Abstract
Control of invasive predators is a priority to protect island biodiversity. Understanding the responses of other species in multi-species invaded food webs is important to avoid unintended consequences. We use an intensive 2-year cat-trapping program in the vicinity of seabird colonies on Bruny Island, Tasmania, to investigate its effectiveness in reducing feral cat density and whether cat control influenced the behaviour and abundance of native and invasive mammal species. Cat density before control was extremely high around this seasonally rich food resource, much higher than on mainlands. Cat density was reduced 5.4-fold by control showing that trapping is effective in reducing cat density in this focussed landscape context. We found no direct effect of cat reduction on the abundance or behaviour of native or invasive mammalian prey species. Recruitment of invasive black rats and native swamp rats increased on the seabird colonies after the shearwater breeding season, and cats responded by increasing their presence on the colonies relative to surrounding areas. This suggests cascading bottom-up effects from a lagged productivity pulse provided by breeding seabirds which would require nutrient sampling to confirm. Our results highlight the complexity of subsequent effects of an invasive predator control on the broader ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high threat to island biodiversity posed by invasive predators, demonstrated by the large number of species extinctions on islands (Bellard et al. 2017), highlights control of invasive predators as a priority for the protection of island biodiversity (Jones et al. 2016). Eradicating invasive predators from an island generally benefits biodiversity (Zavaleta et al. 2001; Jones et al. 2016) by reducing predation on native species, and has been documented to lead to increased breeding success and abundance of threatened seabirds (e.g. terns shearwaters and albatrosses; Ratcliffe et al. 2010; Brooke et al. 2018). Whether control of an invasive predator is beneficial or could have adverse consequences depends on the complexity of the island food web of which it is part, and the relationship of the invasive predator to other species (Zavaleta et al. 2001; Jones et al. 2016).
Island food webs are often complex, comprising multiple native and invasive predators and prey. Control of an invasive predator can have unexpected and adverse consequences where island ecosystems have several invasive species that have novel and complex interactions with native species (Bergstrom et al. 2009; Ortega et al. 2021). Eradicating an invasive predator may remove top-down control on other invasive species, which can result in increased abundance of both herbivores and mesopredators with cascading effects on the ecosystem (Courchamp et al. 1999; Ballari et al. 2016). For example, the successful eradication of feral cats (Felis catus) on subantarctic Macquarie Island in 2001 removed predation pressure on the introduced European rabbit (Oryctolagus cuniculus) at the same time as releases of the Myxoma virus which suppress rabbit populations were reduced and favourable climatic conditions and vegetation recovery increased food supply (Dowding et al. 2009). These multiple factors resulted in high rabbit abundance and overgrazing which denuded the sensitive vegetation of the island (Bergstrom et al. 2009). While cat control can result in mesopredator release of invasive rodent populations on islands with consequent population collapse of seabirds (Rayner et al. 2007), this is not always the case (Russell et al. 2009; Bonnaud et al. 2010), and with modelling indicating complex dynamics between predator size, prey life stage vulnerability and ecosystem productivity (Russell et al. 2009). When both invasive predator and rodent prey are removed together, the conservation outcome can still be adverse, for example in the case where invasive cat and rodent control released a native mesopredator, resulting in increased predation on native prey (Ortega et al. 2021). Understanding top-down and bottom-up control mechanisms in food webs is necessary for effective management of predator populations to protect biodiversity (Elmhagen and Rushton 2007).
Feral cats are recognised as among the most destructive invasive predators globally, particularly on islands (Medina et al. 2011; Doherty et al. 2016). Cats are adaptable and opportunistic predators that can use a broad range of habitats (Doherty et al. 2014). Control or eradication of cats is a major priority for the conservation of colonially nesting seabirds (Holmes et al. 2019), which are among the taxa most threatened by cats on islands (Brooke et al. 2018). Petrel and shearwater species are particularly vulnerable because they nest in colonies, are often naïve to mammalian predators and are clumsy when on the ground (Sih et al. 2010). Since the first cat eradication campaign was carried out in 1924 on Stephens Island in New Zealand (1.5 km2, DIISE 2022), 169 eradication programs have been started on 146 islands with 107 or 63.0% successful. The size of the island is a major factor in the feasibility of eradicating cats. The largest island on which cats have been successfully eradicated is 626.7 km2: Dirk Hartog Island, Western Australia, in 2016 (Algar et al. 2020; DIISE 2022). Although island size influences the outcome of feral cat eradication programs, other factors such as funding and social issues impact implementation (Campbell et al. 2011; Oppel et al. 2011). On larger inhabited islands the risk of reintroduction is as important as the eradication itself (Nogales et al. 2004). Invasive predators like cats are often co-introduced to islands with invasive rodents, such as black rats (Rattus rattus) and house mice (Mus musculus), which are both prey for cats and prey on seabirds and native small vertebrates (Dilley et al. 2016). To reduce the chance of adverse outcomes from cat control on native island fauna, measuring the responses of other species in the food web is an important step before implementing large-scale control. In particular, the mesopredator release hypothesis needs to be taken into account, although controlling cats help conserve native island fauna, especially seabirds, globally (Russell et al. 2009; Bonnaud et al. 2010; Brooke et al. 2018).
Feral cat eradication campaigns on islands could result in cascading effects on the rest of the ecosystem. The objective of our study is to quantify the potential cascading effects of feral cat control on a large island on the mammalian trophic network comprising multiple invasive and native predators and prey. The control program was implemented within and surrounding an area containing seabird colonies, and while intensive in effort was limited in temporal and geographic scope. The intentions of the control program were two-fold: first to provide immediate protection of the seabird colonies from cat predation, and second as a test of the feasibility and challenges of eradicating cats on the entire island. This control program provided the opportunity: 1) to measure the effectiveness of the control effort in reducing the densities of feral cats in the area, and 2) to quantify the effect of reduction in cat density on the abundance and behaviour of other invasive and native mammal species that cats might prey on or compete with. We predicted, based on the theory and studies presented above, that a strong reduction in cat density might lead to predatory release of co-occurring native mesopredators and native and invasive rodents. An increase in invasive rodent populations could create adverse effects by increasing predation on seabirds and other native fauna and would need mitigation in the design of a larger cat control or eradication program on the island. Understanding the consequences of cat control in this complex trophic network will provide a test case for cat eradication on this island and inform cat eradication on large islands globally.
