Abstract
Many species of tilapia (a collection of fish species from the cichlidae family) have biological traits that make them successful invaders. Widespread herbivory has been observed across tilapia species, however knowledge on habitat preferences and tilapia interactions with native fish food webs is limited. This study was conducted three years after spotted (Pelmatolapia mariae) and Mozambique (Oreochromis mossambicus) tilapia were detected in the Mitchell River catchment, northern Australia. Tilapia diet and habitat preferences and their trophic position in native fish food webs was determined using a combination of stomach content, stable isotope, and habitat assessment analyses. Plant material was the most abundant item in tilapia stomachs, and some stomachs also contained other items in small quantities, such as fish eggs, small shrimp, and bivalves. Some niche overlap occurred between P. mariae and native omnivore or herbivore fish species. Macrohabitat type (in-channel (no tilapia), floodplain (no tilapia), weir (tilapia present)) was related to native fish food web structure, with weir food webs showing similarities to floodplain food webs. Floodplain habitats including creeks and wetlands were also most similar to weir habitats and may therefore be most at risk of colonization as tilapia spread throughout the catchment. This study clearly demonstrated that the successful rapid invasion of these two tilapia species is linked to suitable habitat with abundant food that fuels growth and reproduction. These findings increase understanding of diet related habitat use during new tilapia invasions and trophic interactions with native species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapia are a group of nearly 100 tropical freshwater fish species from the Cichlidae family, native to Africa and the southwestern Middle East (Skelton 2001). Nile tilapia Oreochromis niloticus (L.) have been a large component of inland aquaculture fisheries in Asia and South America over the past 50 years (Attayde et al. 2011), whereas several other tilapia species are popular aquarium fishes and have been introduced to waterways outside their native range around the world (Deines et al. 2016). Despite laws against possessing or consuming tilapia in Australia, strict penalties, and expensive education and eradication efforts, Mozambique Oreochromis mossambicus and spotted Pelmatolapia mariae tilapia have successfully invaded many river systems in Australia, particularly in the northeast of the country (Russell et al. 2012a). In many countries the introduction of tilapia has been used to support the provision of high protein food (Canonico et al. 2005). However, there are only a few studies documenting their harm to native species and ecosystems (e.g. Gu et al. 2014; Shuai et al. 2019; Long et al. 2021). Their potential impacts on native fish species are of concern due to the biological traits of tilapia that enable them to be successful invaders in waterways outside their native range (Greiner and Gregg 2008; Deines et al. 2016). Understanding tilapia biology and the niches they occupy in waterways outside their native range is important for informing management strategies that aim to conserve native fish biodiversity in areas where tilapia are present.
Reproductive strategies, tolerance to a range of environmental conditions, and low trophic level feeding are a few of the biological traits that enable tilapia to be successful invaders in river systems outside of their natural range (Canonico et al. 2005; Skelton 2001; Deines et al. 2016). Breeding success of tilapia is enhanced by traits including nest building and protecting, mouthbrooding, and brood guarding (Russell et al. 2012b). They can adapt their reproductive strategies to suit environmental conditions, such as increasing the number of broods but with lower brood efficiency during unfavourable seasons (Reardon and Chapman 2008), and can be aggressive towards native fish during nesting (Bradford et al. 2011). In addition to being able to survive in a range of environmental conditions, including a wide range of salinity and low dissolved oxygen saturation, tilapia can also contribute to poor water quality and eutrophication via excretion of waste, damage to macrophyte habitat during feeding, and substrate disturbance during nest building (Arthington and Blühdorn 1994; Greiner and Gregg 2008). Most tilapia species are primarily herbivorous feeders, with phytoplankton or aquatic macrophytes constituting a large proportion of their diet (Doupé et al. 2010; Semyalo et al. 2011; Idowu et al. 2019).
Strategies including feeding at a low trophic level and generalist feeding may contribute to the invasion success of non-native fish species. Feeding at a low trophic level allows invasives to utilize abundant food sources, particularly when few native herbivores are present (Marshall et al. 2019), whereas generalist feeding allows invasives to adjust their feeding behaviour when niche overlap with native species occurs (Zengeya et al. 2011). As such, extinctions of native species are rare when generalist invasives are present, but lower abundance of native species has been observed (Davis 2003; Bruno et al. 2005; David et al. 2017). However, when food diversity is low, there may be greater competition with native fishes, as both natives and invasives are forced to share resources (Bergstrom and Mensinger 2009; Córdova-Tapia et al. 2015).
Understanding how native fish food webs are shaped by their environment, as well as identifying species that have overlapping niches with invasives, is important for informing management strategies that aim to mitigate impacts of invasive species (Wang et al. 2021). Stomach contents and stable isotope analyses are effective methods to study diet and trophic interactions (Pacioglu et al. 2019). Stomach contents provide a detailed measure of the diet of tilapia in the immediate period (~ 7 h to 1–2 days; Uscanga et al. 2010; de Oliveira et al. 2022) leading up to the sample collection, while stable isotopes provide a coarser measurement of an organism’s diet over a longer time frame (weeks to months, depending on tissue turnover rates; Buchheister and Latour 2010; Vander Zanden et al. 2015; Busst and Britton 2018).
This study was conducted during the early stages of a tilapia invasion (approximately three years after tilapia were detected at multiple locations in the Mitchell River catchment) and involved sampling locations in the catchment where tilapia became established (though densities were still relatively low compared to nearby rivers with longer establishment periods), as well as locations where tilapia were not detected. There is little information available on the diet and habitat ecology of tilapia during new invasions, and such information can be used to better inform targeted management strategies that aim to reduce the spread and minimize impacts of tilapia. The study aimed to (1) determine the diet and trophic position of O. mossambicus and P. mariae in the food webs of the Mitchell River catchment, northern Australia, (2) determine the influence of habitat type on native fish food webs, and (3) identify risk areas in the catchment for further invasion. A combination of stomach contents analysis, stable isotope analysis, and habitat assessments were used to address these aims.
