Abstract
A variety of human activities have been identified as driving factors for the release and spread of invasive earthworm species in North America. Population genetic markers can help to identify locally relevant anthropogenic vectors and provide insights into the processes of population dispersal and establishment. We sampled the invasive European earthworm species Lumbricus terrestris at nine sites and several bait shops within the metropolitan area of Minneapolis-St. Paul in Minnesota, USA. We used microsatellite markers to infer genetic diversity and population structure, and 16S rDNA to address multiple introduction events, including bait dumping, which is a common source of L. terrestris introductions into the wild. Our results indicate multiple introductions but not from current bait dumping. Overall, genetic structure was low and earthworms > 5,000 m apart were genetically differentiated, except for one sampling location, indicating jump-dispersal followed by population establishment. Further, earthworms at one location north of Minneapolis established from one or few founder individuals, suggesting that earthworm invasions are ongoing. We therefore encourage further monitoring of earthworm populations using molecular markers, in order to disentangle the different human-related vectors contributing to the spread of earthworms and their establishment, which is essential to develop adequate management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The invasion of North American forests by European earthworms is indicated by the short- and long-term environmental changes they cause. These lumbricid earthworms actively reduce the litter layer on the soil surface and mix the litter and humus layers, which indirectly reduces soil carbon and nitrogen over the long-term and depletes other soil minerals (Crumsey et al. 2015; Resner et al. 2015; Ferlian et al. 2020). Through their burrowing activity, they also influence the availability of nutrients to plants by disrupting mycorrhizal networks (Paudel et al. 2016; Dobson et al. 2017). Additionally, their presence in North American forests has facilitated the invasion of non-native plant species by selective seed predation and changes in nutrient availability (Nuzzo et al. 2009, 2015; Drouin et al. 2014; Craven et al. 2017). In consequence, non-native earthworms indirectly and directly affect nutrient availability to soil and aboveground communities and have irreversible consequences for forest ecosystems (Eisenhauer et al. 2007; Fisichelli et al. 2013; Fisichelli and Miller 2018; Ferlian et al. 2018; Frelich et al. 2019). Thus, it is important to identify potential introduction pathways in order to protect undisturbed native communities and to prevent future invasions.
The role of human transport for earthworm introductions and dispersal into the wild is well established. Disposal of earthworms used as fishing bait and passive transport by vehicles along roads have been identified as central drivers (Holdsworth et al. 2007; Keller et al. 2007; Cameron et al. 2007, 2008; Klein et al. 2017, 2020). Further, human disturbances seem to provide suitable conditions for non-native earthworm invasions, such that active or historic human settlements, construction sites, agricultural use, and recreational areas and fishing sites contain more non-native earthworms than wilderness and undisturbed sites (Hendrix et al. 2006, 2008; Callaham et al. 2016; Rogers and Collins 2017; Arcese and Rodewald 2019). The identification of vulnerable sites and vectors for earthworm invasions is necessary to formulate containment guidelines, and knowledge of the local earthworm population structure is required to understand how invasive earthworms establish viable populations.
Introduction from distant or locally adapted populations can increase genetic diversity, allowing populations to mitigate founder-effects and increase the potential of adaptation to local environmental conditions (Lawson Handley et al. 2011). Genetic studies of non-native earthworms in northern North America clearly link them to their European or Asian sources (Gailing et al. 2012; Fernandez et al. 2016; Schult et al. 2016) and demonstrate that genetic diversity of populations is generally high (Gailing et al. 2012; Porco et al. 2013; Schult et al. 2016; Keller et al. 2020), particularly in areas used by humans (Cameron et al. 2008; Klein et al. 2017). Notably, North America is experiencing a second earthworm invasion by pheretimoid earthworms of Asian origin. Arrival of Asian species in areas already invaded by European earthworms is not unusual (Greiner et al. 2012; Szlavecz et al. 2018) and may result in changes in community dynamics, ecological processes, and ecosystem functions with unforeseen consequences, depending on the combination of species that interact. Three species, Amynthas agrestis (Goto and Hatai, 1899), Amynthas tokioensis (Beddard, 1892), and Metaphire hilgendorfi (Michaelsen, 1892), have expanded their range north- and westwards into states with cold winters for the last ten to twenty years (Chang et al. 2018; Moore et al. 2018; McAlpine et al. 2022). These Asian species are more flexible in their diet and similar to European species, they also alter soil structure, carbon dynamics, and nutrient concentrations in North American forests (Zhang et al. 2010; Snyder et al. 2011, 2013; Greiner et al. 2012; Qiu and Turner 2017; Chang et al. 2018; McCay et al. 2020). Studies that investigated interspecific effects in the field and in mesocosm-experiments of Asian and European earthworms indicate that, depending on which species interact, Asian earthworms can replace the epigeic species L. rubellus (Zhang et al. 2010; Chang et al. 2016; but see also Greiner et al. 2012), induce different changes in microbial communities (Chang et al. 2017), and show higher leaf litter consumption than each species on its own (Crone et al. 2022). To control further spread, anticipate consequences of the two earthworm invasions, and develop management plans, more field studies on the ecological consequences are needed. The state of establishment and genetic diversity of non-native earthworm populations is also relevant in this context. Permanent populations that have been established for a longer time are genetically more diverse and therefore may be more resilient against additional invasions compared to transient or instable populations, that experience genetic bottlenecks and founder events more strongly. The population structure of free-living earthworms can be investigated with molecular markers that are able to resolve the genetic variability populations and relatedness among individuals, which helps to identify hotspots of multiple introductions, and to infer patterns of dispersal or the direction of transport.
In this study, we investigated the population structure of Lumbricus terrestris Linnaeus, 1758 within the metropolitan area of Minneapolis-St. Paul, Minnesota, USA. This species has been reported in Minnesota since the second half of the twentieth century (Gates 1978; Reynolds et al. 2002), and thus is ideal for studying genetic structure within and among populations. Our sampling sites included recreational and agricultural sites, and were both distant and close to road networks. We sampled earthworms at three locations within a radius of about 50 km, and three subpopulations at each location. Individual earthworms were genotyped with eight microsatellite markers to investigate the incidences and the range of dispersal, as well as genetic diversity within populations. We also included earthworms from five bait shops within the sampling area in order to assess the role of bait dumping as a potential source of free-living earthworm populations. To address multiple introduction events, we sequenced the mitochondrial 16S rDNA gene of all genotyped individuals. First, we analysed population differentiation of L. terrestris, which is a poor disperser that concentrates its activity within a small radius around its burrows (Sims and Gerard 1999; Tiunov et al. 2006; Addison 2009; Potvin and Lilleskov 2017), and therefore relies on passive dispersal to cover larger distances. Second, we analysed population structure to check if alleles are shared among locations, which indicates admixture. Third, we analysed population structure based on 16S rDNA to infer the number of genetic lineages at sampling sites and to identify potential hotspots of multiple introductions.