Materials and methods
Study area
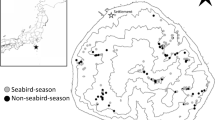
We conducted the study on Lunawanna-allonah/Bruny Island, a climatically mild temperate island located off the south-east of Tasmania (− 43.27 S, 147.34 E) and the fourth largest offshore island of Lutruwita/Tasmania, Australia (Fig. 1). The island is 362 km2, has a permanent resident human population supported by agriculture and tourism and is connected to mainland Tasmania by a frequent and regular car ferry. Bruny Island comprises two distinct continental land masses, North and South Bruny, which are connected by a narrow sand isthmus called “The Neck” which supports a large seabird colony, one of many on the island.
Bruny Island supports a rich diversity of native terrestrial fauna. This includes the endangered native mesopredator, the eastern quoll (0.7–2.0 kg, Family Dasyuridae, Dasyurus viverrinus; https://www.iucnredlist.org/) and an omnivorous native rodent, the Tasmanian subspecies of the swamp rat (Rattus lutreolus velutinus) that may be affected by predation and competition from feral cats (Fancourt et al. 2015; Lazenby et al. 2021). Swamp rats are present mainly in denser vegetation and unlikely to prey on seabirds (Watts and Braithwaite 1978). The rakali/water rat (0.8–1.3 kg, Hydromys chrysogaster), Australia’s largest native rodent, forages within the seabird colonies on Bruny Island and may prey on seabirds; they have been observed on Tasmanian islands entering shearwater burrows (V. Scoleri personal communication). The two larger native marsupial predators that occur on the adjacent Tasmanian mainland, the Tasmanian devil (Sarcophilus harrisii) and spotted-tailed quoll (D. maculatus), are absent from the island. Bruny Island has been designated of global importance for bird conservation, on the basis of the presence of all twelve Tasmanian endemic bird species, a significant proportion of the populations of two threatened Australian endemic woodland birds (Swift Parrot, Lathamus discolor, and Forty-spotted Pardalote, Pardalotus quadragintus), and breeding colonies of migratory seabird species (including the Short-tailed Shearwater, Puffinus tenuirostris, and Little Penguin, Eudyptula minor) (Dutson et al. 2009). Adult shearwaters are present from September to late March. Chicks fledge in early May and leave the island soon after. Penguins are present all year around.
Cats were introduced to Bruny Island by the mid-1800s and feral cats (1.7–4.8 kg, Scomparin unpublished data for Bruny cats) occur across the entire island. While studies of feral cat diet on Bruny Island are not yet completed, cats are documented as major predators of shearwaters on Maria Island, Tasmania (Scoleri et al. 2020), and have been observed feeding on seabirds on Bruny Island (N. Mooney, personal communication). Feral cats in Tasmania also present a predatory threat to the survival of the threatened eastern quoll (Fancourt et al. 2015) and the swamp rat (Lazenby et al. 2021). There are two invasive rodent species on the island, the black rat (Rattus rattus) and house mouse (Mus musculus). Both species are numerous, particularly in the seabird colonies, and are known to be prey of feral cats and predators of birds, including colonially nesting seabirds, on other islands (Angel et al. 2009; Dilley et al. 2016). Both these invasive rodent species are listed amongst the 100 most destructive invasive species (http://www.iucngisd.org/gisd/100_worst.php). The invasive European rabbit is also recorded on Bruny Island from the early years of European occupation (Lawrence and Tucker 2002).
Cat control was implemented in the vicinity of “The Neck” isthmus, including within the seabird breeding colonies (shearwater and little penguins) at The Neck and at Cape Queen Elizabeth 7 km to the north, as well as in areas adjacent to and north and south (which we refer to hereafter as North and South) of The Neck (Fig. 1). A control site, where there was no cat control, was established at Whalebone Point (WBP), located in far South Bruny, which also supports a seabird colony (shearwaters and penguins) (Fig. 1). This site is smaller due to a smaller area of suitable habitat on a peninsula. The vegetation at both sites is a mosaic of coastal heathland, dry coastal forest, saltmarsh and wetland [sourced TASVEG, the state government GIS vegetation layer (Department of Primary Industries 2013)].
Field study design and methods
The field study was carried out in 2017 and repeated in 2019, with paired time periods in each year: during the short-tailed shearwater breeding season “BS” and post shearwater breeding season “PBS”. We deployed 93 cameras stations at The Neck and 20 at Whalebone Point for an average of 36 consecutive days, in a grid array with cameras spaced on average 150 m apart (Appendix S1 & S2). This spacing was used so that each individual feral cat could encounter more than one camera station within its home range (Despres-Einspenner et al. 2017). A systematic design was used to maximize the overall benefits of the survey and allow monitoring of several species, including larger and smaller prey species, at different levels of detail (Head et al. 2013; Despres-Einspenner et al. 2017). The difference in the number of cameras per site reflects the area of each site. The cameras covered an area of 10.25 and 0.05 km2 in The Neck and Whalebone Point, respectively. Only 91 cameras were used in 2019 due to two camera malfunctions. The number of days per deployment ranged from 24 to 87 consecutive days depending on camera malfunction and field limitation, however all data were used for analysis.
Each camera station was set up with a fresh lure secured in a vented PVC canister, suspended 60 cm above the ground. We used lures to maximize the time that cats spent in front of the cameras to aid individual identification. A single canister per camera station was filled with food attractants for predators (sardines, liver treats, and tuna oil), omnivores (truffle oil and walnut oil) and herbivores (peanut butter and rolled oats).
We monitored the lure stations using passive infrared-triggered cameras (Reconyx Hyperfire Professional HC600 and PC800) programmed to record three consecutive images (3.1-megapixels) at high sensitivity each time the sensor was triggered, with one second interval between pictures, and no break between sets. Cameras were strapped to a tree or post ~ 60 cm above ground and ~ 2.5 m from the lure. The cameras operated 24 h a day, with most images obtained at night as all species were nocturnally active.
Following the first paired survey in 2017, the Bruny Island Cat Management Program (BICMP) undertook a 2-year intensive control program of cats at The Neck, from July 2017 until July 2019, with the intention to reduce cat numbers around the shearwater colonies. This intensive control program deployed a total trapping effort of 4185 cage trap-night across the 2 years. Cats were captured using large wire cage traps (710 × 305 × 305 mm) baited with either tuna or chicken. This intensive control program followed many years of opportunistic cage trapping and removal of feral cats at The Neck seabird colony during the shearwater breeding season. It was, however, much larger in geographic extent, much longer in duration and more intensive than the previous ad hoc control and provided more effective reduction of cat numbers on and around the seabird colonies.
Individual cat identification
Individual feral cats were uniquely identified from camera images through a combination of coat pattern and morphological characteristics (McGregor et al. 2015). All discrimination of individuals was done by one experienced observer (C. Scomparin). We first grouped the images of feral cats as marked or unmarked (black) individuals. Although some black cats had small white patches on the neck or chest, these were not always visible depending on orientation of the image, and so all black cats were considered unmarked to avoid double-counting. The marked portion were tabby cats with naturally unique coat markings. We identified individual cats from this group based on matches in unique markings. Due to poor image quality, some images were considered as unidentifiable.