Methods
Study location
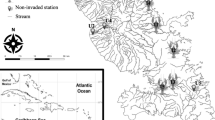
The Mitchell River catchment is situated in the wet-dry tropics of northern Queensland, Australia, and covers an area of approximately 72,000 km2 (Petheram et al. 2018). The westward flowing main channel of the Mitchell River stretches from the headwaters in the Daintree rainforest in the east to the river mouth in the Gulf of Carpentaria in the west. Major tributaries that flow into the Mitchell River include the Walsh, Lynd, Alice, and Palmer rivers (Fig. 1). The geology and river form varies throughout the catchment, with higher elevations to the east comprised of mostly bedrock (Batlle-Aguilar et al. 2014). An alluvial delta megafan at lower elevation spreads west from the confluence of the Mitchell and Palmer Rivers, producing a network of braided channels and creeks on the floodplain (Rustomji et al. 2010). There are several Indigenous Australian communities residing within the catchment (making up ̴ 23% of the catchment population) who harvest fish and other animals from wetlands, creeks and river channels as an important part of their diet (Jackson et al. 2014).
Rainfall in the Mitchell catchment is highly seasonal, with only 4% of annual rainfall (on average) falling across the catchment during the dry season, from May to October (Petheram et al. 2018). A mostly unregulated flow regime ensures wet season flows provide lateral connectivity to floodplain habitats such as wetlands and creeks that become disconnected from the river channel in the dry season. Recent research has shown that fish move onto the Mitchell floodplain to feed during the wet season (Jardine et al. 2012; O’Mara et al. 2021), especially larger bodied species (Jardine et al. 2017). Fish retreat to the main channel and wetlands during the prolonged dry season and sustain biomass with autochthonous food sources (Venarsky et al. 2020) such as aquatic macroinvertebrates (O’Mara et al. 2022).
Tilapia were first detected in the Mitchell River catchment in 2008 in Eureka Creek (a side creek of the upper Walsh River) and were eradicated in 2008 with rotenone poisoning (DAF 2011). In 2017, a report was made to the Queensland Government by community members stating tilapia had been observed in the upper Walsh River. In subsequent physical and eDNA surveys both O. mossambicus and P. mariae were detected in the Walsh River, with 2019 surveys finding established groups of tilapia in three locations, including Eureka Creek and two weirs (Bruce and Leafgold weirs) on the upper Walsh River (www.daf.qld.gov.au).
Field sampling
Fish were collected for stable isotope samples using either a 2.5 GPP Smith-Root boat-mounted electrofishing unit or a Smith-Root LR24 back-pack in 2017/2018 and a 7.5GPP Smith-Root electrofisher, mounted in a 4.1 m aluminium boat or a Smith-Root LR24 back-pack in 2020/2021. Backpack electrofishing was used at sites where low water levels prevented boat use. We conducted between five to eight electrofishing passes at each site, depending on the reach length. Each electrofishing shot was between 120 and 200 s. Sites sampled in 2017, 2018, and 2020 did not contain tilapia and were chosen to study native fish food webs across the catchment, and sites visited in 2021 contained tilapia and were chosen in response to tilapia detections during Queensland Department of Agriculture and Fisheries surveys to locate tilapia conducted in 2019 (Table S1).
A total of 254 fish samples were collected for stable isotope analysis. Between 1 and 10 individuals (depending on site abundance) of four common native fish species from a range of known trophic guilds (Sternberg and Kennard 2014) were collected from each site to compare food web structure among macrohabitat types across the catchment (see Table S2 for site x species n). Species collected included one herbivore/detritivore species (Nematalosa erebi—Clupeidae family), two omnivore species from the Terapontidae family (Amniataba percoides and Leiopotherapon unicolor) and invertivore/piscivore species from the Eleotridae family, Oxyeleotris genus, including Oxyeleotris lineolatus or Oxyeleotris selheimi. Fish collected for stable isotope analysis in 2017, 2018, and 2020 were euthanized in an ice slurry, and muscle tissue was dissected. To minimize disturbance to the fish community at tilapia invaded sites, fin clips were taken using non-lethal sampling of native fish in 2021, as differences in stable isotope values between muscle and fin samples for fish from the Mitchell River catchment have been previously shown to be negligible (Jardine et al. 2011). Samples were dissected and frozen in the field before being transported to Griffith University and stored in a − 20 °C freezer. Tilapia caught in the Bruce and Leafgold weirs in 2021 were placed in an ice slurry after capture to preserve the structure and consistency of stomach contents. Stomachs were dissected in the field and placed in sample bags before being frozen and transported to Griffith University for stomach contents analysis.
Several habitat metrics were recorded at each site, including, percent substrate type (mud, sand, gravel, and bedrock), and percent macrohabitat type (root mass, overhanging vegetation, undercut bank, and large [> 10 cm dia.] and small [< 10 cm dia.] wood). Depending on the size of the site sampled, which varied with geomorphology and macrohabitat type (reach lengths of sites ranged between 190 and 1138 m), water depth and the following physiochemical variables were measured at one to four locations within the site: temperature, conductivity, turbidity, and dissolved oxygen. Water column profile physicochemical measurements were taken at sites with stratification, though most sites had well mixed physicochemical profiles. Physicochemical variables were measured using a YSI handheld sonde.
Stomach contents and stable isotope analysis
Stomach contents
Stomach content analyses were performed on 39 tilapia stomachs using a microscope and petri dish. Stomachs were cut open and stomach fullness (0 (empty)–100 (full) %) was recorded before emptying the stomach contents onto a petri dish. Stomach contents were separated in water and examined under a microscope to identify prey items. A percentage of the total contents was assigned to each item using a 5 mm × 5 mm grid under the petri dish. A Type II PERMANOVA (Euclidean resemblance, arcsine transformed percentages of items compared to total contents (2/PI*arcsin(sqrt(x))) was performed in PRIMER to test for differences in stomach contents composition between species.