Methods
Collection sites, collecting methods and DNA isolation
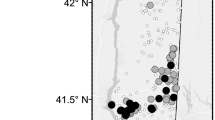
Three populations of L. terrestris were collected in forest habitats between May and July 2014 at a distance of 25–75 km from the urban area of Minneapolis-St.-Paul, MN, USA (Table 1, Fig. 1). At each sampling site (labelled as subpopulation _1), two additional replicates were taken at 5 m (labelled as subpopulation _2) and 5,000 m (labelled as subpopulation _3) distance, dividing each population into three subpopulations. Adult and juvenile earthworms were collected from plots of 0.5 × 0.5 m using mustard extraction (Gunn 1992) and hand-sorting. Sampling site MN_0 was about 70 km south of Minneapolis and located in a woodland, intersected by a single gravel road that was surrounded by farmland, near Interstate 35 (Fig. 1). Sampling site MN_50 was located in a suburban area of Minneapolis approximately 100 km north-west from MN_0. Several lakes and parks for recreational activities are located in this area. The third sampling location MN_100 was about 100 km north-east from MN_0 and located in an area characterised by agricultural fields and various lakes that are frequently visited by the local population for fishing and camping, and only accessible by small roads. The sampling site of MN_100_3 was located on a ridge that slopes down to the St. Croix River.
To investigate the importance of bait dumping as potential source for introductions, L. terrestris fishing baits were included from five different bait shops (five individuals per bait shop) within the sampling region. One centimetre of tail tissue of each sample was shipped from the United States to Germany for barcoding and further molecular analyses. Remaining body parts were stored as vouchers at the University of Minnesota (Department of Forest Resources).
16S rDNA sequencing and genotyping
Total genomic DNA was extracted using the Qiagen Blood and Tissue kit (Qiagen; Hilden, Germany) following the manufacturer’s protocol. To verify species identity of juvenile individuals, an 800 bp fragment of 16S rDNA was amplified using the primers 16S-LumbF and Ho_16Sra (Pèrez-Losada et al. 2009), which contain a barcoding region that reliably distinguishes lumbricid species (Bienert et al. 2012; Klarica et al. 2012). All PCR reactions for sequencing were performed in 25 μl volumes containing 11.75 μl ultrapure H2O, 1.25 μl BSA (∼4%), 2.5 μl Buffer with KCl, 1 μl dNTPs (10 mM), 3.5 μl MgCl2 (25 mM), 0.5 μl Taq polymerase (5 U/μl; Thermo Scientific; Dreieich, Germany), 1 μl of each primer (10 mM), and 2.5 μl template DNA. The PCR protocol consisted of an initial activation step at 95 °C for 3 min, 40 amplification cycles (denaturation at 95 °C for 30 s, annealing at 53 °C for 60 s, and elongation at 72 °C for 60 s), and a final elongation step at 72 °C for 10 min. PCR products were analysed with multi-capillary electrophoresis using QIAxcel (Qiagen; Hilden, Germany). Some of the PCR products performed inadequately in terms of amplification success and PCRs were repeated in 25 μl volumes containing 2 μl ultrapure H2O, 1.5 μl MgCl2 (25 mM), 12.5 μl HotStarTaq Mastermix (Genaxxon; Ulm, Germany), 2 μl of each primer (10 mM), and 5 μl template DNA. This PCR protocol consisted of an initial activation step at 92 °C for 15 min, 40 amplification cycles as described above but with an annealing temperature of 56 °C. PCR products were checked using QIAxcel and positive products were purified using the QIAquick PCR Purification kit (Qiagen; Hilden, Germany) and were either sequenced at the Göttingen Genome Sequencing Laboratory (University of Göttingen) or by Macrogen Europe Inc. (Amsterdam, The Netherlands). All 16S rDNA sequences were checked and ambiguous positions were corrected using electropherograms in Geneious 8.0.5 (https://www.geneious.com), and are available with the accession numbers ON506389 to ON506413 (bait shop individuals) and ON506269 to ON506388 (field collected animals) at NCBI GenBank (www.ncbi.nlm.nih.gov).
We amplified eight highly polymorphic microsatellite loci from L. terrestris (LTM 026, LTM 128, LTM 163, LTM 165, LTM 187, LTM 193, LTM 252, and LTM 278; Velavan et al. 2007), using Taq polymerase (Thermo Scientific; Waltham, MA, USA) or the HotStarTaq Mastermix (Genaxxon; Ulm, Germany) following the protocol of Velavan et al. (2007) and Klein et al. (2017). Genotyping of multi locus genotypes (MLGs) was performed at the Department of Animal Sciences, Veterinary Medicine, University of Göttingen, using FAM-labelled forward primers (Sigma Aldrich; St. Louis, MO, USA). Peak calling and allele binning was performed in Geneious, potential genotyping errors (scoring errors, stutter, and presence of null alleles) were checked with MICRO-CHECKER v2.2.3 (http://www.microchecker.hull.ac.uk/; van Oosterhaut et al. 2004). Three markers showed stuttering, and were subsequently checked and corrected manually in Geneious.