Statistical analysis
We estimated the effect of lethal control on the population density of feral cats over the two paired surveys using two different methods. First, we applied spatial mark-resight (SMR) models, a form of spatially explicit capture-recapture (SECR) that is used when only a part of the population is uniquely identifiable (McClintock et al. 2009; Efford and Hunter 2018). Second, we used dynamic N-mixture models (Royle 2004) for open populations (Dail and Madsen 2011) to estimate relative abundance, detection probability and population dynamics of four species that may have been affected by the reduction in the cat population.
Population density estimates of feral cats: spatial mark-resight models
We estimated the density of cats for each of the eight deployments (two sites, 2 years, paired breeding/post-breeding season—BS/PBS) using a spatial mark-resight model, performed using the “secr” package (Borchers and Efford 2008) in R version 4.0.2 (R Core Team 2020). Such models have been used elsewhere in Australia to estimate abundance and density of feral cats (McGregor et al. 2015; Cunningham et al. 2020). To estimate the contribution of unmarked individuals to the overall population, the model assumes that marked and unmarked individuals have identical sighting probabilities (McClintock et al. 2009).
We constructed a set of capture histories for each of the eight deployments by dividing each survey period into a series of 5-day intervals for each camera, in which each the number of separate individual cats detected was counted (Appendix S1). This approach provided a sufficient number of days within each session to achieve high detection probabilities, while allowing a sufficient number of survey periods to construct encounter histories. Because photographic detections of feral cats were sparse, combining the data in this manner helps to avoid estimated detection rates close to 0, because this can sometimes lead to estimation problems.
To aid estimation of model parameters, a ‘mask’ has to be chosen as the likely distance from an individual’s home-range centre at which its probability of detection is essentially zero (Balme et al. 2009). In our case, the mask serves several purposes: (i) to distinguish habitat sites from non-habitat sites (i.e., the sea) within the outer limit, and (ii) to define a region for which a post-hoc estimate of population size is required (i.e., North and South of The Neck). We chose a mask width of 2000 m around the cameras with a spacing of 100 m between the cells, as this is the estimated maximum width of home ranges of male cats in similar habitat type on the island (personal observation from GPS data), clipped to exclude the ocean. The resulting masks covered an area of 20.14 (51% covered by the cameras) and 7.92 (1% covered by the cameras) km2 in The Neck and Whalebone Point, respectively.
A detection function relates the probability of detection (referred to as “g0”) for an animal to the distance of a detector from a point usually thought of as its home-range centre, even though the centres themselves are unknown (Efford et al. 2016). To estimate the detection function, we first assessed which functional form, half-normal HN or negative exponential EX, best fitted the observed data. In these models, detection probabilities at the home-range centre, and the shoulder of the detection function (“sigma”) were constant. Models were compared using information-theoretic multi-model inference, and the detection function with the lowest AIC was used as the basis for further modelling. Next, we constructed biologically plausible models, influenced or not by the seabird breeding season, on sigma and compared these with the null model. Finally, we constructed models to compare estimates of cat densities across: i) The Neck (treatment site where cat removal occurred) and Whalebone Point (control site), and ii) across the area to the North of The Neck seabird colony, The Neck colony, and the area to the South of The Neck colony. We divided The Neck area into three zones to nuance the cat densities of the seabird colony itself on The Neck isthmus compared to the adjacent land to the north and south of the isthmus (Appendix S2).
Estimates of relative abundance of mesopredators: open N-mixture models
We employed the extended Dail-Madsen open population model, also called an open N-mixture model (Dail and Madsen 2011; Hostetler and Chandler 2015) with a Poisson distribution to estimate variation in demographic parameters of four native and invasive mesopredator and prey species: eastern quoll, swamp rat, black rat, and house mouse. The European rabbit and the water rat were not considered in our analysis because there were too few detections of these species. The Dail-Madsen model relies on temporally and spatially replicated detection histories, which are counts in the N-mixture model (counts of detections need to often exceed one). We defined a detection as independent if it was separated by at least 30 min from the next detection of that species at that camera site, as is commonly done in similar studies (e.g., Brook et al. 2012; Cunningham et al. 2019). We created species-specific detection histories for each camera, allowing us to assess factors that may affect species-specific detection. Missing data during a sampling occasion resulted from cameras malfunctioning and was accounted for in the detection histories. The four structural model parameters are initial abundance (lambda; λ), recruitment rate (gamma; γ), apparent survival (omega; ω) and detection probability (given presence; p). Apparent survival represents animals in site i at time t who survived in that site since time t − 1, and recruitment denotes gains at site i since time t − 1. In other words, in these N-mixture models, recruitment and survival refer to the number of camera sites that gained or lost detections of the species over the survey periods. Note that because individual animal identities are not recorded, no distinction is possible between either deaths and emigrants for the animals departing (apparent survival), or between births and immigrants for new arrivals (recruitment) (Dail and Madsen 2011).
The four parameters (λ, γ, ω and p) can be modelled as functions of covariates. The probability of detection (p) was modelled in relation to lure (started at 1 in the first day of each camera station and increased from 1 in each consecutive day), moon phase, seabird breeding season, and presence of cat, the initial abundance (λ) was modelled in relation to site, and the recruitment (γ) and survival (ω) in function of site, seabird breeding season, and year. Meteorological variables were not considered in our models. Due to the large number of covariate combinations possible among the four demographic parameters in the models, our approach was to do the modelling in four stages. In stage 1, we built a set of models to estimate p (see below for model selection). Using the model with the lowest AICc resulting from stage 1, we then built a set of stage 2 models to estimate λ. Using the same procedure, we then estimated γ and ω during stage 3 and 4 of the modelling stages, respectively.
In each of the four modelling stages, model candidates were compared using multi-model inference in an information theoretic framework (Anderson and Burnham 2004), and all models with ∆AICc > 6 were discarded as non-competitive fits to the data (Richards 2015). However, models with ∆AICc ≤ 6 may lead to retention of overly complex models (Richards 2008). An alternative is, of the remaining competitive models, the more complex versions of models that had simpler, nested versions with smaller AICc values were also excluded to yield the final model set (Richards 2008, 2015). Although we used the top model with the lowest AICc in the successive stages of the best model construction and to visualise fitted values, we used the best model set to describe the relative contribution of each of the covariates for each parameter.