Stable isotopes
Fish muscle tissue samples were freeze dried for 48 h. Tilapia samples were visibly richer in lipids than native fish tissue. The lipid content in the tilapia samples was lowered to be comparable with the other species (C:N ratio of approximately 4.0 or less) by submerging samples in chloroform methanol (2:1) in glass test tubes in a fume cupboard overnight (Groß et al. 2021). The solution was then poured off and this process was repeated before rinsing the sample in deionized water and oven drying at 60 °C for 48 h. All lipid extracted samples and a subset of unextracted samples were analyzed, and C:N ratios of the same individuals were compared to ensure the lipid extraction method was sufficient for the desired reduction in lipids to C:N of ~ 4.0 or less. All samples were ground into a fine powder using a stainless-steel Retch Mixer Mill grinder. Ground and dried tissue was weighed (9–10 mg) into tin capsules and an oxidizing agent (vanadium pentoxide) was added to aid combustion of S for δ34S analysis.
Fish samples were analyzed using a Sercon Europa EA-GSL elemental analyzer and a Hydra-20-20 continuous flow isotope ratio mass spectrometer. Relative differences of isotope ratios were expressed in delta (δ) notation as deviations from international standards in per mil (‰): δX = [(Rsample/Rstandard) − 1], where X is 13C, 15N, or 34S, Rsample is the ratio (13C/12C, 15N/14N, or 34S/32S) in the sample, and Rstandard is the ratio in the standard. Reference material Vienna Pee Dee Belemnite (VPDB) was used for carbon, air N2 for nitrogen, and Vienna Cañon Diablo troilite (VCDT) for sulfur. Primary standards were IAEA-N and IAEA-N2 for 15N, IAEA-CH-6 for 13C, and IAEA-S-1, IAEA-S-2, and IAEA-S-3 for 34S. The reference standard was NBS-127 and working standards were Hi Mix and Low Mix. The analytical precision for deviation from the standards used is listed in Table 1.
Data analysis
We used the software Microsoft Excel and PRIMER (v6) (Clarke and Gorley 2006) for all data transformations and analyses. ArcMap 10.8 (ESRI 2011) and R version 4.0.2 (R Core Team 2021) in the RStudio IDE (RStudio Team 2020) were used for mapping.
Stable isotopes data analysis
The R package ‘nicheROVER’ (Swanson et al. 2015; Lysy et al. 2021) was used to estimate niche size and probability of isotopic niche overlap among native species and tilapia at sites where tilapia were present. Both niche size and overlap were estimated from δ13C, δ15N, and δ34S isotope values for the 95% region using a 1000 random draws from the posterior distribution with Normal-Inverse-Wishart prior (Lysy et al. 2021).
Carbon, nitrogen, and sulfur stable isotope values were standardized to local isotope baseline conditions at each site. The standardization converted the isotope values to comparable values among the sites by re-scaling measured δ13C, δ15N, and δ34S isotope data to modified Z-scores to equalize the effects of baselines and average food inputs (Fry and Davis 2015). Because the same species were collected from each site, it was possible to rescale individual values using the species means, community means and community standard deviation for each site (Fry and Davis 2015).
To determine if there were statistical differences in the food web structure among macrohabitat types, a PERMANOVA was used in PRIMER, following transformation of the standardized isotope data into a Euclidean distance matrix. Single and interactive effects of the fixed factors ‘Species’ and ‘Macrohabitat type’ on food web structure were analyzed in the PERMANOVA with unrestricted permutations of the raw data. A pairwise PERMANOVA of the same terms was then used to examine macrohabitat type differences for each species.
Habitat similarity assessment
We used minimally correlated environmental variables in the analysis (excluding one variable from each pair of variables with Pearson’s r > 0.75) that described habitat characteristics and water physical and chemical measurements (depth, water temperature, conductivity, turbidity, dissolved oxygen, mud substrate (%), sand substrate (%), bedrock (%), small wood (%), large wood (%), macrophyte cover (%), overhanging vegetation (%), steep banks (%), undercut banks (%)). Physical and chemical variables were averaged throughout the water column for stratified sites and all environmental variables (except depth) were averaged across replicates within each site. The value used for site depth was the deepest observation at each site. Water physical and chemical variables and depth were log(x + 1) transformed while habitat characteristics (recorded as percentages) were arcsine transformed using 2/PI*arcsin(sqrt(x)).
The environmental data were imported into PRIMER where all variables were normalized, and a PCA was used to visualize the environmental data. The data were then transformed into a Euclidean distance matrix, and a SIMPER analysis was performed using a factor, which grouped the sites where tilapia were present (weir habitats) and kept all other sites separate. The weir sites were grouped due to having highly similar PCA values and a low average squared distance between them of 2.04 (SIMPER). The averaged squared distance between the tilapia site group and each other site was mapped to identify sites around the catchment that had an aquatic environment similar to sites inhabited by tilapia. A high similarity would suggest the site may provide suitable tilapia habitat.
Results
Tilapia diet
Stomachs of Oreochromis mossambicus (n = 7) and Pelmatolapia mariae (n = 32) caught in the Bruce and Leafgold weirs in 2021 were examined. Most tilapia stomachs contained food items, with only five out of 39 stomachs found to be empty (Fig. 2), suggesting that tilapia feed regularly in this system. There was no significant difference between the stomach contents of O. mossambicus and P. mariae (Type II PERMANOVA of stomach contents composition: df = 1, F = 2.76, p = 0.092). The stomach contents analysis clearly identified plant material (including macrophytes, filamentous algae, and epiphyton) as the primary prey item for these tilapia species. Macrophytes and filamentous algae were the dominant plant types present in tilapia stomachs, with most stomachs containing a mix of filamentous algae and macrophytes. The ratio of filamentous algae to macrophytes varied between stomachs.
Plant material was found in all stomachs that were not empty, sediment/detritus (decomposed organic matter) was found in most stomachs, and other small food items were found in some stomachs (Fig. 3a). However, plant material made up a much greater proportion of the contents of each stomach than sediment/detritus and other food items (Fig. 3b). Sediment and detritus can accumulate in weirs as water flow is slowed and inflowing particles become trapped. It is therefore possible that sediment and detritus may have also been incidentally ingested by tilapia when consuming plants. While several other prey items, such as atyid shrimp and fish eggs, were found in some stomachs (Fig. 3a), these items were all small (most were less than 0.5 mm, with the largest items (atyid shrimp and fish eggs) less than 2 mm in size). It is therefore possible that these items were also incidentally ingested when consuming plants (Fig. 3b).