Population structure analyses
All 16S rDNA sequences were blasted using the nucleotide BLAST algorithm provided by NCBI GenBank to verify species identity of L. terrestris, which was particularly relevant for juvenile individuals included in this dataset. All sequences were corrected by eye and aligned in AliView v1.25 (Larsson 2014) using default settings and remaining wobble positions were conservatively corrected by eye i.e., we manually assigned the nucleotide that was present in all other sequences at this position in the alignment. The alignment was trimmed to the shortest sequence (516 bp) and collapsed to haplotypes with FaBox (Villesen 2007). Population structure was assessed with a haplotype network based on observed p-distances in R (R Core Team, 2020) using the ape and pegas packages (Paradis 2010; Paradis and Schliep 2019). Additionally, we calculated a Maximum Likelihood tree with 1,000 bootstrap replicates to check phylogenetic relatedness of 16S haplotypes using the ape and phangorn packages (Schliep 2011; Paradis and Schliep 2019). Additionally, we checked the identity of the sampled earthworm haplotypes with a phylogenetic analysis that included 628 16S sequences from NCBI database (L. castaneus = 10 sequences, L. centralis = 8 sequences, L. friendi = 10 sequences, L. herculeus = 6 sequences, L. polyphemus = 1 sequence, L. rubellus = 148 sequences, L. terrestris = 445 sequences). All NCBI sequences were aligned together with the 36 haplotypes of this study, trimmed to the shortest sequences, and positions that contained only Ns were excluded, resulting in a total length of 333 positions. The alignment was subsequently collapsed to haplotypes with FaBox resulting in 145 haplotypes (alignment files with accession numbers and haplotype datasets are available together with the R code in Dryad, https://doi.org/10.5061/dryad.w0vt4b8v2). Alignment and Maximum Likelihood analyses were performed as described above. Completeness of sampled haplotypes was analysed with a rarefaction analysis based on abundance data using the iNEXT package (Hsieh et al. 2016).
Population genetic analyses of microsatellites were performed using the adegenet package (Jombart 2008, Jombart and Ahmed 2001) in R. All earthworms from bait shops were assigned to a single population (Bait_Shop, 25 individuals) due to their high genetic similarity. For each microsatellite marker, we calculated the mean number of alleles (Na), and observed (Ho) and expected (He) heterozygosity. We tested for significant deviations from expected heterozygosity using the Bartlett test of homogeneity and for significant deviations from Hardy–Weinberg equilibrium (HWE) using a chi-square test. To estimate population differentiation and inbreeding, we calculated the standard diversity indices FST and FIS (fixation and inbreeding index, Weir and Cockerham F statistics), and pairwise FST values for four populations (MN_0, MN_50, MN_100 and Bait_Shop) and ten subpopulations (three subpopulations per population and Bait_Shop) using the hierfstat package (Goudet and Jombart 2020). To identify at which hierarchical level the most genetic variation existed, we performed an Analysis of Molecular Variance (AMOVA) using the poppr package v2.9.3 (Kamvar et al. 2014, 2015), and a Monte-Carlo test with 9,999 replications to test for significance. To identify genetic clusters, we did a Discriminant Analysis of Principal Components (DAPC, Jombart et al. 2010), using the xvalDAPC function for cross validation to decide how many axes to retain. This approach partitions genetic variation into a between- and a within-group component, and maximises the first component, thereby reducing the within-group variability, without relying on population genetic models.
We investigated population genetic structure using STRUCTURE v2.3.4 (Pritchard et al. 2000) which, in contrast to DAPC, assigns population to groups that best represent Hardy–Weinberg equilibrium, using a Bayesian sampling approach. Several preliminary runs were performed using various model configurations for optimal prior settings using the LOCPRIOR option. All other settings remained in default mode. Each run comprised ten iterations of the value of K that ranged between 1 and 10 with 105 MCMC burn-in steps and 106 replicates. The most likely number of genetic clusters (ΔK) for all runs of K was calculated in STRUCTURE HARVESTER (Evanno et al. 2005). The greedy algorithm in CLUMPP v1.12 (Jakobsson and Rosenberg 2007) was used to obtain a single mode of K corresponding to the maximum ΔK. Barplots were generated with DISTRUCT v1.1 (Rosenberg 2004).
Results
Mitochondrial 16S rDNA
Clear 16S rDNA sequences were obtained for 145 earthworms, 120 from the three sites and 25 from bait shops (Table 1). All haplotypes of this study clustered with L. terrestris’ sequences from NCBI but never with other congeners (Supplementary Information, Fig. S1), supporting correct identification of all sampled individuals. However, it is still possible that L. terrestris is a species complex (Martinsson and Erséus 2017), which cannot be discerned ultimately with this nucleotide alignment because the aligned and trimmed 16S fragment is relatively short. Notably, many haplotypes of this study were identical to earthworms sampled from Alberta (Klein et al. 2017, 2020) and some with earthworms from Klarica et al. (2012). Sanger sequences of 15 individuals were of bad quality and were not included in the 16S rDNA dataset, but microsatellites amplified well for all individuals. These failed 16S PCRs corresponded to few individuals across all sampling sites, except MN_0_1 and individuals collected from bait shops. Sequencing failures can have many reasons, ranging from buffer or ethanol remains to mutations at the primer binding sites or in the fragment causing poor primer annealing or secondary structures that hamper the sequencing reactions, respectively. We cannot exclude that these missing individuals were genetically distant or even cryptic species of L. terrestris, but we consider this as unlikely, as microsatellite markers worked well for these individuals and did not result in unusual genotypes. Overall, the 16S rDNA sequences showed a maximum p-distance of 3.9%; and the 145 individual sequences were collapsed into 36 haplotypes (Table 1). Rarefaction analysis showed that about 50% of potential haplotypes were sampled and the extrapolation curve started to reach saturation at expected 60 haplotypes if 500 individuals were sampled (Supplementary Information, Fig. S2).
Three haplotypes were very common. The most common (18 individuals) was present in two bait shop individuals and in all subpopulations except MN_100_3. Two other haplotypes were also very common (both with 17 individuals), but were less widespread. One occurred in four subpopulations (MN_0_3, MN_50_1, MN_50_2, MN_100_1) and in one bait shop individual. The other was present in six subpopulations (MN_0_3, MN_50_1, MN_50_2, MN_50_3, MN_100_1, MN_100_3) and in one bait shop individual. Subpopulation MN_50_3 had the highest number of different haplotypes relative to the number of individuals sampled (14 individuals with nine haplotypes), followed by subpopulations MN_100_1 and MN_100_2 (seven haplotypes in twelve and 13 individuals, respectively). Subpopulation MN_100_3 had the lowest number of haplotypes, all but one individual had identical 16S rDNA sequences, the other haplotype in this subpopulation was genetically distinct (p-distance = 1.9%) and co-occurred only in a single bait shop individual.
The haplotype network showed no clear structure among populations or bait-shop and field-collected individuals (Fig. 2). Subpopulations included both unique and frequent haplotypes that co-occurred in other subpopulations. Earthworms obtained from bait shops carried six haplotypes that were not identical with field collected haplotypes, but also seven haplotypes that were identical with individuals from the field. The maximum likelihood analysis showed seven well-supported clades (bootstrap support > 90%, Supplementary Information, Fig. S3), five of these clades included bait-shop and field-collected individuals and two clades comprised five individuals that were exclusively sampled from bait shops but which were distinct from field-collected individuals.