To fit the open N-mixture model, we used the unmarked package (Fiske and Chandler 2011) in R (R Core Team 2020), which provides a unified modelling framework for hierarchical models. Data were modelled using maximum likelihood methods with the function pcountOpen, specifically written to use the Dail and Madsen (2011) model in unmarked (Chandler and King 2011). We estimated seasonal abundance for each species at each site using empirical Bayes methods, using the function ranef from unmarked and demographic parameters from the best-supported model.
Results
Changes in feral cat densities
During the 2 years of cat control a total of 43 cats were trapped and removed (euthanized) from the study area: 22 from The Neck seabird colony, 20 from the Cape Queen Elizabeth seabird colony, one from South of The Neck and none the site North of The Neck. We recorded a total of 2,186 cat passes in front of the cameras from which individual cats were identifiable in 1277 cases, across the four deployments of camera traps from 2017 to 2019 at two sites (The Neck and Whalebone Point), representing a total of 35 individually identifiable cats (Appendix S3). These detections were spread over 3,658 trap-nights, with an average of 750 and 165 per deployment for The Neck and the control site respectively.
Detection probability of cats declined exponentially with distance from the centre of the home-range, as indicated by the stronger support for the exponential detection function than the half-normal detection function (Appendix S4). The best-supported model describes the detection parameter sigma (shoulder of the detection function) varying with the breeding season (Appendix S4).
Cat densities at The Neck (including the control area at the seabird colony, and the areas immediately to the North and South) decreased from 1.4 to 0.56 cats/km2 between the shearwater breeding seasons of 2017 and 2019, and from 1.9 to 0.45 cats/km2 between the post-breeding season surveys of 2017 and 2019 (Fig. 2a and Appendix S5a). For The Neck shearwater colony alone, cat densities decreased following cat control (between the 2017 and 2019 surveys) from 8.03 ± 0.72 cats/km2 to 1.48 ± 0.28 cats/km2 during the shearwater breeding season and from 15.51 ± 1.07 cats/km2 to 3.20 ± 0.42 cats/km2 after the shearwater breeding season (Fig. 2b; Appendix S5b). In the adjacent areas, cat densities decreased from the 2017 to the 2019 survey, but to a lesser extent, from 0.93 ± 0.01 to 0.62 ± 0.09 cats/km2 during the breeding season and from 0.70 ± 0.04 to 0.39 ± 0.01 cats/km2 after the breeding season in the North of the Neck and from 1.52 ± 0.19 to 0.58 ± 0.09 cats/km2 during the breeding season and from 0.61 ± 0.05 to 0.38 ± 0.06 cats/km2 after the breeding season in the South of the Neck. The cat density within the Neck seabird colony was always higher than in the adjacent areas (Fig. 2b).
Feral cat densities across, a The Neck (treatment site) and Whalebone Point (control site) and b the North, South and the colony sites of the Neck, on Lunawanna-allonah/Bruny Island, Lutruwita/Tasmania, Australia, during the shearwater breeding season (BS) and post-breeding season (PBS), in 2017 and 2019
Cat densities did not considerably change at the seabird colony at Whalebone Point (where there was no cat control) over the same time period. The mean density of cats at Whalebone Point was on average 0.39 ± 0.067 cat/km2 (0.40 and 0.39 during and post breeding season respectively; Fig. 2a; Appendix S5a), lower than the lowest cat density recorded, following control, in the vicinity of The Neck.
Changes in abundance of mammalian mesopredators and prey
Sufficient data were obtained for four other species of mesopredators and prey to model associations between their abundance and changing cat density and environmental parameters. The number of detections we obtained for each of these species was: 2205 black rats, 332 swamp rats, 1312 house mice and 818 eastern quolls. The initial abundance of black rats, swamp rats, house mice and eastern quoll was strongly associated with site, with the null model more than 6 ∆AICc points above the model containing site (Table 1). Cat control and density reduction at The Neck colony and adjacent areas did not explain changes observed in the relative abundance of these four species (Fig. 3). Cat presence on a given night, however, was negatively associated with the probability of detecting black rats (estimate parameter for detection probability p = − 0.24 [− 0.37; − 0.11] IC95%, s.e. = 0.07) and, to a lesser extent, swamp rats (estimate parameter for detection probability p = − 0.52, [− 1.28; 0.23] IC95%, s.e. = 0.39).
Relative abundance estimated by the best N-mixture model selected for black rat (a), swamp rat (b), house mouse (c) and eastern quoll (d) populations. The abundances are estimated per camera on four sites on Lunawanna-allonah/Bruny Island-Lutruwita/Tasmania, Australia during the shearwater breeding season (BS) and post-breeding season (PBS), in 2017 and 2019
The final set of models that described the probability of detection and estimated abundance of black rats for each survey and each site had five parameters and included two interaction terms: between year and site, and between breeding season and site (Table 1, Appendix S6a). As well as detection probability being negatively affected by the presence of cats, there was evidence that rats were more detectable during the full moon, and during the shearwater breeding season. Recruitment of black rats was higher, and apparent survival lower, at both seabird colonies than in the areas adjacent to The Neck that did not have breeding seabirds, and recruitment and survival were both higher in 2019 than in 2017.
The candidate models describing the probability of detection and the estimated abundance of swamp rats for each survey and each site had six parameters (Table 1, Appendix S6b). The presence of cat on a given night on a given camera had only a weak negative effect on swamp rat detectability. Swamp rats were less detectable during full moon and after the seabird breeding season, and detection probability decreased with lure age. Recruitment of swamp rats was higher in 2019 than in 2017 and was higher in the Whalebone Point shearwater colony than The Neck and adjacent areas. Recruitment was slightly higher (weak effect) and apparent survival lower after the seabird breeding season, relative to the breeding season.
The final model set that described the probability of detection and estimated abundance of house mice for each survey and each site had four parameters and included the interaction terms between year and site (Table 1, Appendix S6c). Mice were more detectable during the waning moon and less detectable during the waxing moon. Recruitment of house mice was higher, and apparent survival was lower, after the seabird breeding season. Recruitment was also higher in the Whalebone Point colony during year 2017, but not different among other sites.
Finally, the candidate model set describing the estimated abundance of eastern quoll for each survey and each site had three parameters (Table 1, Appendix S6d). Eastern quolls were more detectable during the waning moon than any other moon phase. Recruitment of quolls was lower on the site South of the Neck compared to all other sites. The apparent survival was not influenced by any variables we tested.