The percentage of stomachs (excluding empty stomachs) containing each prey item (a), and the percentage of the total contents taken up by each prey item, averaged across stomachs that included the same item (b). Detritus refers to decomposed organic matter. Error bars represent standard deviation from the mean
Nitrogen stable isotope values suggest a low trophic level diet for O. mossambicus and Nematalosa erebi within the weirs, with P. mariae δ15N values intermediate between N. erebi and the omnivores Amniataba percoides and Leiopotherapon unicolor (Fig. 4). The higher δ15N values of P. mariae than O. mossambicus may indicate more frequent feeding on prey other than plants by P. mariae, such as fish eggs or atyid shrimp, which were found in some of the stomachs of both tilapia species (Fig. 3). In Leafgold Weir, P. mariae had low δ13C values similar to that of O. mossambicus and N. erebi, whereas δ13C values of L. unicolor and Oxyeleotris lineolatus were consistently higher, and A. percoides had intermediate δ13C values (Fig. 4). Tilapia had similar δ34S values to the omnivore and invertivore/piscivore species in the weirs, while N. erebi had lower mean δ34S values and a larger range of δ34S values, suggesting that δ34S sources for N. erebi may include some sediment derived sulfur, while the other species may have derived more δ34S from sulfate in the water column (Croisetiere et al. 2009).
Carbon and nitrogen stable isotopes of fish from (a) Bruce Weir and (b) Leafgold Weir, and carbon and sulfur stable isotopes of fish from (c) Bruce Weir and (d) Leafgold Weir. Circles represent fish classified as herbivores/detritivores, squares represent fish classified as omnivores and triangles represent fish classified as invertivore/piscivores. Trophic guild classifications taken from Sternberg and Kennard (2014). O. mossambicus and P. mariae are the introduced tilapia species present in the Bruce and Leafgold weirs. Error bars are standard deviation from the mean
Niche sizes of native species varied among trophic guilds; the invertivore/piscivore (O. lineolatus) had the smallest niche size in both weirs (A. percoides niche was smallest, but only calculated for Bruce Weir), and the niche size of the herbivore/detritivore (N. erebi) was large in both weirs (Table 2). The niche size of the omnivore (L. unicolor) varied between the two weirs. Within the food webs of each weir, the niche sizes of the tilapia species were intermediate between those of O. lineolatus and N. erebi (Table 2).
Tilapia caught in the Bruce and Leafgold weir pools appeared to fit within the existing food webs of the common native fish species, shown by isotope values of tilapia falling within the range of the native species sampled. Within the community sampled, there was some overlap with native species (Table 3). The two tilapia species (considered to be primarily herbivorous) had minimal niche overlap with the native herbivore/detritivore N. erebi in Bruce Weir (Table 3; Fig. 4). However, there was considerable overlap of P. mariae and N. erebi in Leafgold Weir, with a 77% chance of P. mariae feeding within the niche of N. erebi (Table 3). Niche overlap of P. mariae in Bruce Weir was mostly with L. unicolor, with a 66% chance of P. mariae feeding within the niche of L. unicolor. In Bruce Weir, the Mozambique tilapia O. mossambicus overlapped more with P. mariae than any of the native species sampled, indicating that P. mariae may be a greater competitive threat to native omnivores and invertivores than O. mossambicus.
Native fish food webs
The structure of common native fish food webs (N. erebi, A. percoides and L. unicolor, and Oxyeleotris sp (including Oxyeleotris lineolatus or Oxyeleotris selheimi)) appeared to be influenced by macrohabitat type, suggesting that these fish utilize available resources (PERMANOVA: Species df = 3, F = 14.7, p = 0.001; Macrohabitat type df = 2, F = 0.14, p = 0.989; Species x Macrohabitat type df = 6, F = 3.45, p = 0.001). Food web position of each species relative to the other species remained the same across macrohabitat types, however there were significant differences in food web position among the macrohabitat types for N. erebi and Oxyeleotris sp. and smaller, non-significant differences for A. percoides and L. unicolor, indicating that some species may have larger diet shifts in response to food availability than others (Table 4; Fig. 5). Additionally, higher δ15N values of the omnivores A. percoides and L. unicolor than the invertivore/piscivore Oxyeleotris sp suggests that A. percoides and L. unicolor are mostly secondary consumers in this system.
Despite considerable variation within and among sites and unbalanced sample sizes, which was expected when sampling species with wide distributions across habitat types, rescaling to correct for background isotope values revealed that food webs in river channel sites may be more constrained (species have more similar diets) than weir or floodplain sites across the three isotopes. Complementing the PERMANOVA analysis, the δ13C versus δ15N and δ13C versus δ34S biplots of rescaled isotope values show the differences between the river channel and the floodplain or weir pool food web structure (Fig. 5). Species means of weir pool food webs were more similar to that of floodplain sites than to that of natural river channel sites, suggesting that changing river channel environments from lotic to more lentic by installing weirs also changes the food web structure through altering food availability to fishes. Given the similarity of weir pool and floodplain food webs, it is not possible to ascertain whether tilapia presence has shifted the diet of these common native species without sampling weir pools in the system that do not contain tilapia.
Food web structure across habitat types. Points represent species means of rescaled stable isotope values of individual fish, averaged across each habitat type. Tilapia stable isotope values were not included in this analysis. Error bars are species x habitat type standard errors of rescaled isotope values
Habitat similarity risk assessment
Tilapia were found in sites with high macrophyte coverage, including a tributary creek and two weir pools. Within the weir sites, tilapia showed clear habitat preferences to deeper areas (0.5–2 m). Both weir pools had emergent macrophytes from the Hymenachne genus along the edges of the riverbank and around in-channel islands, with beds of submerged macrophytes in some areas of the channel. In Bruce Weir, O. mossambicus were mostly caught along the edges of the Hymenachne, while P. mariae were mostly caught in submerged macrophyte beds. In Leafgold Weir, these habitat preferences also occurred, and an additional school of smaller P. mariae were caught along Hymenachne in a backwater area close to the weir.