Haplotype network of 16S rDNA sequences collected from the field and bait shops in the St. Paul-Minneapolis area, MN (USA). The network shows that seven distinct lineages are present in the field, some are rare (small circles representing one individual), others are widely distributed (large circles representing several individuals from different subpopulations). The genetic distinctness among haplotypes is reflected by the length of the lines connecting circles, and dots on lines represent hypothetical mutation steps. The size of circles is relative to the number of individuals represented by this haplotype. The dashed boxes outline well-supported clades from the Maximum Likelihood tree, black numbers in boxes are bootstrap support values
Nuclear microsatellites
The microsatellite dataset contained 160 individuals and eight loci. The number of alleles per locus across all populations was similar and ranged from 17 (LTM128) to 22 (LTM278), except for locus LTM252 which had 34 alleles (Supplementary Information, Fig. S4a). In all but two loci (LTM026 and LTM187), the observed heterozygosity was lower than the expected heterozygosity (Supplementary Information, Fig. S4b). Further, all loci deviated significantly from HWE and the mean observed heterozygosity varied significantly among loci (Bartlett’s test: p = < 0.05). The PCR reactions for microsatellite markers performed well on the collected specimens for eight out of the ten published microsatellite primers developed by Velavan et al. (2007). This contrasts with other studies that used these markers in Lumbricus terrestris individuals across a larger geographic range i.e., from Michigan, Maryland and Maine in the United States and Europe (Gailing et al. 2012), France and Poland (Souleman et al. 2016) and Vermont, USA (Keller et al. 2020). However, MICRO-CHECKER detected potential null alleles for five markers (LTM 128, LTM 163, LTM 165, LTM 252 and LTM 278), and potential genotyping errors (stuttering) for the three markers LTM 128, LTM163 and LTM165 (see Supplementary Information Tables S2, S3 for number of alleles per locus and populations and comparison with Velavan et al. 2007), which were checked and corrected manually in Geneious. We continued the analyses with all loci because deviations from Hardy–Weinberg equilibrium can have other causes than null alleles, including inbreeding or Wahlund effect, in particular for small populations (Dabrowski et al. 2013; De Meeûs 2017).
Each field collected population consisted of 45 individuals (15 individuals per subpopulation, three subpopulations per site), and the pooled Bait_Shop population consisted of 25 individuals. Population MN_100 had the highest numbers of alleles (Na = 117; Supplementary Information, Fig. S4c), closely followed by populations MN_0 (Na = 112) and MN_50 (Na = 111). Bait shop individuals had considerably fewer numbers of alleles (Na = 90), but sample size was also lower. When comparing the ten subpopulations (Supplementary Information, Fig. S4d), MN_100_1 had the highest number of alleles (Na = 87) and MN_100_3 had the lowest number of alleles (Na = 62). The number of alleles of the three subpopulations at MN_0 and subpopulation MN_50_3 ranged between 72 and 75. The number of alleles of the remaining subpopulations were lower with Na = 66 (MN_50_1) and Na = 65 (MN_50_2 and MN_100_2). The global FST and FIS values, that take all genotypes and all loci into account, were smaller for the four populations (Supplementary Information, Fig. S4e), with FST = 0.045 and FIS = 0.089 compared to the ten subpopulations dataset (Supplementary Information, Fig. S4f) with values of FST = 0.068 and slightly lower FIS = 0. 0.062.
The mean pairwise FST values between populations were also very close to zero, ranging between 0.033 and 0.058 (Supplementary Information, Table S1). However, the range of pairwise FST values was greater at subpopulation level, ranging between 0.013 and 0.139 (Table 2).
The AMOVA was significant at all levels and showed that genetic variance was quite low between populations and subpopulations. The majority of variance (89%) was found within samples (individuals). Variance between subpopulations within populations was low (4.9%), and variance between individuals within sites was even lower (3.7%). The variance was lowest between populations (2.0%).
The DAPC cross-validation peaked around 40 PC axes. Closer examination showed that the clustering patterns were consistent after reducing the number of PC axis around that value for both, four populations (33 PC axes retained, eigenvalues of LD1 = 181.3 and LD2 = 145.0) and for ten subpopulations (33 PC axes retained, eigenvalues of LD1 = 80.2 and LD2 = 74.3). Clustering patterns showed that most subpopulations were very similar and overlapped in one cluster (Fig. 3), except for subpopulation MN_100_3, which separated from all other subpopulations at the first axis, and bait shop individuals, which separated from all other subpopulations at the second axis. The analysis with four populations (Supplementary Information, Fig. S5) showed similar clustering patterns in which population MN_0 and MN_50 overlapped in one cluster, and MN_100 and bait shop individuals represented the most distinct genetic clusters on the first and second axes.
Discriminant analysis of principal components (DAPC) for 160 individuals of Lumbricus terrestris from ten subpopulations in the urban area of Minneapolis-St. Paul, MN (USA). The axes represent the first two Linear Discriminants (LD). Each circle represents a cluster and each dot represents an individual. The subpopulations weakly differentiated along the first two axes, but rather overlap with each other, except for subpopulation MN_100_3 and individuals collected from bait shops (MN_Baits)
Population assignment in STRUCTURE and STRUCTURE HARVESTER indicated that the sampling area most likely comprised five genetically differentiated populations (K = 5, Fig. 4). Four clear patterns occurred. First, subpopulations 5 m apart (subpopulations _1 and _2 per sampling site) were most similar. Second, at sampling sites MN_50 and MN_100, subpopulations at 5,000 m distance were considerably differentiated from the other two subpopulations at the same site. Third, sampling sites MN_0_1-3 and subpopulation MN_50_3 represented one genetic cluster, which was consistent across analyses with other K-values. Finally, subpopulation MN_100_3 and bait shop individuals (Bait_Shop) also represented discrete clusters.