Discussion
Trapping in the vicinity of the shearwater colonies around The Neck isthmus on Bruny Island reduced the cat density by 5.4-fold. There was no direct measured effect on the relative abundance or behaviour of native and invasive mammal species that cats might prey on or compete with. There is, however, evidence of avoidance (spatial and temporal, indicated by lower probability of detection on a given camera and night) by swamp rats and black rats of cats and of lower survivorship of these species at times of year when cat densities are highest. Importantly, recruitment of both invasive and native rodents and density of cats increased after the seabird breeding season, indicating a potential lagged response to bottom-up productivity from the breeding seabirds. We cannot confirm this without measuring nutrients and plant productivity. We reiterate that in these N-mixture models, recruitment and survival refer to the number of camera sites that gained or lost detections of the species over the survey periods. Our data suggest that the ecological system around the shearwater colony could be driven more by bottom-up resourcing than the ability of cats to control rodent densities, particularly of invasive rodents. We show a complex but subtle cascade of ecological effects amongst native and invasive mesopredator species, that also prey for cats, and prey species in response to an intensive 2-year program of cat control in an island ecosystem.
Feral cat densities are exceptionally high (1.7–0.5 cat/km2 pre–post-control) at the shearwater colony at The Neck on Bruny Island, greater than the average density of cats on mainland Tasmania and Australia (0.27 [IC 95% 0.18–0.45] cats/km2) (Legge et al. 2017), although density at the smaller Whalebone Point shearwater colony (0.39 cat/km2) was similar to mainland densities. While cat densities are often higher on islands than on mainlands due to abundant food resources, including colonially-nesting seabirds and ongoing inputs of food from washed-up marine life (Legge et al. 2017), two features specific to Bruny Island may explain the very high densities of cats at The Neck. First, Bruny Island lacks larger mammalian predators, in particular the Tasmanian devil, that are able to limit the abundance of feral cats elsewhere (Hollings et al. 2016; Cunningham et al. 2020). Second, the geographical particularity of The Neck, an isthmus creating a bottleneck between two land masses, may concentrate the movements of feral cats travelling between the northern and southern areas, giving an apparent elevated local density. It is unlikely that the densities recorded on The Neck isthmus are found elsewhere on Bruny Island, as indicated by the lower densities in the areas adjacent to The Neck and at the control site Whalebone Point.
Feral cat control through targeted trapping across a large area around The Neck seabird colony on Bruny Island was effective in reducing cat density. A total control effort of 4185 trap nights over 2 years was effective at reducing cat density by an average of 3.3-fold, a control efficiency of 54% (~ 81% of cats removed—22 cats removed of the 27 cats identified in 2017—versus ~ 44% reduction of the population—27 cats identified in 2017 and 12 cats in 2019). The control efficiency on Bruny Island was better than that modelled by McCarthy et al. (2013), in which population dynamics simulations found that to achieve a 44% cat control efficiency would require the removal of at least 57% of cats annually to sustain a 25% decrease in population size. To achieve close to or zero cats on the Neck of Bruny Island would require 3500 trap-nights, given the capture rate of one cat per 190 trap-nights and assuming the control efficiency would stay constant. This seems feasible and the result is encouraging, although it does not consider issues such as declining capture success as cat density decreases, learned aversion of traps by cats through time, and immigration from adjacent regions (Palmas et al. 2020), all of which would likely increase the number of trap-nights. For the eradication to be useful to protect the seabird colony in the long term, an ongoing cat control in this area or consideration for eradicated cat across the entire island would be required (Doherty et al. 2017).
The presence of the shearwater breeding colony at The Neck would provide a seasonal nutrient and productivity pulse (Polis and Hurd 1996), which may trigger a lagged bottom-up response in recruitment of rodents and local movement and density of cats, independent of the cat control. Seabird colonies are areas of high productivity, contributing pulses of nutrients to islands during the breeding season, including live prey, guano, fish scraps, feathers, unhatched eggs and dead chicks and adults (Polis and Hurd 1996), which also cascade through the trophic network, increasing soil nutrient levels and plant productivity (Anderson et al. 2008). A lagged response in rodent recruitment to the pulse of nutrients in seabird colonies, after the breeding season, has been reported from both island and continental systems (Russell and Ruffino 2012). Attributing causation for these potential bottom up effects is beyond the scope of this study, because we did not measure soil nutrients or plant growth or seeding. The data do, however, show increased recruitment of both black rats and swamp rats after but not during the breeding season on the two seabird colonies (potential productivity pulse) relative to surrounding areas (general habitat productivity). This increase was greater in invasive black rats than in swamp rats, which were detected less frequently possibly due to a preference for denser, wetter habitats than occur on the seabird colonies (Lunney 2023). Cats seem to be responding to the increased recruitment of rodents, with cat density on The Neck seabird colony in 2017, prior to control operations, increasing between the shearwater breeding and post-breeding seasons, and correspondingly decreasing, albeit to a lesser extent, in the areas adjacent to the colony.
While cats seem to track increasing rodent recruitment, an important question is whether cats have a predatory impact on rodent behaviour or populations. Black rats and swamp rats are both sensitive to the presence of feral cats. Both species reduced their visitation to camera stations (becoming less detectable) on nights when cats were present, indicating risk-sensitive behaviour in response to cats (Bedoya-Pérez et al. 2021). House mice did not behaviourally avoid cats at the camera stations (no influence of cats on nightly detectability). Anderson and Polis (1999) found that house mice will ignore the risk of predation to maximise foraging at high population density and low food supply. The patterns of survival of the three rodent species across the 2 years, the breeding and post-breeding seasons and the different geographic areas may reflect changes and differences in cat density, although the results are complex and there is no direct statistical association between the numerical density of cats and the population dynamics of these rodent species. Survival of all three species was lower post-breeding, when cat densities were higher in the seabird colonies than during seabird breeding, although this effect was seen at the control site. For black rats, survival was lower in the seabird colonies than the areas away from the colonies. That this effect was evident at both The Neck, which had high cat density, and at Whalebone Point, which had low cat density, suggests a change in cat diet or behaviour that we didn’t measure. As a result of the relationship between recruitment and survival, the estimated abundance of black rats is higher post-breeding season of the seabirds in both colonies, but this increase seems higher in the Neck colony, which could be a consequence of the reduction of cat predation (Fig. 3).
Eastern quolls, a marsupial mesopredator of invertebrate and vertebrate prey, smaller than and at risk of predation by cats, did not respond to changes in cat density, despite their populations declining on mainland Tasmania where cat densities are high (Fancourt et al. 2015). Despite experimental confirmation of anti-predator responses to cats (Jones et al. 2004), the nightly detection probabilities of eastern quolls, which can reflect risk-sensitive behavioural avoidance, were not influenced by the same-night presence of cats at camera stations. Nor were population parameters responsive to cat density or to site. The values for initial abundance reflected the distribution of eastern quolls on Bruny Island, where they are abundant in the drier north and uncommon in the south (Parker 2016). Quolls do not seem to focus on seabirds as a primary prey resource, although quolls are present in the seabird colony and have been observed killing shearwaters (Author personal observation). In this prey-rich environment, top-down influences from cats of intraguild predation or interference or exploitation competition may not be important drivers of quoll behaviour or population dynamics, as suggested by Fancourt et al. (2015).