Characteristics of the aquatic environment varied across the Mitchell catchment and with macrohabitat type (Fig. 6). Although the weirs are located along the river channel in the upper Walsh sub-catchment, the PCA showed that the environment in the weir pools was more similar to off-channel habitats such as floodplain creeks and wetlands than unmodified river channel habitat. The weir pools, floodplain creeks, and wetlands contained more macrophytes and were typically deeper and muddier (more silt/clay in substrate) than unmodified river channel habitats. River channels often had more rocky or sandy substrate and steeper riverbanks. Most river channel sites had at least one bank with overhanging vegetation.
A SIMPER analysis between the habitat of individual sites and grouped weir sites showed that of the sites that do not currently have tilapia present, some sites were more likely to provide favourable tilapia habitat than others (Fig. 7). Off-channel wetlands and creeks, as well as some headwater sites, had the most similar aquatic environment to weir pool sites of all the sites in this study, indicating that these sites are likely to be most at risk of invasion as they provide habitat that can facilitate the successful establishment of tilapia.
Map of Mitchell River catchment sites where habitat assessments were performed. Symbol shape depicts macrohabitat type and colour scale shows average squared distances (environmental dissimilarity) from weir habitats from SIMPER analysis. The distances given in the 0–40 scale are equal to the square of the number of standard deviations of each site away from weir habitat sites. Sites with lower values have habitat characteristics that are more similar to weir habitats than sites with higher values
Discussion
This study identified key elements of tilapia diet-ecology that are likely to contribute to their invasion success in a river into which they have only recently invaded. Firstly, they fed mostly on plant material, including macrophytes and filamentous algae, an abundant basal food web source in parts of the Mitchell River catchment. Tilapia fitted into the existing native fish food web, occupying the niche between the common native herbivore/detritivore and omnivore species, with the probability of niche overlap with these species varying between the two weirs. Secondly, the habitat assessment showed that while the weir pools inhabited by tilapia were located along river channels, the habitat in the weirs was different from river channels and more similar to that of off-channel habitats, such as wetlands. Assuming that the presence of both tilapia species in weir pools indicates these are suitable habitat for their establishment, this finding suggests that other in-channel impoundments and off-channel habitats are likely to provide ideal tilapia habitat with abundant food to fuel growth and reproduction. This has implications for the environmental impacts of potential new dams in the Mitchell catchment, which may contribute to the establishment and spread of invasive tilapia as well as posing threats to the movement of fish assemblages throughout the catchment (O’Mara et al. 2021). Additionally, stable isotopes revealed insights into how common native fish species in this system respond to the availability of food resources across macrohabitat types. Given that increased tilapia biomass has the potential to change both habitat quality and food availability, understanding the diet-habitat ecology of both the invasive and native species is important for predicting and understanding responses of native fish as the tilapia invasion progresses.
Tilapia are known to be highly herbivorous, with grinding teeth and long digestive tracts that aid in the breakdown of macrophytes and other plant material (Bradford et al. 2011). Stomachs of P. mariae and O. mossambicus collected in this study contained mostly macrophytes, filamentous algae, and detritus/sediment. Similar stomach contents proportions were found in the stomachs of P. mariae collected from another northern Australian catchment, the Mulgrave River (Rayner et al. 2009), suggesting that macrophytes and filamentous algae provide the required nutrition for tilapia to thrive in the tropical rivers of northern Australia. Nitrogen stable isotopes of tilapia and common native fish showed that tilapia in the Mitchell catchment fed at low trophic levels, with O. mossambicus lower than P. mariae. Fish feeding at lower trophic levels are thought to have higher invasion success because there are more resources available at the bottom of the food web (Gido and Franssen 2007).
Stable isotopes also showed that P. mariae may adjust its trophic position in response to food availability. Omnivory is widespread in northern Australian freshwater fish food webs and P. mariae may be a competitive threat to omnivorous species for obtaining higher quality food resources (Douglas et al. 2005; Pusey et al. 2010). There are few solely herbivorous native species present in the Mitchell catchment, and direct competition of these species with tilapia is therefore less likely to be a problem. Instead, it is possible that by feeding at the base of the food web, tilapia will monopolize and retain sufficient basal food resources to alter energy availability to other fishes, even if not directly competing with them for food. This has been identified as one of the main mechanisms by which invasive common carp, which also monopolize and retain basal food resources, limit native fish biomass in Australian rivers where they have established (Marshall et al. 2019). Mitchell River fish have been shown to have a high dependency on algal feeding macroinvertebrates for their nutrition (O’Mara et al. 2022), so by feeding on algae, tilapia may limit this food source for grazing invertebrates and subsequently limit food quantity and quality for native fish assemblages.
The trophic position of O. mossambicus in the weirs was close (with some overlap) to that of N. erebi, and P. mariae was likely to feed within the niche of N. erebi in Leafgold Weir. Nematalosa erebi is a widespread and abundant herbivore/detritivore in the Mitchell River catchment, and niche overlap of N. erebi with invasive fish species has been observed in other Australian freshwater environments (Kelleway et al. 2010), though it is unclear whether competition occurs between N. erebi and invasive species when food resources are limited. The relationships between food availability and feeding behaviour of both native and invasive fish species may contribute to whether competition occurs (Sampson et al. 2009; Juncos et al. 2015). Fine scale resource partitioning within a trophic level can occur when species with overlapping niches are present, facilitating the co-existence of invasive and native species (Pen et al. 1993; Zengeya et al. 2011; Juncos et al. 2015).
Rescaling of stable isotope values to correct for differences in baseline conditions showed that at current tilapia densities in the Mitchell catchment, common native fish species do not appear to significantly adjust their trophic position in response to tilapia presence. Similarity of weir pool and floodplain food webs indicated that habitat type was likely to be a greater driver of food web structure than tilapia presence. However, tilapia densities were low compared to other rivers, and further research is needed to determine whether impacts occur when tilapia densities are higher, as observed by Shuai et al. (2019). Additionally, the short time frame since detection at these sites may mean that ecological impacts are still developing.
