Abstract
Invasive species can trigger profound effects on recipient ecosystems, namely through the food web. Despite being recognized as one of the worst invasive species, little is known about the feeding ecology of the Mozambique tilapia Oreochromis mossambicus. To understand how this invasive species might impact food webs, we applied metabarcoding to analyze its diet’s composition in two African mangroves, in the Obô Natural Park in the oceanic island of São Tomé. Given the particular importance of mangroves as fish nurseries, we specifically aimed to determine if this invader might predate on other fish species. However, we found that tilapia were mostly phytoplanktivorous and indication on predation of other fish was very limited. Instead, due to their local high densities, tilapia may impact basal trophic levels and nutrient availability with the potential to cascade through the food web by means of bottom-up disruption. In addition, we recorded important changes in the taxonomic composition of the diet, linked to locations and life stages, suggesting that its opportunistic feeding associated with its aggressive territorial behavior may result in resource competition with native species with which it has overlapping dietary niches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Mozambique tilapia, Oreochromis mossambicus (Peters, 1852) is a cichlid fish native to Eastern Africa, being among the worst and most widely distributed invasive fish species and occurring in at least 92 countries beyond its native range (Froese and Pauly 2022). Its introduction was mostly driven by high demand for aquaculture and biological control, which combined with its life-history traits has allowed successful establishment in many new areas (Canonico et al. 2005). Early maturation, highly successful parental care, wide tolerance to environmental conditions (e.g., high salinity variations), and opportunistic feeding behavior enable its invasion of highly variable environments, such as estuaries (Félix et al. 2017). Although the Mozambique tilapia (herein tilapia) pose serious threats to native aquatic communities worldwide through predation competition for food, habitat and spawning sites (Russell et al. 2012), little is known about the impacts exerted by its omnivore-detritivore feeding behavior. A primary concern pertains to the consequences of tilapia introductions through trophic interactions, given the mounting empirical evidence on the detrimental effects of invasive fish species on food webs (Wainright et al 2021). Thus, it is critical to understand the feeding ecology of the tilapia in its invasion range, as this may affect the food web and native assemblages.
The presence of tilapia has been recently described for two mangroves in the São Tomé Obô Natural Park (Félix et al. 2017). Despite the well-established importance of mangrove forests as the source of countless ecosystem services (Primavera et al. 2019), and the general recognition of the negative impacts of invasive species, little attention has been paid towards this problem on type of ecosystems. Furthermore, the few studies published regarding invasions in mangroves mostly focused on plant species (Chen and Ma 2015). Moreover, only 3% of all publications on aquatic biological invasions between 1972 and 2012 were derived from African case-studies (Thomsen et al. 2014). This study aims to evaluate the diet of the invasive Mozambique tilapia in two mangroves in São Tomé Island. Specifically, we aim to (1) determine its diet and (2) examine intraspecific diet variations related to habitat and life stages. To this aim, we applied DNA metabarcoding using two genetic markers. Metabarcoding (i.e. the simultaneous identification of many taxa within one sample through next-generation sequencing of a DNA barcode) is regarded as a powerful, reliable, high-resolution method that allows for broad taxonomic coverage being particularly useful when dealing with microscopic prey items (de Sousa et al. 2019). The application of this tool will thus enable us to circumvent the difficulties of studying an omnivore-detritivore fish and infer about its putative impact on native biodiversity.
Materials and methods
Field collection
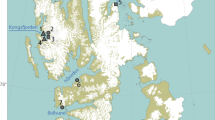
In 2020, we collected 50 tilapia specimens in the mangroves of Malanza (0.046111 N, 6.53 E) and Praia das Conchas (0.405833 N, 6.622778 E) of São Tomé Island (Democratic Republic of São Tomé and Principe, Gulf of Guinea, Central Africa). Both mangroves are characterized in Félix et al. (2017). Tilapia were captured using cast nets (mesh size 30 mm) and anesthetized using clove oil. All specimens were measured for body mass (± 1 g) and total length (± 1 mm), and then separated into adults or juveniles based on total length (threshold size: 144 mm; Froese and Pauly 2022). The digestive system of all specimens was extracted through dissection and preserved in 96% alcohol until further processing.