A holistic approach to invasive species management is an important pre-requisite to implementing successful wildlife management (Lurgi et al. 2018). This approach includes understanding the functional roles of predators in a whole of ecosystem context, and integrated control of multiple species, applying several methods and including control or eradication of invasive prey (Marlow and Croft 2016). Invasive predators are frequently co-introduced with invasive prey, species of rodents and European rabbits, which have amongst the highest intrinsic rates of increase of any mammals (Ballari et al. 2016). Control of feral cats has had unexpected outcomes in complex, multispecies food webs, due to predatory or competitive release of invasive mesopredators and prey species with further cascading effects on ecosystems (Rayner et al. 2007; Bergstrom et al. 2009; Brook et al. 2012). Control measures not only reduce predator density and predatory impact but can lead to behavioural changes in mesopredators and prey in response to relaxed top-down pressure (Sih et al. 2010). Responses can be rapid (Peckarsky et al. 2008), with mesopredators or prey shifting to more optimal foraging areas or time periods, with fitness benefits translating to increases in abundance (Lima 1998).
The ecological implications of the demographic responses of invasive and native rodents to seasonal changes in seabird presence, the consequent nutrient pulses, and the subsequent focus of cats on The Neck isthmus are not known and need further investigation. Simultaneous control of populations of invasive prey species, in this case black rats and house mice, could increase the success of the current cat control by reducing overall food availability and the potential for prey switching, especially when the seabirds are not breeding. Also important is investigating the predatory and competitive relationships between these invasive prey species because control or eradication of only one might increase the number of another (Zavaleta et al. 2001; Courchamp et al. 2003; Ballari et al. 2016). As mesopredators and aggressive competitors, rats can regulate mouse populations on islands where both mammals have been introduced (Caut et al. 2007). Rat eradication can result in the release of mouse populations, if mice are not eradicated in the same time, which can lead to increased mouse damage to seabirds, plants or insects (Caut et al. 2007; Ruscoe et al. 2011). The predatory impact of black rat and house mouse on seabirds or other species on Bruny Island is not known.
Seabird life histories are characterised by high longevity and low fecundity (Weimerskirch 2001), which means that responses to cat control will be slow but also probably strongly positive, and will require long-term monitoring to detect. Although we did not measure the impact of cat predation on shearwaters in this study, the shearwater colony at The Neck is being monitored (Eric Woehler and Mary-Ann Lea, personal communication). Cats prey on both adults and chicks in shearwater colonies on Tasmanian islands (Scoleri et al. 2020) but population growth in long-lived seabirds is more sensitive to changes in adult survival than to changes in breeding success (Bonnaud et al. 2010).
Conclusions
Our study demonstrates that cat density on islands, particularly around seasonally rich food resources such as seabird colonies can be very high, much higher than on mainland areas, and that control using trapping is effective in this focussed landscape context at reducing cat density. The rate of reduction was encouraging and suggests that eradication might be possible under sustained control. The control of cats did not have a direct measurable impact on the abundance of prey or other mesopredator species. The substantial reduction in cat density may have impacted the invasive rodent populations, though, mediated through complex trophic cascades, with lagged effects of nutrient pulses working through primary productivity to support recruitment in rodents, which also subsequently attracted cats to the site of the colony after the seabirds had left on migration. The complexity of this system indicates the need for further research into ecological dynamics between cats, native mesopredators, and invasive and native rodents. Integrated control of multiple invasive predator and prey species is indicated, particularly as invasive rodents are known to prey on seabird eggs and chicks. Eradication or control programs for invasive predators need to be based on research that provides an understanding of community dynamics to reduce unexpected adverse consequences in complex communities with multiple species of native and invasive predators and prey.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Algar D, Morris K, Asher J, Cowen S (2020) Dirk Hartog Island ‘Return to 1616’project—the first six years (2014 to 2019). Ecol Manag Restor 21(3):173–183
Anderson D, Burnham K (2004) Model selection and multi-model inference, vol 63, 2nd edn. Springer, New York, p 10
Anderson WB, Polis GA (1999) Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118(3):324–332
Anderson WB, Wait DA, Stapp P (2008) Resources from another place and time: responses to pulses in a spatially subsidized system. Ecology 89(3):660–670
Angel A, Wanless RM, Cooper J (2009) Review of impacts of the introduced house mouse on islands in the Southern Ocean: Are mice equivalent to rats? Biol Invasions 11(7):1743–1754
Ballari SA, Kuebbing SE, Nuñez MA (2016) Potential problems of removing one invasive species at a time: a meta-analysis of the interactions between invasive vertebrates and unexpected effects of removal programs. PeerJ 4:e2029
Balme GA, Hunter LT, Slotow R (2009) Evaluating methods for counting cryptic carnivores. J Wildl Manag 73(3):433–441
Bedoya-Pérez MA, Le A, McGregor IS, Crowther MS (2021) Antipredator responses toward cat fur in wild brown rats tested in a semi-natural environment. Behav Ecol
Bellard C, Rysman J-F, Leroy B, Claud C, Mace GM (2017) A global picture of biological invasion threat on islands. Nat Ecol Evol 1(12):1862–1869
Bergstrom DM, Lucieer A, Kiefer K, Wasley J, Belbin L, Pedersen TK, Chown SL (2009) Indirect effects of invasive species removal devastate World Heritage Island. J Appl Ecol 46(1):73–81. https://doi.org/10.1111/j.1365-2664.2008.01601.x
Bonnaud E, Zarzoso-Lacoste D, Bourgeois K, Ruffino L, Legrand J, Vidal E (2010) Top-predator control on islands boosts endemic prey but not mesopredator. Anim Conserv 13(6):556–567. https://doi.org/10.1111/j.1469-1795.2010.00376.x
