiliations
Contributions
PC, MKD, WA and AZ conceived the ideas and designed methodology. PC, MKD, KKK and OK collected the data; PC and MKD analysed the data. The first draft of the manuscript was written by PC and all remaining authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
The authors agreed to be included as co-authors of the manuscript.
Consent for publication
The authors declare agreement for publication of the manuscript in the Biological Invasions journal.
Ethics approval
The study did involve human or animal participants as a study subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to the special characters were incorrectly denoted as "?" symbol throughout the entire article. The original article has been corrected with correct special characters.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Czortek, P., Adamowski, W., Kamionka-Kanclerska, K. et al. Patterns of Prunus cerasifera early invasion stages into a temperate primeval forest. Biol Invasions 26, 633–647 (2024). https://doi.org/10.1007/s10530-023-03188-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03188-z