Laboratory processing
DNA extractions of gut content samples were performed using the E.Z.N.A. Tissue DNA Kit (Omega Bio-Tek, Norcross, GA, USA), following manufacturer instructions. Prior to DNA isolation, stomach contents were homogenized with a tissue lyser (TissueLyser LT, Qiagen, Venlo, Netherlands). PCR amplifications of two barcodes were prepared in separate PCR reactions. We targeted the 313 bp mitochondrial cytochrome c oxidase subunit I (COI) region with the primer pair of NexF_m1COIintF and NexR_jgHCO2198 (Leray et al. 2013) due to their suitability across a wide range of metazoan species. To identify other eukaryotic prey items, we used the 528F/706R primer set which amplifies a 350 bp region of the 18S ribosomal RNA gene (Ho et al. 2017). Illumina library preparation and sequencing were performed at CIBIO-InBIO's Centre for Molecular Analysis (for details see Appendix 1 in ESM).
Bioinformatic analysis
The sequencing data from each barcode was processed separately, and it involved (1) trimming adapter and primer regions and exclude short sequences (< 80 bp) using cutadapt (Martin 2011); (2) merging pair-ended reads and defining Amplicon Sequence Variants (ASVs), using dada2 (Callahan et al. 2016); (3) performing blastn runs (Camacho et al. 2009) against the nucleotide NCBI GenBank database (https://www.ncbi.nlm.nih.gov), as well as the SILVA (https://www.arb-silva.de/) and the BOLD (https://boldsystems.org/) databases for the 18S and COI, respectively. A detailed description of taxonomy assignment and exclusion criteria to account for sequencing artifacts, secondary predation and contamination (including from the host’s DNA) can be found in Appendix 1 in ESM.
Diet analysis
A total of 40 samples passed the quality control steps described above (37 and 35 samples for 18S and COI barcodes, respectively; Table S1). We decided to retain samples regardless of whether they yielded results for both markers or not, since we cannot determine whether the lack of amplification of a particular marker resulted from a protocol failure or from the absence of the target prey item (e.g. animals for COI). We merged the two datasets by collapsing the taxonomic assignment of ASVs down to the lowest identifiable common taxonomic level (da Silva et al. 2019). The combined read count data set from 40 individuals (26 adults and 14 juveniles) was transformed into binary occurrence data, from which the weighted Percentage Of Occurrence (wPOO) was calculated (Deagle et al. 2019). The wPOO simply scales the weight of an occurrence according to the number of food items in the sample. This is a way to account for the relative abundance of each prey per individual without calculating it directly, given the non-linear relationship between read counts and prey abundance (Deagle et al. 2019). Non-prey taxa such as Fungi, Parasites and Protists were excluded. We arranged the remaining ASVs into 11 broad taxonomic categories, based on their share of the whole diet composition and taxonomic affinities. The category “Other prey” comprised rare taxa (wPOO < 1.6%). We calculated the average wPOO of each food category and compared them between locations and life stages. To this aim, data were square root transformed to build a Bray–Curtis distance matrix, which served as the input to the non-metric multidimensional scaling (NMDS) with 40% standard ellipses representing the core population diet niche (de Santis and Volta 2021) of tilapia occurring at different sites and different size classes. We conducted Permutational Multivariate Analysis of Variance (PERMANOVA) tests with 999 permutations to examine overall differences considering two factors, site and life stage. Pairwise dissimilarities between groups were assessed using Analysis of Similarity (ANOSIM), following the protocol described by Gkenas et al. (2021). Similarity Percentage (SIMPER) analysis was used to identify which prey contributed the most to the difference between the diets of different groups, following Gkenas et al. (2021). Diet diversity at the population level was determined by the Shannon–Wiener index (H') using 95% bootstrapped confidence intervals (Gkenas et al. 2021). An extended protocol of the statistical treatment of diet composition data can be found in Appendix 1 in ESM. The ANOSIM and SIMPER analyses were carried out in PRIMER (Clarke and Gorley 2006). PERMANOVA was performed using the function adonis2 from the R package Vegan (v2.6.6., Oksanen et al. 2022) and the rest of the analyses were conducted in R (v4.2.2., R Development Core Team 2021).
Results and discussion
Out of the reads of acceptable quality, we were able to assign a taxonomic identification to 99.9% of the reads for 18S and to 94.5% for COI. Analysis of these reads revealed that tilapia’s DNA constituted 22% (between 0 and 79%) for the 18S dataset and 15% (between 0 and 91%) for COI (Table S2 and S3). We identified 102 ASVs assigned to 72 taxonomic groups (17 species, 23 genera, 16 families, and 16 higher-than-family level; Table S4) for 18S and 143 ASVs matched to 33 taxa (8 species, 4 genera, 3 families, and 18 higher-than-family level; Table S5) for COI. Only 56% of the taxa identified using 18S and 36% of the taxa identified using COI were resolved down to genus. By merging the two datasets down to the lowest identifiable common taxonomic level and subsequently removing non-food taxa, we obtained 54 taxa for downstream analyses. The taxonomic resolution ranged from species to phylum, with most taxa resolved down to family level (5 species, 2 genera, 26 families, 1 suborder, 4 orders, 1 subclass, 7 classes, 8 phyla; Table S6). The lower than ideal taxonomic resolution resulted from a lack of a well curated and comprehensive reference database specific to the study sites.