Discussion
Using nuclear and mitochondrial markers, this study investigated the genetic structure of the invasive earthworm species L. terrestris in the metropolitan area of Minneapolis-St.Paul. Our results show that allelic differentiation and genetic variance of the nuclear markers was rather low between populations of L. terrestris, indicating that these populations are a homogeneous group of genetically similar individuals. This suggests that these populations are established and likely interbreed. However, across all populations, the numbers of alleles and mitochondrial lineages was high, demonstrating that overall genetic diversity is substantial in L. terrestris, which is consistent with findings of earlier genetic studies in this species (Gailing et al. 2012; Klein et al. 2017; Keller et al. 2020). Populations of invasive species may initially exhibit low genetic diversity due to small propagule size. In cases of multiple introductions from different origins, populations can quickly overcome founder effects and can demonstrate even higher genetic diversity than in their home ranges, which in turn increases their adaptative potential to new environments (Sakai et al. 2001; Lawson Handley et al. 2011; Keller et al. 2020). The combination of low genetic structure but high genetic diversity and presence of characteristic genotypes in populations is interesting. First, earthworms represented one, well admixed population at five metre distance, showing that earthworms migrate and interbreed at short distances on their own. At five kilometres distance, genotypes changed except for one population, indicating that new populations start from few individuals after being transported by chance. Second, the high genetic diversity and low genetic structure among populations at larger distances suggests that transport (passive or active) occurs in all directions, eventually mixing populations genetically when newly arriving and established individuals interbreed. Third, the low genetic structure among populations indicates that transport of individuals occurred frequently and that populations with well mixed genotypes have been established for a long time.
We could not identify hotspots of introductions, because several mitochondrial lineages were common and widespread among populations. This suggests that dispersal or short-distance transport of earthworms may occur at random in many directions, and that individuals may interbreed after transport with already present earthworms. Apart from human mediated transport, earthworm predators such as birds, shrews or snakes also likely function as vectors for random transport over short distances. Further, isolated mitochondrial haplotypes that were only present in single individuals were also common, suggesting recurrent transport or introductions of earthworms in the field which was not followed by successful breeding.
Alternative to multiple introductions with admixture, the high number of mitochondrial haplotypes accompanied by low genetic variance at nuclear markers within populations could be a result of the special reproductive mode of earthworms. Lumbricus terrestris is a hermaphrodite with reciprocal insemination, resulting in fertilisation of eggs and production of cocoons in each individual after mating (Edwards and Bohlen 1996). Consequently, it may be that a pair of earthworms may produce offspring that carries similar, admixed nuclear genomes but different mitochondrial lineages, due to the maternal transmission of mitochondria as opposed to the biparental inheritance of nuclear genes. It is also possible that some earthworms invest more in sperm and less in eggs, or mate unsuccessfully, which may result in the presence of isolated mitochondrial lineages.
The population structure found for nuclear markers indicates that different dispersal processes acted on these earthworm populations. First, one subpopulation (MN_50_3) belonged genetically to a population 100 km south-east of its sampling site (MN_0), and not to the nearby subpopulations 5 km away, indicating effective jump dispersal, which is common in earthworms (Cameron et al. 2008; Klein et al. 2020). Second, one subpopulation in the north-west of the sampling area (MN_100_3) was also genetically differentiated from the nearby subpopulations 5 km away, but remained isolated in the population structure analyses and was dominated by one mitochondrial lineage, suggesting a recent founder event by passive dispersal of one or few individuals or a genetic bottleneck as this site is prone to recurrent flooding by the nearby St. Croix River. Third, individuals collected from bait shops separated genetically from all other subpopulations indicating that the free-living populations did not establish from recent or current cases of bait-dumping. Baits sold from shops or from private vermicultures that were not included in our sampling could also be a possible source for free-living earthworms. However, the high genetic similarity of earthworms sold in the five local bait shops suggests that local retailers purchased their products from a common source, such as a central distributor or earthworm farm. The genetic similarity of all bait shop individuals and their clear differentiation from free-living earthworms instead suggests that free-living earthworm populations derived from other sources or from releases of baits that were sold in the past. These patterns strongly contrast with findings from a study in Calgary, Alberta, Canada (Klein et al. 2017), which analysed L. terrestris populations from the field and bait shops using a similar study design and markers. They found that bait shop genotypes formed a genetic cluster with the urban population, indicating that bait disposal was an important source for free-living earthworm populations. Further, their populations were considerably more differentiated from each other than populations in this study, suggesting that free-living populations in Canada are rather recent introductions and with lower incidences of effective dispersal (dispersal with breeding), which is consistent with the more recent history of earthworm invasions in Alberta compared to Minnesota (Hale 2008; Klein et al. 2017).
The relevance of human activities for dispersal and establishment of earthworm populations is well known (Holdsworth et al. 2007; Keller et al. 2007; Cameron et al. 2007, 2008; Klein et al. 2017, 2020). The next step for investigating earthworm invasions is to disentangle the different anthropogenic vectors acting on local populations in order to manage these invasions. Once established, it is impossible to remove earthworm populations, and the development of management strategies to confine the invasion therefore remains the primary option when faced with established populations (Keller et al. 2007; Lawson Handley et al. 2011). The well-known link between baits and free-living populations (Holdsworth et al. 2007; Keller et al. 2007; Cameron et al. 2008; Klein et al. 2017) could not be identified in this study. Possibly, public awareness and educational campaigns reduced bait disposal in the past decade (Callaham et al. 2006; Keller et al. 2007). These campaigns are dedicated to education around earthworms and their effect and exist in Minnesota and the upper Midwest (e.g., https://wormwatch.d.umn.edu). They also distribute information to anglers about the importance of proper bait disposal. Other human-related factors that correlate with earthworm invasions are farming (Keller et al. 2007; Klein et al. 2020), logging and proximity to roads (Dymond et al 1997; Gundale et al. 2005; Suarez et al. 2006; Holdsworth et al. 2007; Hendrix et al. 2008; Cameron et al. 2008; Callaham et al. 2016; Roger and Collins 2017). Further, genetic differentiation between populations at small scale (0.6–13 km) is common in L. terrestris (Klein et al. 2017; Keller et al. 2020), but was not present in the population we sampled in the south from an area of intense agriculture (MN_0). Non-directional transport of earthworms with farm equipment and soil-movement by tillage practices could explain the genetic homogeneity and connectivity of this population, suggesting that farming facilitates the dispersal of L. terrestris and likely that of other invasive earthworm species such as Aporrectodea caliginosa, that are less sensitive to disturbances (Ivask et al. 2007; Smith et al. 2008).