Native fish food webs appeared to be more constrained at river channel sites compared to floodplain and weir pool sites, showing that native fish converge on the lower diversity of available food in river channels (Pusey et al. 2010). The larger separation between species in food webs in floodplain and weir pool habitats than in river channels is likely to result from a greater diversity of available food in the more lentic environments. Habitat heterogeneity and diversity of aquatic macrophytes may influence the diversity of periphyton species present at a location (Algarte et al. 2017). Additionally, periphyton and phytoplankton respond differently to flow, and seasonal flood pulses restrict the diversity of macrophytes in river channels to flow tolerant species; therefore, different basal food sources can be expected among habitats of varying flow (Munn et al. 2002; Bunn et al. 2006; Mihaljević et al. 2013). Macrophytes house high densities of macroinvertebrates and epiphytic algal species, and macrophyte rich wetlands are the main source of primary production in the Mitchell catchment (Thomaz et al. 2008; Molinari et al. 2021). The success of tilapia invasion at various locations throughout the catchment is likely to be influenced by the availability of basal food sources, and the number of herbivorous, omnivorous and piscivorous species present, which can change with connectivity (Baiser et al. 2010). A reduction in macrophyte coverage via consumption by tilapia could impact the productivity of the system and may impact omnivorous fish via a shift in the diet of omnivorous fish from a mix of plant and animal sources to a primarily animal diet and increased competition with P. mariae (David et al. 2017). Macrophyte reduction could also impact native fish through loss of egg laying substrate and refuge for juvenile native fish to hide from predators (Enge et al. 2013).
Each of the common native fish species collected in this study appeared to respond to food availability across habitat types whilst maintaining their position within the food web with respect to the other species, suggesting that these species coexist in organized niches. Fish may be able to adjust their feeding within the range of their niche when an invasive species is present (David et al. 2017), however species with small ranges of resource use may be less adaptable (Juncos et al. 2015). Impacts of invasive fish on native fish communities are likely to be lower when native fish biodiversity is high, with greater impacts observed when human interference in regards to flow, habitat quality, and water quality is prevalent (Marks et al. 2010; Kim et al. 2021). Habitat and water quality related impacts on food webs can also be caused by invasive species (Jackson et al. 2012). Gu et al. (2019) found that poor fishery management, water quality degradation and biodiversity loss facilitated the tilapia invasion in Guangdong Province, China. They also observed a reduction in Nile tilapia biomass when native fish biodiversity was high (Gu et al. 2014). Therefore, management of invasive species to minimize impacts should address environmental issues in addition to understanding the trophic and ecological interactions of invasive species with native fish communities.
Managing emerging invasions of species like tilapia and the risks to native biodiversity that they entail is very challenging. However, our findings provide a knowledge base to guide management of such an invasion in a relatively unmodified catchment. We have identified the types of habitats (particularly wetlands on the floodplain) beyond their current range that are more likely to be suitable for these tilapia species to flourish, based on their habitat and dietary preferences. There are many parts of the catchment that appear to be less suitable for these tilapia species, primarily the riverine habitats between the study area and the floodplain wetlands downstream, as they tend to have rockier substrates with relatively low densities of aquatic macrophytes. The development of dams and weirs throughout the catchment that create standing water that favours aquatic macrophyte growth is likely to result in additional habitats that favour tilapia and may facilitate, rather than hinder, their further spread. Integrating these findings into regional planning decisions may help natural resource managers reduce the speed and severity of the invasion and contribute to protecting native biodiversity.
Data availability
The datasets generated during and/or analysed during the current study are available from IsoBank.
References
Algarte VM, Siqueira T, Landeiro VL et al (2017) Main predictors of periphyton species richness depend on adherence strategy and cell size. PLoS One 12:e0181720
Arthington AH, Blühdorn DR (1994) Distribution, genetics, ecology and status of the introduced cichlid, Oreochromis mossambicus, in Australia. Int Ver für Theor Und Angew Limnol Mitt 24:53–62
Attayde JL, Brasil J, Menescal RA (2011) Impacts of introducing Nile tilapia on the fisheries of a tropical reservoir in North-eastern Brazil. Fish Manag Ecol 18:437–443
Baiser B, Russell GJ, Lockwood JL (2010) Connectance determines invasion success via trophic interactions in model food webs. Oikos 119:1970–1976
Batlle-Aguilar J, Harrington GA, Leblanc M et al (2014) Chemistry of groundwater discharge inferred from longitudinal river sampling. Water Resour Res 50:1550–1568
Bergstrom MA, Mensinger AF (2009) Interspecific resource competition between the invasive round goby and three native species: logperch, slimy sculpin, and spoonhead sculpin. Trans Am Fish Soc 138:1009–1017
Bradford M, Kroon FJ, Russell DJ (2011) The biology and management of Tilapia mariae (Pisces: Cichlidae) as a native and invasive species: a review. Mar Freshw Res 62:902–917
Bruno JF, Fridley JD, Bromberg KD, Bertness MD (2005) Insights into biotic interactions from studies of species invasions. Species Invasions Insights into Ecol Evol Biogeogr, 13–40
Buchheister A, Latour RJ (2010) Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Can J Fish Aquat Sci 67:445–461