Borchers DL, Efford M (2008) Spatially explicit maximum likelihood methods for capture–recapture studies. Biometrics 64(2):377–385
Brook LA, Johnson CN, Ritchie EG (2012) Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. J Appl Ecol 49(6):1278–1286. https://doi.org/10.1111/j.1365-2664.2012.02207.x
Brooke MDL, Bonnaud E, Dilley B, Flint E, Holmes N, Jones H, Provost P, Rocamora G, Ryan P, Surman C (2018) Seabird population changes following mammal eradications on islands. Anim Conserv 21(1):3–12
Campbell KJ, Harper G, Algar D, Hanson CC, Keitt BS, Robinson S (2011) Review of feral cat eradications on islands. Island invasives: eradication and management, pp 37–46
Caut S, Casanovas JG, Virgos E, Lozano J, Witmer GW, Courchamp F (2007) Rats dying for mice: modelling the competitor release effect. Austral Ecol 32(8):858–868
Chandler RB, King DI (2011) Habitat quality and habitat selection of golden-winged warblers in Costa Rica: an application of hierarchical models for open populations. J Appl Ecol 48(4):1038–1047
Courchamp F, Langlais M, Sugihara G (1999) Cats protecting birds: modelling the mesopredator release effect. J Anim Ecol 68(2):282–292
Courchamp F, Chapuis J-L, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78(3):347–383
Cunningham CX, Scoleri V, Johnson CN, Barmuta LA, Jones ME (2019) Temporal partitioning of activity: rising and falling top-predator abundance triggers community-wide shifts in diel activity. Ecography 42(12):2157–2168
Cunningham CX, Johnson CN, Jones ME (2020) A native apex predator limits an invasive mesopredator and protects native prey: Tasmanian devils protecting bandicoots from cats. Ecol Lett
Dail D, Madsen L (2011) Models for estimating abundance from repeated counts of an open metapopulation. Biometrics 67(2):577–587
Department of Primary Industries, Parks, Water and Environment (2013) TASVEG 3.0. Tasmanian vegetation monitoring and mapping program, resource management and conservation division
Despres-Einspenner ML, Howe EJ, Drapeau P, Kuhl HS (2017) An empirical evaluation of camera trapping and spatially explicit capture-recapture models for estimating chimpanzee density. Am J Primatol. https://doi.org/10.1002/ajp.22647
DIISE (2022) The database of Island invasive species eradications, developed by Island Conservation, Coastal Conservation Action Laboratory UCSC, IUCN SSC Invasive Species Specialist Group, University of Auckland and Landcare Research New Zealand. http://diise.islandconservation.org. Accessed 16 Oct 2022
Dilley BJ, Schoombie S, Schoombie J, Ryan PG (2016) ‘Scalping’ of albatross fledglings by introduced mice spreads rapidly at Marion Island. Antarct Sci 28(2):73–80
Doherty TS, Bengsen AJ, Davis RA (2014) A critical review of habitat use by feral cats and key directions for future research and management. Wildlife Res. https://doi.org/10.1071/wr14159
Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR (2016) Invasive predators and global biodiversity loss. Proc Natl Acad Sci 113(40):11261–11265
Doherty TS, Dickman CR, Johnson CN, Legge SM, Ritchie EG, Woinarski JC (2017) Impacts and management of feral cats Felis catus in Australia. Mammal Rev 47(2):83–97
Dowding JE, Murphy EC, Springer K, Peacock AJ, Krebs CJ (2009) Cats, rabbits, Myxoma virus, and vegetation on Macquarie Island: a comment on Bergstrom. J Appl Ecol 46(5):1129–1132. https://doi.org/10.1111/j.1365-2664.2009.01690.x
Dutson GC, Garnett ST, Gole C (2009) Australia’s Important Bird Areas: key sites for bird conservation. Birds Australia
Efford MG, Hunter CM (2018) Spatial capture–mark–resight estimation of animal population density. Biometrics 74(2):411–420. https://doi.org/10.1111/biom.12766
Elmhagen B, Rushton SP (2007) Trophic control of mesopredators in terrestrial ecosystems: Top-down or bottom-up? Ecol Lett 10(3):197–206
Efford MG, Dawson DK, Jhala YV, Qureshi Q (2016) Density-dependent home-range size revealed by spatially explicit capture–recapture. Ecography 39(7):676–688. https://doi.org/10.1111/ecog.01511
Fancourt BA, Hawkins CE, Cameron EZ, Jones ME, Nicol SC (2015) Devil declines and catastrophic cascades: is mesopredator release of feral cats inhibiting recovery of the eastern quoll? PLoS ONE 10(3):e0119303
Fiske I, Chandler R (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43(10):1–23
Head JS, Boesch C, Robbins MM, Rabanal LI, Makaga L, Kühl HS (2013) Effective sociodemographic population assessment of elusive species in ecology and conservation management. Ecol Evol 3(9):2903–2916. https://doi.org/10.1002/ece3.670
Hollings T, Jones M, Mooney N, McCallum H (2016) Disease-induced decline of an apex predator drives invasive dominated states and threatens biodiversity. Ecology 97(2):394–405
Holmes ND, Spatz DR, Oppel S, Tershy B, Croll DA, Keitt B, Genovesi P, Burfield IJ, Will DJ, Bond AL (2019) Globally important islands where eradicating invasive mammals will benefit highly threatened vertebrates. PLoS ONE 14(3):e0212128
Hostetler JA, Chandler RB (2015) Improved state-space models for inference about spatial and temporal variation in abundance from count data. Ecology 96(6):1713–1723
Jones ME, Smith GC, Jones SM (2004) Is anti-predator behaviour in Tasmanian eastern quolls (Dasyurus viverrinus) effective against introduced predators? Anim Conserv 7(2):155–160. https://doi.org/10.1017/S136794300400126X
Jones HP, Holmes ND, Butchart SHM, Tershy BR, Kappes PJ, Corkery I, Aguirre-Muñoz A, Armstrong DP, Bonnaud E, Burbidge AA (2016) Invasive mammal eradication on islands results in substantial conservation gains. Proc Natl Acad Sci 113(15):4033–4038
Lawrence S, Tucker C (2002) Sources of meat in colonial diets: faunal evidence from two nineteenth century Tasmanian whaling stations. Environ Archaeol 7(1):23–34
Lazenby B, Mooney N, Dickman C (2021) Raiders of the last ark: the impacts of feral cats on small mammals in Tasmanian forest ecosystems. Ecol Appl 31(6):e02362
Legge S, Murphy BP, McGregor H, Woinarski JCZ, Augusteyn J, Ballard G, Baseler M, Buckmaster T, Dickman CR, Doherty T, Edwards G, Eyre T, Fancourt BA, Ferguson D, Forsyth DM, Geary WL, Gentle M, Gillespie G, Greenwood L, Hohnen R, Hume S, Johnson CN, Maxwell M, McDonald PJ, Morris K, Moseby K, Newsome T, Nimmo D, Paltridge R, Ramsey D, Read J, Rendall A, Rich M, Ritchie E, Rowland J, Short J, Stokeld D, Sutherland DR, Wayne AF, Woodford L, Zewe F (2017) Enumerating a continental-scale threat: How many feral cats are in Australia? Biol Conserv 206:293–303. https://doi.org/10.1016/j.biocon.2016.11.032
Lima SL (1998) Nonlethal effects in the ecology of predator–prey interactions. Bioscience 48(1):25–34