Apart from identifying source populations and disentangling invasion routes, it is also important to understand what determines the success of invasive non-native species in order to implement successful management strategies (Lawson Handley et al. 2011). Here, earthworm populations consisted of both common and widespread, but also rare and isolated mitochondrial lineages, which is consistent with other molecular studies on invasive earthworm species in North America (Cameron et al. 2008; Gailing et al. 2012; Porco et al. 2013; Schult et al. 2016; Klein et al. 2017, 2020; Keller et al. 2017, 2020). Why some mitochondrial lineages thrive and reproduce and others do not represents one of the most fundamental questions of invasion biology (Lawson Handley et al. 2011). Repeated sampling of populations that contained the most widespread haplotypes, but also of populations that contained many isolated haplotypes would allow to observe changes in the frequency of genetic lineages over time and thereby monitor the viability of earthworm lineages.
This study revealed that earthworm populations in the metropolitan region of Minneapolis-St. Paul, Minnesota, are established and that different patterns of population structure, introductions and dispersal co-occurred, demonstrating that molecular markers are a valuable tool to study invasion patterns of earthworms. However, this study also showed that additional sampling is required to disentangle different human-related mechanisms of earthworm dispersal. Transcriptome screening for selective sweeps in different genetic lineages could eventually help to identify whether different lineages acquired adaptations that correlate with local invasions and how populations cope with founder effects, bottlenecks and local inbreeding (Gailing et al. 2012; Klein et al. 2017, 2020; Keller et al. 2020). The advances in sequencing technologies provide a valuable toolkit to launch new studies on invasive earthworm populations in order to infer the invasion success of some lineages as opposed to others, and will ultimately aid in developing management strategies to limit earthworm invasions.
Availability of data and material
All 16S rDNA sequences are available at GenBank (www.ncbi.nlm.nih.gov) under the accession numbers ON506389 to ON506413 (bait shop individuals) and ON506269 to ON506388 (field collected animals). Please, also check information in Code availability.
Code availability
The R code used for data analysis is available at Dryad (https://doi.org/10.5061/dryad.w0vt4b8v2) or from the corresponding author on request. The files include the following data and analyses: aligned 16S rDNA dataset of this study for phylogeny and haplotype networks, aligned 16S rDNA dataset of this study and sequences downloaded from NCBI, rarefaction analysis, microsatellite data and analyses of this study and microsatellite data from Klein et al. 2017 to compare population structures.
References
Addison JA (2009) Distribution and impacts of invasive earthworms in Canadian forest ecosystems. Biol Inv 11:59–79
Arcese P, Rodewald AD (2019) Predictors and consequences of earthworm invasion in a coastal archipelago. Biol Inv 21:1833–1842
Bienert F, De Danieli S, Michel C, Coissac E, Poillot C, Brun J-J, Taberlet P (2012) Tracking earthworm communities from soil DNA. Mol Ecol 21:2017–2030
Callaham MA, González G, Hale CM, Heneghan L, Lachnicht SL, Zou X (2006) Policy and management responses to earthworm invasions in North America. Biol Inv 8:13317–21329
Callaham MA, Snyder BA, James SW, Oberg ET (2016) Evidence for ongoing introduction of non-native earthworms in the Washington, DC metropolitan area. Biol Inv 18:3133–3136
Cameron EK, Bayne EM, Clapperton MJ (2007) Human-facilitated invasion of exotic earthworms into northern boreal forests. Ecoscience 14:482–490
Cameron EK, Bayne EM, Coltman DW (2008) Genetic structure of invasive earthworms Dendrobaena octaedra in the boreal forest of Alberta: insights into introduction mechanisms. Mol Ecol 17:1189–1197
Chang C-H, Szlavecz K, Filley T, Buyer JS, Bernard MJ, Pitz S (2016) Belowground competition among invading detritivores. Ecology 97:160–170
Chang C-H, Szlavecz K, Buyer JS (2017) Amynthas agrestis invasion increases microbial biomass in Mid-Atlantic deciduous forests. Soil Biol Biochem 114:189–199
Chang C-H, Johnston MR, Görres JH, Dávalos A, McHugh D, Szlavecz K (2018) Co-invasion of three Asian earthworms, Metaphire hilgendorfi, Amynthas agrestis and Amynthas tokioensis in the USA. Biol Inv 20:843–848
Chang C-H, Bartz MLC, Brown G et al (2021) The second wave of earthworm invasion in North America: biology, environmental impacts, management and control of invasive jumping worms. Biol Inv 23:3291–3322
Craven D, Thakur MP, Cameron E et al (2017) The unseen invaders: introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis). Global Change Biol 23:1065–1074
Crone ER, Sauer EL, Herrick BM, Drake D, Preston DL (2022) Effects of invasive jumping worms (Amynthas spp.) on microhabitat and trophic interactions of native herpetofauna. Biol Inv 24:2499–2512
Crumsey JM, Capowiez Y, Goodsitt MM, Larson S, Le Moine JM, Bird JA, Kling GW, Nadelhoffer KJ (2015) Exotic earthworm community composition interacts with soil texture to affect redistribution and retention of litter-derived C and N in northern temperate forest soils. Biogeochemistry 126:379–395
Dabrowski MJ, Pilot M, Kruczyk M, Zihorsi M, Umer HM, Gliwicz ZJ (2013) Reliability assessment of null detection: inconsistencies between and within different methods. Mol Ecol Res 14:361–373
De Meeûs T (2017) Revisiting Fis, Fst, Wahlund effects, and null alleles. J Heredity 109:446–456
Dobson AM, Blossey B, Richardson JB (2017) Invasive earthworms change nutrient availability and uptake by forest understory plants. Plant Soil 421:175–190
Drouin M, Bradley R, Lapointe L, Whalen J (2014) Non-native anecic earthworms (Lumbricus terrestris L.) reduce seed germination and seedling survival of temperate and boreal tree species. Appl Soil Ecol 75:145–149
Dymond P, Scheu S, Parkinson D (1997) Density and distribution of Dendrobaena octaedra (Lumbricidae) in aspen and pine forests in the Canadian Rocky Mountains (Alberta). Soil Biol Biochem 29:265–273