Bunn SE, Thoms MC, Hamilton SK, Capon SJ (2006) Flow variability in dryland rivers: boom, bust and the bits in between. River Res Appl 22:179–186
Busst G, Britton JR (2018) Tissue-specific turnover rates of the nitrogen stable isotope as functions of time and growth in a cyprinid fish. Hydrobiologia 805:49–60
Canonico GC, Arthington A, McCrary JK, Thieme ML (2005) The effects of introduced tilapias on native biodiversity. Aquat Conserv Mar Freshw Ecosyst 15:463–483
Clarke KR, Gorley RN (2006) PRIMER-e, Plymouth
Córdova-Tapia F, Contreras M, Zambrano L (2015) Trophic niche overlap between native and non-native fishes. Hydrobiologia 746:291–301
Croisetiere L, Hare L, Tessier A, Cabana G (2009) Sulphur stable isotopes can distinguish trophic dependence on sediments and plankton in boreal lakes. Freshw Biol 54:1006–1015
DAF (2011) Stop the spread. Tilapia training package. Module 2: overview and history of pest fish management. Department of agriculture and fisheries
David P, Thebault E, Anneville O et al (2017) Impacts of invasive species on food webs: a review of empirical data. Adv Ecol Res 56:1–60
Davis MA (2003) Biotic globalization: Does competition from introduced species threaten biodiversity? Bioscience 53:481–489
de Oliveira CG, López-Olmeda JF, Costa LS et al (2022) Gastrointestinal emptying and daily patterns of activity of proteinolytic enzymes in Nile tilapia (Oreochromis niloticus). Aquaculture 546:737338
Deines AM, Wittmann ME, Deines JM, Lodge DM (2016) Tradeoffs among ecosystem services associated with global tilapia introductions. Rev Fish Sci Aquac 24:178–191
Douglas MM, Bunn SE, Davies PM (2005) River and wetland food webs in Australia’s wet-dry tropics: general principles and implications for management. Mar Freshw Res 56:329–342
Doupé RG, Knott MJ, Schaffer J et al (2010) Experimental herbivory of native Australian macrophytes by the introduced Mozambique tilapia Oreochromis mossambicus. Austral Ecol 35:24–30
Enge S, Nylund GM, Pavia H (2013) Native generalist herbivores promote invasion of a chemically defended seaweed via refuge-mediated apparent competition. Ecol Lett 16:487–492
ESRI (2011) ArcGIS desktop: release 10. Environmental Systems Research Institute, Redlands
Fry B, Davis J (2015) Rescaling stable isotope data for standardized evaluations of food webs and species niches. Mar Ecol Prog Ser 528:7–17. https://doi.org/10.3354/meps11293
Gido KB, Franssen NR (2007) Invasion of stream fishes into low trophic positions. Ecol Freshw Fish 16:457–464
Greiner R, Gregg D (2008) Tilapia in North Queensland waterways: risks and potential economic impacts. River Consulting, Townsville
Groß J, Fry B, Burford MA, Bengtson Nash S (2021) Assessing the effects of lipid extraction and lipid correction on stable isotope values (δ13C and δ15N) of blubber and skin from southern hemisphere humpback whales. Rapid Commun Mass Spectrom 35:e9140
Gu DE, Luo D, Xu M et al (2014) Species diversity defends against the invasion of Nile tilapia (Oreochromis niloticus). Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2014018
Gu DE, Yu FD, Yang YX et al (2019) Tilapia fisheries in Guangdong Province, China: socio-economic benefits, and threats on native ecosystems and economics. Fish Manag Ecol 26:97–107. https://doi.org/10.1111/fme.12330
Idowu F, Olusegun E, Oghenebrorhie MT, Tunde I (2019) Diet composition and length-weight relationship of Tilapia mariae in Lower River Ogun, Akomoje water reservoir, Nigeria. Egypt J Aquat Biol Fish 23:43–51
Jackson MC, Donohue I, Jackson AL et al (2012) Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS One 7:e31757
Jackson S, Finn M, Scheepers K (2014) The use of replacement cost method to assess and manage the impacts of water resource development on Australian indigenous customary economies. J Environ Manag 135:100–109
Jardine TD, Hunt RJ, Pusey BJ, Bunn SE (2011) A non-lethal sampling method for stable carbon and nitrogen isotope studies of tropical fishes. Mar Freshw Res 62:83–90
Jardine TD, Pusey BJ, Hamilton SK et al (2012) Fish mediate high food web connectivity in the lower reaches of a tropical floodplain river. Oecologia 168:829–838. https://doi.org/10.1007/s00442-011-2148-0
Jardine TD, Rayner TS, Pettit NE et al (2017) Body size drives allochthony in food webs of tropical rivers. Oecologia 183:505–517
Juncos R, Milano D, Macchi PJ, Vigliano PH (2015) Niche segregation facilitates coexistence between native and introduced fishes in a deep Patagonian lake. Hydrobiologia 747:53–67
Kelleway J, Mazumder D, Wilson GG et al (2010) Trophic structure of benthic resources and consumers varies across a regulated floodplain wetland. Mar Freshw Res 61:430–440
Kim JY, Atique U, An K-G (2021) Relative abundance and invasion dynamics of alien fish species linked to chemical conditions, ecosystem health, native fish assemblage, and stream order. Water 13:158
Long KE, Schneider L, Connor SE et al (2021) Human impacts and anthropocene environmental change at Lake Kutubu, a Ramsar wetland in Papua New Guinea. Proc Natl Acad Sci 118:e2022216118
Lysy M, Stasko AD, Swanson HK, Lysy MM (2021) Package ‘nicheROVER’
Marks JC, Haden GA, O’Neill M, Pace C (2010) Effects of flow restoration and exotic species removal on recovery of native fish: lessons from a dam decommissioning. Restor Ecol 18:934–943
Marshall JC, Blessing JJ, Clifford SE et al (2019) Ecological impacts of invasive carp in Australian dryland rivers. Aquat Conserv Mar Freshw Ecosyst 29:1870–1889
Mihaljević M, Žuna Pfeiffer T, Stević F, Špoljarić D (2013) Dynamics of phytoplankton and periphytic algae in a danubian floodplain lake: a comparative study under altered hydrological conditions. Fresenius Environ Bull 22:2516–2523
Molinari B, Stewart-Koster B, Malthus TJ, Bunn SE (2021) Assessing spatial variation in algal productivity in a tropical river floodplain using satellite remote sensing. Remote Sens 13:1710
Munn MD, Black RW, Gruber SJ (2002) Response of benthic algae to environmental gradients in an agriculturally dominated landscape. J N Am Benthol Soc 21:221–237