Overall, we found tilapia were mostly planktivorous. Diatoms, including Melosiraceae and Stephanodiscaceae, constituted the major component of the diet both in Malanza (28.3% wPOO) and in Praia das Conchas (33.4% wPOO—Fig. 1). Another major component was green algae (Malanza: 23.9% wPOO; Praia das Conchas: 24.4% wPOO) including taxa in the class Trebouxiophyceae and family Pycnococcaceae. In addition, land plants, such as Fabaceae and Lindsaeineae, were important components in Malanza (13.1% wPOO), and dinoflagellates, such as Prorocentraceae and Dinophyceae, were important in Praia das Conchas (16.6% wPOO—Fig. 1). With respect to dinoflagellates, we identified several taxa known to be harmful (e.g., Blixaea quinquecornis) or potentially toxic (e.g., Prorocentrum sp.), which could be of concern given that tilapia represent a vital source of protein for local human populations. The PERMANOVA identified significant differences in the diet composition between tilapia from different sites (p = 0.004; Table S7) and between life stages (p = 0.035; Table S7). In Malanza, juvenile and adult tilapia preyed mainly on diatoms (27.6%; 29.8% wPOO) and used similar prey but different proportions of green algae (27%; 16.7%; wPOO) and land plants (11.7%; 16.3% wPOO). Some other taxa were detected in smaller proportions, including rotifers (9.1% wPOO) and dinoflagellates (10.4% wPOO) for juvenile and adult tilapia respectively (Fig. 1). In Praia das Conchas, diets of juvenile and adult tilapia were mainly composed of diatoms (36.1%; 30.8% wPOO), followed by green algae (20.9%; 27.8%; wPOO). Smaller proportions of rotifers (13.7% wPOO) and mollusks (8.7% wPOO) were detected in juveniles. Additionally, dinoflagellates were detected in higher (25.6% wPOO) but nematodes (5.1% wPOO) in lower amounts in adults.
There was some variation in use of prey between juvenile and adult tilapia, being most notably in Malanza (Fig. 2). ANOSIM corroborated the existence of a significant but moderate overlap between juveniles and adults in Malanza (R = 0.26, p = 0.008) but no significant differences were found in Praia das Conchas (R = 0.20, p > 0.05). SIMPER analysis indicated that land plants contributed most to the dissimilarity between juveniles and adults, with diatoms and green algae being also important for the differentiation in Malanza. Dinoflagellates had the highest contribution in the comparison between juveniles and adults in Praia das Conchas, with other contributors including rotifers and mollusks (Table 1). Diet breadth was considerable higher for juveniles than adults in Malanza, but patterns remained similar between these groups in Praia das Conchas (Fig. S1). Considering the high density of tilapia reported for these mangroves (Dias 2022), the differences in feeding behavior between life-stage groups in Malanza might be driven by competition avoidance between the juveniles and adults whereas in Praia das Conchas, the lack of habitat heterogeneity does not allow for the diversification of tilapia diet. One of the few studies on the diet of this species in its native range, using volumetric data, found a shift from a carnivorous to a phytoplanktivorous-detritivorous diet as tilapia mature from juveniles to adults (de Moor et al. 1986). We did not observe such a trend, as phytoplankton was the major component in the diet of both juveniles and adults. Fish prey (Anguilliformes) was only detected once, in a juvenile specimen (Table S8), and given the low prevalence, our results do not corroborate previous studies (Canonico et al. 2005) on the possibility of significant predation from tilapia on fish eggs, larvae and juveniles. Nevertheless, this first study examined a limited number of specimens, during a restricted time period, and considering the key role of these mangroves as nurseries to a high number of species, including commercially valuable ones such as Megalops atlanticus (Félix et al. 2017; Dias 2022), it would be advisable to perform additional studies with larger sample sizes before drawing definitive conclusions. Namely, it would be important to explore the effect of seasonality, which influences mangrove connectivity and regulates fish reproduction seasons. Furthermore, the planktivorous diet of tilapia can also affect the growth and survival of larval fish via habitat changes, competition for shared prey, and resource depletion. The presence of invasive planktivores can dramatically reduce the growth rate and delay ontogenetic habitat shifts during the early life stage of native fish (Fletcher et al. 2019). Given the high tilapia densities documented for these areas (Dias 2022), it is expected that its proliferation might significantly impacts the availability of its food source, thus careful attention should be placed into monitoring these populations. Moreover, the competition for food resources associated with the aggressive territorial behavior of this species puts them in a favorable feeding dominance. Gkenas et al. (2022) observed that a non-native cichlid was an extremely effective competitor in face of limited resources against two different species. Finally, since the methodology we employed does not allow differentiating prey from host’s DNA, we did not assess cannibalism, which is documented for this species (de Moor et al. 1986).