Edwards CA, Bohlen PJ (1996) Biology and ecology of earthworms. Chapman and Hall, London
Eisenhauer N, Partsch S, Parkinson D, Scheu S (2007) Soil Biol Biochem 39:1099–1110
Evanno G, Regnaut, s., Goudet, J. (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Ferlian O, Eisenhauer N, Aguirrebengoa M, Camara M, Ramirez-Rojas I, Santos F, Tanalgo K, Thakur MP (2018) Invasive earthworms erode soil biodiversity: a meta-analysis. J Animal Ecol 87:162–172
Ferlian O, Thakur MP, Castañeda González A, San Emeterio LM, Marr S, Da Silva RB, Eisenhauer N (2020) Soil chemistry turned upside down: a meta-analysis of invasive earthworm effects on soil chemical properties. Ecology 101:e02936
Fernández R, Novo M, Marchán DF, Díaz Cosín DJ (2016) Diversification patterns in cosmopolitan earthworms: similar mode but different tempo. Mol Phyl Evol 94:701–708
Fisichelli NA, Miller KM (2018) Weeds, worms, and deer: positive relationships among common forest understory stressors. Biol Inv 20:1337–1348
Fisichelli NA, Frelich LE, Reich PB, Eisenhauer N (2013) Linking direct and indirect pathways mediating earthworms, deer, and understory composition in Great Lakes forests. Biol Inv 15:1057–1066
Frelich LE, Blossey B, Cameron EK, Dávalos A, Eisenhauer N, Fahey T, Ferlian O, Groffmann PM, Larson E, Loss SR, Maerz JC, Nuzzo V, Yoo K, Reiche PB (2019) Side-swiped: ecological cascades emanating from earthworm invasion. Front Ecol Environ 17:502–510
Gailing O, Hickey E, Lilleskov E, Szlavecz K, Richter K, Potthoff M (2012) Genetic comparisons between North American and European populations of Lumbricus terrestris L. Biochem Syst Ecol 45:23–30
Gates GE (1978) The earthworm genus Lumbricus in North America. Megadrilogica 3:81–116
Goudet J, Jombart T (2020) hierfstat: estimation and tests of hierarchical F-statistics. R package version 0.5–7
Greiner HG, Kashian DR, Tiegs SD (2012) Impacts of invasive Asian (Amynthas hilgendorfi) and European (Lumbricus rubellus) earthworms in a North American temperate deciduous forest. Biol Inv 14:2017–2027
Gundale MJ, Jolly WM, Deluca TH (2005) Susceptibility of a northern hardwood forest to exotic earthworm invasion. Cons Biol 19:1075–1083
Gunn A (1992) The use of mustard to estimate earthworm populations. Pedobiologia 36:65–67
Hale CM (2008) Evidence for human-mediated dispersal of exotic earthworms: support for exploring strategies to limit further spread. Mol Ecol 17:1165–1169
Hale CM, Frelich LE, Reich PB (2005) Exotic European earthworm invasion dynamics in northern hardwood forests of Minnesota, USA. Ecol Appl 15:848–860
Hendrix PF, Baker GH, Callaham MA Jr, Damoff GA, Fragoso C, González G, James SW, Lachnicht SL, Winsome T, Zou W (2006) Invasion of exotic earthworms into ecosystems inhabited by native earthworms. Biol Inv 8:1287–1300
Hendrix PF, Callaham MA Jr, Drake JM, Huang C-Y, James SW, Snyder BA, Zhang W (2008) Pandora’s box contained bait: the global problem of introduced earthworms. Annu Rev Ecol Evol Syst 39:593–613
Holdsworth AR, Relich LE, Reich PB (2007) Effects of earthworm invasion on plant species richness in Northern hardwood forests. Cons Biol 21:997–1008
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for interpolation and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456
Ivask M, Kuu A, Sizov E (2007) Abundance of earthworm species in Estonian arable soils. Europ J Soil Biol S39-S42
Jakobsson M, Rosenberg NA (2007) CLUMM: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806
Jombart T (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405
Jombart T, Ahmed I (2011) adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics 27:3070–3071
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:94
Kamvar ZN, Tabima FJ, Grünwald NJ (2014) poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2:e281
Kamvar ZN, Brooks JC, Grünwald NJ (2015) Novel R tools for analysis of genome-wide population genetic data with emphasis on clonality. Front Genet 6:208
Keller RP, Cox AN, van Loon C, Lodge DM, Herborg L-M, Rothlisberger J (2007) From bait shops to the forest floor: earthworm use and disposal by anglers. Am Midl Nat 158:321–328
Keller EL, Göres JH, Schall JJ (2017) Genetic structure of wo invasive earthworms, Amynthas agrestis and Amynthas tokioensis (Oligochaeta, Megascolecidae), and a molecular method for species identification. Megadrilogica 22:140–149
Keller EL, Conolly ST, Görres JH, Schall JJ (2020) Genetic diversity of an invasive earthworm, Lumbricus terrestris, at a long-term trading crossroad, the Champlain Valley of Vermont, USA. Biol Inv 22:1723–1735
Klarica J, Kloss-Brandstätter A, Traugott M, Juen A (2012) Comparing four mitocondrial genes in earthworms – implications for identification, phylogenetics, and discovery of cryptic species. Soil Biol Biochem 45:23–30
Klein A, Cameron EK, Heimburger B, Eisenhauer N, Scheu S, Schaefer I (2017) Changes in the genetic structure of an invasive earthworm species (Lumbricus terrestris, Lumbricidae) along an urban-rural gradient in North America. Appl Soil Ecol 120:265–272
Klein A, Eisenhauer N, Schaefer I (2020) Invasive lumbricid earthworms in North America – different life histories but common dispersal? J Biogeogr 47:674–685
Larsson A (2014) AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30:3276–3278
Lawson Handley Estoup A, Evans DM, Thomas CE, Lombaert E, Facon B, Aebi A, Roy HE (2011) Ecological genetics of invasive alien species. Biol Control 56:409–428
Martinsson S, Erséus C (2017) Cryptic speciation and limited hybridization within Lumbricus earthworms (Clitellata: Lumbricidae). Mol Phyl Evol 106:18–27
McAlpine DF, Reynolds JW, Manzer LR, Elton K (2022) First reports of invasive pheretimoid earthworms (Oligochaeta: Megascolecidae) of Asian origin in Atlantic Canada. BioInv Rec 11:830–838
McCay TS, Brown G et al (2020) Tools for monitoring and study of peregrine pheretimoid earthworms (Megascolecidae). Pedobiologia 83:150669
Moore J-D, Görres JH, Reynolds JW (2018) Exotic Asian pheretimoid earthworms (Amynthas spp., Metaphire spp.): potential for colonisation of south-eastern Canada and effects on forest ecosystems. Environ Rev 26:113–120