O’Mara K, Venarsky M, Stewart-Koster B et al (2021) Connectivity of fish communities in a tropical floodplain river system and predicted impacts of potential new dams. Sci Total Environ 788:147785
O’Mara K, Venarsky M, Stewart-Koster B et al (2022) Trophic transfer of lipids and fatty acids across habitats in tropical river food webs. Freshw Biol 67:893–911
Pacioglu O, Zubrod JP, Schulz R et al (2019) Two is better than one: combining gut content and stable isotope analyses to infer trophic interactions between native and invasive species. Hydrobiologia 839:25–35
Pen LJ, Potter IC, Calver MC (1993) Comparisons of the food niches of three native and two introduced fish species in an Australian river. Environ Biol Fishes 36:167–182
Petheram C, Watson I, Bruce C, Chilcott C (eds) (2018) Water resource assessment for the Mitchell catchment. A report to the Australian government from the CSIRO Northern Australia Water Resource Assessment, part of the National Water Infrastructure Development Fund: Water Resource Assessments. CSIRO, Australia
Pusey BJ, Arthington AH, Stewart-Koster B et al (2010) Widespread omnivory and low temporal and spatial variation in the diet of fishes in a hydrologically variable northern Australian river. J Fish Biol 77:731–753
R Core Team (2021) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. https://www.R-project.org/
Rayner TS, Pusey BJ, Pearson RG (2009) Spatio-temporal dynamics of fish feeding in the lower Mulgrave River, north-eastern Queensland: the influence of seasonal flooding, instream productivity and invertebrate abundance. Mar Freshw Res 60:97–111
Reardon EE, Chapman LJ (2008) Reproductive seasonality in a swamp-locked African cichlid. Ecol Freshw Fish 17:20–29
RStudio Team, RStudio (2020) RStudio: integrated development for R. PBC, Boston, MA URL. http://www.rstudio.com/
Russell DJ, Thuesen PA, Small FE (2012a) Tilapia in Australia-development of management strategies for the control and eradication of feral tilapia populations in Australia
Russell DJ, Thuesen PA, Thomson FE (2012b) Reproductive strategies of two invasive tilapia species Oreochromis mossambicus and Tilapia mariae in northern Australia. J Fish Biol 80:2176–2197
Rustomji P, Shellberg J, Brooks A et al (2010) A catchment sediment and nutrient budget for the Mitchell River, Queensland. Tropical Rivers and Coastal Knowledge (TRACK)
Sampson SJ, Chick JH, Pegg MA (2009) Diet overlap among two Asian carp and three native fishes in backwater lakes on the Illinois and Mississippi rivers. Biol Invasions 11:483–496
Semyalo R, Rohrlack T, Kayiira D et al (2011) On the diet of Nile tilapia in two eutrophic tropical lakes containing toxin producing cyanobacteria. Limnologica 41:30–36
Shuai FM, Li XH, Liu QF et al (2019) Nile tilapia (Oreochromis niloticus) invasions disrupt the functional patterns of fish community in a large subtropical river in China. Fish Manag Ecol 26:578–589. https://doi.org/10.1111/fme.12368
Skelton PH (2001) A complete guide to the freshwater fishes of southern Africa. Struik Publishers, Cape Town
Sternberg D, Kennard MJ (2014) Phylogenetic effects on functional traits and life history strategies of Australian freshwater fish. Ecography (Cop) 37:54–64
Swanson HK, Lysy M, Power M et al (2015) A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 96:318–324
Thomaz SM, Dibble ED, Evangelista LR et al (2008) Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshw Biol 53:358–367
Uscanga A, Moyano FJ, Alvarez CA (2010) Assessment of enzymatic efficiency on protein digestion in the tilapia Oreochromis niloticus. Fish Physiol Biochem 36:1079–1085
Vander Zanden MJ, Clayton MK, Moody EK et al (2015) Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS One 10:e0116182
Venarsky MP, Stewart-Koster B, Hunt RJ et al (2020) Spatial and temporal variation of fish community biomass and energy flow throughout a tropical river network. Freshw Biol 65:1782–1792
Wang Y, Tan W, Li B et al (2021) Habitat alteration facilitates the dominance of invasive species through disrupting niche partitioning in floodplain wetlands. Divers Distrib 27:1861–1871
Zengeya TA, Booth AJ, Bastos ADS, Chimimba CT (2011) Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environ Biol Fishes 92:479–489
Acknowledgements
This study was funded by the Australian Government Department of Agriculture, Water and the Environment (Grant # 4-D2WBD9L) and the National Environment Science Programme, Northern Australia Environmental Resources Hub. We acknowledge the traditional owners of the country from which the samples were collected in the Mitchell and River catchment. We acknowledge and thank the Mitchell River Traditional Custodian Advisory Group for their contribution of local knowledge and engagement with the project. Colton Perna, Cameron Schulz, Peter Negus, Glenn McGregor, Kate Hodges, Doug Harding, and John Bowlen assisted with data collection in 2017 and 2018. Terry Vallance from Tropical River Consulting, Chantal Saint Ange, and Bianca Molinari assisted with field sampling during 2020 and 2021 field trips. Vanessa Fry and Rad Bak (Stable Isotope Laboratory, Griffith University) conducted the stable isotope analysis. General Fisheries Permit (198642) and animal ethics approval (ENV/06/21/AEC, Griffith University) were used in this study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by the Australian Government Department of Agriculture, Water and the Environment (Grant # 4-D2WBD9L) and the National Environment Science Programme, Northern Australia Environmental Resources Hub.
Author information
Authors and Affiliations
Contributions
MV and JM conceived and designed the study and BS-K and KO collected the data. BS-K and KO conceptualized data analysis. KO conducted the analysis and is the primary author of the manuscript. All authors contributed to draft manuscripts and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Mara, K., Venarsky, M., Marshall, J. et al. Diet-habitat ecology of invasive tilapia and native fish in a tropical river catchment following a tilapia invasion. Biol Invasions 26, 489–504 (2024). https://doi.org/10.1007/s10530-023-03185-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03185-2