Non-Metric Multidimensional Scaling (NMDS) ordination for the diet composition of Oreochromis mossambicus (n = 40) in the mangroves of Malanza (9 adult and 21 juvenile specimens) and Praia das Conchas (5 adult and 5 juvenile specimens), in São Tomé, displaying dietary niches as 40% standard ellipse area per size class, based on Bray–Curtis dissimilarity index for weighted percentage of occurrence (wPOO) data obtained from the metabarcoding markers
The current work constitutes the first analysis of the diet of the Mozambique tilapia in particularly important habitats, such as mangroves, representing one of the first attempts to assess the feeding ecology of their invasive populations through the application of an innovative technology. Through the use of DNA metabarcoding we demonstrated that tilapia are mostly planktivorous but can adjust their diet to different environments, which can contribute to their ability to thrive and spread rapidly within an invaded ecosystem. This also calls for further studies to gain a deeper understanding of the impact of tilapia on ecosystems. The conservation of these mangroves ensures the long-term delivery of its ecosystem services, which is of the utmost importance for local human populations. Building further knowledge and disseminating empirical evidence on the impacts of tilapia amongst stakeholders, such as conservation managers, governmental agencies and communities living near impacted ecosystems is vital for the control and prevention of further spread of this invader.
Data availability
Raw sequence data for the samples used in this study are deposited in NCBI’s short read archive (SRA) repository under the Project Number PRJNA924239 (BioSample IDs: SAMN32755195- SAMN32755256).
References
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/NMETH.3869
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinformatics 10:1–9. https://doi.org/10.1186/1471-2105-10-421
Canonico GC, Arthington A, Mccrary JK, Thieme ML (2005) The effects of introduced tilapias on native biodiversity. Aquat Conserv Mar Freshwat Ecosyst 15:463–483. https://doi.org/10.1002/aqc.699
Chen Q, Ma K (2015) Research overview and trend on biological invasion in mangrove forests. Chin J Plant Ecol 39:283–299. https://doi.org/10.17521/cjpe.2015.0028
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
da Silva LP, Mata VA, Lopes PB, Pereira P, Jarman SN, Lopes RJ, Beja P (2019) Advancing the integration of multi-marker metabarcoding data in dietary analysis of trophic generalists. Mol Ecol Resour 19:1420–1432. https://doi.org/10.1111/1755-0998.13060
de Santis V, Volta P (2021) Spoiled for choice during cold season? Habitat use and potential impacts of the invasive Silurus glanis L. in a deep, large, and Oligotrophic Lake (Lake Maggiore, North Italy). Water 13:2549–2567. https://doi.org/10.3390/w13182549
de Moor FC, Wilkinson RC, Herbst HM (1986) Food and feeding habits of Oreochromis mossambicus (Peters) in hypertrophic Hartbeespoort Dam, South Africa. South Afr J Zool 21:170–176. https://doi.org/10.1080/02541858.1986.11447976
de Sousa LL, Silva SM, Xavier R (2019) DNA metabarcoding in diet studies: unveiling ecological aspects in aquatic and terrestrial ecosystems. Environ DNA 1:199–214. https://doi.org/10.1002/edn3.27
Deagle BE, Thomas AC, McInnes JC, Clarke LJ, Vesterinen EJ, Clare EL, Kartzinel TR, Eveson JP (2019) Counting with DNA in metabarcoding studies: How should we convert sequence reads to dietary data? Mol Ecol 28:391–406. https://doi.org/10.1111/mec.14734
Dias D (2022) Understanding the role of insular African mangroves as nursery areas for the early life stages of fish. Master’s Thesis. University of Lisbon
Félix PM, Chainho P, Lima RF, Costa JL, Almeida AJ, Domingos I, Brito AC (2017) Mangrove fish of São Tomé Island (Gulf of Guinea): new occurrences and habitat usage. Mar Freshw Res 68:123–130. https://doi.org/10.1071/MF15392
Fletcher CM, Collins SF, Nannini MA, Wahl DH (2019) Competition during early ontogeny: effects of native and invasive planktivores on the growth, survival, and habitat use of bluegill. Freshw Biol 64:697–707. https://doi.org/10.1111/fwb.13255