Nuzzo VA, Maerz JC, Blossey B (2009) Earthworm invasion as the driving force behind plant invasion and community change in Northeastern North American forests. Cons Biol 23:966–974
Nuzzo V, Dávalos A, Blossey B (2015) Invasive earthworms shape forest seed bank composition. Diversity Distrib 21:560–570
Paradis E (2010) pegas: an R package for population genetics with an integrated-modular approach. Bioinformatics 26:419–420
Paradis E, Schliep K (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528
Paudel S, Longcore T, MacDonal B, McCormick MK, Szlavecz K, Wilson GWT, Loss SR (2016) Belowground interactions with aboveground consequences: invasive earthworms and arbuscular mycorrhizal fungi. Ecology 97:605–614
Pérez-Losada M, Ricoyb M, Marscshal JC, Domíngues J (2009) Phylogenetic assessment of the earthworm Aporrectodea caliginosa species complex (Oligochaeta: Lumbricidae) based on mitochondrial and nuclear DNA sequences. Mol Phyl Evol 52:293–302
Porco D, Decaens T, Deharveng L, James SW, Skarzynski D, Erséus C, Butt KR, Richard B, Hebert PDN (2013) Biological invasions in soil: DNA barcoding as a monitoring tool in a multiple taxa survey targeting European earthworms and springtails in North America. Biol Inv 15:899–910
Potvin LR, Lilleskov EA (2017) Introduced earthworm species exhibited unique patterns of seasonal activity and vertical distribution, and Lumbricus terrestris burrows remained usable for at least 7 years in hardwood and pine stands. Biol Fertil Soils 53:187–198
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using genotype data. Genetics 155:945–959
Qiu J, Turner M (2017) Effects of non-native Asian earthworm invasion on temperate forest and prairie soils in the Midwestern US. Biol Inv 19:73–88
Resner K, Yoo K, Sebestyen SD, Aufdenkampe A, Hale C, Lyttle A, Blum A (2015) Invasive earthworms deplete key soil inorganic nutrients (Ca, Mg, K, and P) in a Northern hardwood forest. Ecosystems 18:89–102
Reynolds JW, Linden DR, Hale CM (2002) The earthworms of Minnesota (Oligochaeta: Acanthrodrilidae: Lumbricidae and Megascolecidae). Megadrilogica 8:85–100
Rogers JA, Collins CD (2017) Ecological predictors and consequences of non-native earthworms in Kennebec County, Maine. Northeast Nat 24:121–136
Rosenberg NA (2004) DISTRUCT: a program for the graphical display of population structure. Mol Ecol Notes 4:137–138
Sakai AK, Allendorf FW et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Schliep KP (2011) Phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593
Schult N, Pittenger K, Davalos S, McHugh D (2016) Phylogeographic analysis of invasive Asian earthworms (Amynthas) in the northeast United States. Invertebrate Biol 135:314–327
Sims RW, Gerard BM (1999) Earthworms-synopses of the British fauna, No 31. The Dorset Press, Dorchester, Great Britain
Smith RG, McSwiney CP, Grandy AS, Suwanawaree P, Snider RM, Robertson GP (2008) Diversity and abundance of earthworms across an agricultural land-use intensity gradient. Soil Tillage Res 100:83–88
Snyder BA, Callaham MA, Hendrix PF (2011) Spatial variability of an invasive earthworm (Amynthas agrestis) population and potential impacts on soil characteristics and millipedes in the Great Smoky Mountains National Park, USA. Biol Inv 13:349–358
Snyder BA, Callaham MA, Lowe CN et al (2013) Earthworm invasion in North America: food resource competition affects native millipede survival and invasive earthworm reproduction. Soil Biol Biochem 57:212–216
Souleman D, Grumiaux F, Frérot H, Vandenbulcke F, Pauwels M (2016) Isolation and characterization of eight polymorphic microsatellites markers for the earthworm Lumbricus terrestris. Eur J Soil Biol 74:76–80
Suárez ER, Tierney GL, Fahey TJ, Fahey R (2006) Exploring patterns of exotic earthworm distribution in a temperate hardwood forest in south-central New York, USA. Landscape Ecol 21:297–306
Szlavecz K, Chang C-H, Bernard MJ, Pitz SL, Xia L, Ma Y, McCormick MK, Filley T, Yarwood SA, Yesilonis ID, Csuzdi C (2018) Litter quality, dispersal and invasion drive earthworm community dynamics and forest soil development. Oecologia 188:237–250
Tiunov AV, Hale CM, Holdsworth AR, Vsevolodova-Perel TS (2006) Invasion patterns of Lumbricidae into the previously earthworm-free areas of northeastern Europe and the western Great Lakes region of North America. Biol Inv 8:1223–1234
van Oosterhaut C, Hutchinson WF, Willis DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Velavan TP, Schulenburg H, Michiels NK (2007) Development and characterization of novel microsatellite markers for the common earthworm (Lumbricus terrestris L.). Mol Ecol Notes 7:1060–1062
Villesen P (2007) FaBox: an online toolbox for fasta sequences. Mol Ecol Notes 7:965–968
Zhang WX, Hendrix PF, Snyder BA, Molina M, Li JX, Rao XQ, Siemann E, Fu SL (2010) Dietary flexibility aids Asian earthworm invasion in North American forests. Ecology 91:2070–2079
Acknowledgements
We are grateful to Sabrina Pach for her support in genotyping the microsatellites at the Veterinary Medicine (University of Göttingen, Germany) and for permissions to collect specimens which were provided by the Department of Natural Resources Minnesota and the Warner Nature Center.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the German Research Foundation (DFG Grant: Ei 862/7‐1, SCHA1671/5‐1). NE acknowledges funding by the European Research Council (European Union’s Horizon 2020 research and innovation program; grant no 677232) and the German Centre for Integrative Biodiversity Research Halle-Jena-Leipzig, funded by the German Research Foundation (DFG; FZT 118, 202548816).
Author information
Authors and Affiliations
Contributions
IS, SS, NE conceived and designed the study, AR, AK and BH did the field work, BH performed the laboratory work and analysed the data. BH and IS drafted the manuscript. All authors read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest are declared.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heimburger, B., Klein, A., Roth, A. et al. Population structure and genetic variance among local populations of a non-native earthworm species in Minnesota, USA. Biol Invasions 25, 2361–2375 (2023). https://doi.org/10.1007/s10530-023-03048-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03048-w