Lunney D (2023) Swamp rat Rattus lutreolus. In: Baker AM, Gynther IC (eds) Strahan’s mammals of Australia, 4th edn. Reed New Holland Publishers
Lurgi M, Ritchie EG, Fordham DA (2018) Eradicating abundant invasive prey could cause unexpected and varied biodiversity outcomes: the importance of multispecies interactions. J Appl Ecol. https://doi.org/10.1111/1365-2664.13188
Marlow N, Croft D (2016) The effect of rabbit-warren ripping on the consumption of native fauna by foxes in the arid zone of New South Wales. Conserv Sci West Aust 10:1–13
McCarthy RJ, Levine SH, Reed JM (2013) Estimation of effectiveness of three methods of feral cat population control by use of a simulation model. J Am Vet Med Assoc 243(4):502–511
McClintock BT, White GC, Antolin MF, Tripp DW (2009) Estimating abundance using mark–resight when sampling is with replacement or the number of marked individuals is unknown. Biometrics 65(1):237–246
McGregor HW, Legge S, Potts J, Jones ME, Johnson CN (2015) Density and home range of feral cats in north-western Australia. Wildl Res 42(3):223–231. https://doi.org/10.1071/Wr14180
Medina FM, Bonnaud E, Vidal E, Tershy BR, Zavaleta ES, Josh Donlan C, Keitt BS, Corre M, Horwath SV, Nogales M (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Change Biol 17(11):3503–3510. https://doi.org/10.1111/j.1365-2486.2011.02464.x
Nogales M, Martin A, Tershy BR, Donlan CJ, Witch D, Puerta N, Wood B, Alonso J (2004) A review of feral cat eradication on islands. Conserv Biol 18(2):310–319. https://doi.org/10.1111/j.1523-1739.2004.00442.x
Oppel S, Beaven BM, Bolton M, Vickery J, Bodey TW (2011) Eradication of invasive mammals on islands inhabited by humans and domestic animals. Conserv Biol 25(2):232–240
Ortega S, Rodríguez C, Mendoza-Hernández B, Drummond H (2021) How removal of cats and rats from an island allowed a native predator to threaten a native bird. Biol Invas 1–13
Palmas P, Gouyet R, Oedin M, Millon A, Cassan J-J, Kowi J, Bonnaud E, Vidal E (2020) Rapid recolonisation of feral cats following intensive culling in a semi-isolated context. NeoBiota 63:177–200
Parker R (2016) Spatial distributions of eastern quolls and cats: an analysis of the occupancy and abundance of eastern quolls (Dasyurus viverrinus) and cats (Felis cattus) on Bruny Island. Unpublished Honours Thesis, University of Tasmania, Hobart
Peckarsky BL, Abrams PA, Bolnick DI, Dill LM, Grabowski JH, Luttbeg B, Orrock JL, Peacor SD, Preisser EL, Schmitz OJ (2008) Revisiting the classics: considering nonconsumptive effects in textbook examples of predator–prey interactions. Ecology 89(9):2416–2425
Polis GA, Hurd SD (1996) Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am Nat 147(3):396–423
Ratcliffe N, Bell M, Pelembe T, Boyle D, Benjamin R, White R, Godley B, Stevenson J, Sanders S (2010) The eradication of feral cats from Ascension Island and its subsequent recolonization by seabirds. Oryx 44(1):20–29
Rayner MJ, Hauber ME, Imber MJ, Stamp RK, Clout MN (2007) Spatial heterogeneity of mesopredator release within an oceanic island system. Proc Natl Acad Sci 104(52):20862–20865
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Richards SA (2015) Likelihood and model selection
Richards SA (2008) Dealing with overdispersed count data in applied ecology. J Appl Ecol 45(1):218–227
Royle JA (2004) N-mixture models for estimating population size from spatially replicated counts. Biometrics 60(1):108–115
Ruscoe WA, Ramsey DS, Pech RP, Sweetapple PJ, Yockney I, Barron MC, Perry M, Nugent G, Carran R, Warne R, Brausch C, Duncan RP (2011) Unexpected consequences of control: competitive vs. predator release in a four-species assemblage of invasive mammals. Ecol Lett 14(10):1035–1042. https://doi.org/10.1111/j.1461-0248.2011.01673.x
Russell JC, Ruffino L (2012) The influence of spatio-temporal resource fluctuations on insular rat population dynamics. Proc R Soc B Biol Sci 279(1729):767–774
Russell JC, Lecomte V, Dumont Y, Le Corre M (2009) Intraguild predation and mesopredator release effect on long-lived prey. Ecol Model 220(8):1098–1104
Scoleri VP, Johnson CN, Vertigan P, Jones ME (2020) Conservation trade-offs: Island introduction of a threatened predator suppresses invasive mesopredators but eliminates a seabird colony. Biol Conserv 248:108635
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119(4):610–621
Watts C, Braithwaite R (1978) The diet of Rattus lutreolus and five other rodents in southern Victoria. Wildlife Res 5(1):47–57
Weimerskirch H (2001) Seabird demography and its relationship with the marine environment. Biol Mar Birds 115–136
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16(8):454–459. https://doi.org/10.1016/S0169-5347(01)02194-2
Acknowledgements
We thank Kaylene Allen, Brett Woodruff, Conrad Daniels, of the Bruny Island Cat Management Program, Kingborough Council, and many volunteers for assistance in the field. We thank also Tasmanian Land Conservancy, Bruny Island Coastal Retreats, South Bruny Islands Parks and Wildlife and private landowners for access to properties.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The Project was funded by the Australian Research Council (ARC DP170101653 to MJ). CS was supported by a University of Tasmania PhD Scholarship and a top-up scholarship from Kingborough Council, Bruny Island Coastal Retreats, and Pennicott Wilderness Journeys. Funding sources had no involvement in study design, collection, analysis and interpretation of data, in writing of the report, or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
CS: Conceptualization, methodology, formal analysis, investigation, writing—original draft, visualization. CG: Conceptualization, methodology, formal analysis, investigation. CNJ: Conceptualization, methodology, writing—review and editing, supervision. MEJ: Conceptualization, methodology, writing—review and editing, supervision, funding acquisition. All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CS and CG. The first draft of the manuscript was written by CS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics statements
This work was carried out under the University of Tasmania Animal Ethics Permits: A0016370 & A0017365, Department of Primary Industries, Parks, Water and Environment Permits: FA17040 & FA18156 and with authority to undertake research on Forest, Crown and Park lands.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scomparin, C., Geale, C., Johnson, C.N. et al. Limited top–down effects of feral cats on rodent dynamics in a seabird colony. Biol Invasions 25, 3965–3981 (2023). https://doi.org/10.1007/s10530-023-03152-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03152-x