Froese R, Pauly D (eds) (2022) FishBase. World Wide Web electronic publication. www.fishbase.org. Accessed 30th December 2022
Gkenas C, Magalhães MF, Campos-Martin N, Ribeiro F, Clavero M (2021) Desert pumpkinseed: diet composition and breadth in a Moroccan river. Knowl Manag Aquat Ecosyst 422:34–39. https://doi.org/10.1051/kmae/2021033
Gkenas C, Kodde A, Ribeiro F, Magalhães MF (2022) Warming affects the feeding success of invader and native fish in Iberian streams. Aquat Ecol 56:319–324. https://doi.org/10.1007/s10452-021-09888-9
Ho TW, Hwang JS, Cheung MK, Kwan HS, Wong CK (2017) DNA-based study of the diet of the marine calanoid copepod Calanus sinicus. J Exp Mar Biol Ecol 494:1–9. https://doi.org/10.1016/j.jembe.2017.04.004
Leray M, Yang JY, Meyer CP, Mills SC, Agudelo N, Ranwez V, Boehm JT, Machida RJ (2013) A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Front Zool 10:1–14. https://doi.org/10.1186/1742-9994-10-34
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet.journal 17:10–12
Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, Barbour B, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista HBA, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill MO, Lahti L, McGlinn D, Ouellette M-H, Cunha ER, Smith T, Stier A, Ter Braak CJF, Weedon J (2022) vegan: Community Ecology Package. R package version 2.6-2. https://CRAN.R-project.org/package=vegan
Primavera JH, Friess DA, Van Lavieren H, Lee SY (2019) Chapter 1 - The mangrove ecosystem. In: Sheppard C (ed) World Seas: an environmental evaluation (second edition), pp 1–34. Academic Press, London. https://doi.org/10.1016/B978-0-12-805052-1.00001-2
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Russell DJ, Thuesen PA, Thomson FE (2012) A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Rev Fish Biol Fish 22:533–554. https://doi.org/10.1007/s11160-011-9249-z
Thomsen MS, Wernberg T, Olden JD, Byers JE, Bruno JF, Silliman BR, Schiel DR (2014) Forty years of experiments on aquatic invasive species: are study biases limiting our understanding of impacts? 22:1–22. https://doi.org/10.3897/neobiota.22.6224
Wainright CA, Muhlfeld CC, Elser JJ, Bourret SL, Devlin SP (2021) Species invasion progressively disrupts the trophic structure of native food webs. Proc Natl Acad Sci 118:e2102179118. https://doi.org/10.1073/pnas.2102179118
Acknowledgements
We thank the reviewers for their time and effort in reviewing the manuscript as well as their helpful comments which seriously improved the quality of the manuscript. We thank Dr. Judite Alves for granting us access to the laboratory facilities at the National Museum of Science & Natural History (Lisbon) and for the support throughout the project, without which this study would not have been possible.
Funding
Open access funding provided by FCT|FCCN (b-on). This study was funded by the Critical Ecosystem Partnership Fund (CEPF) with the Project GFWA-2018-LG-02, entitled “Participatory Management of Malanza and Praia das Conchas Mangroves in São Tomé”. Additional support was provided by Fundação para a Ciência e a Tecnologia (FCT) through the project (UIDB/04292/2020 and UIDP/04292/2020) awarded to MARE, cE3c (UID/BIA/00329/2023) and through project LA/P/0069/2020 granted to the Associate Laboratory ARNET.
Author information
Authors and Affiliations
Contributions
MC, PMF and FR contributed to the study conception and design. Sample collection was performed by FA, DD and JM. Laboratory work was performed by SN. Data analysis was performed by SN, MC and CG. PMF and FR secured funding. The first draft of the manuscript was written by SN, MC and CG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nogueira, S., Curto, M., Gkenas, C. et al. DNA metabarcoding reveals the diet of the invasive fish Oreochromis mossambicus in mangroves of São Tomé Island (Gulf of Guinea). Biol Invasions 26, 17–23 (2024). https://doi.org/10.1007/s10530-023-03170-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03170-9