Abstract
Nutria (Myocastor coypus) were introduced to the eastern shore of Chesapeake Bay, USA in the 1940s. They reached peak densities in the late 1990s, causing massive wetland loss. Beginning in 2002, a systematic plan to eradicate nutria from the 1.7M ha Delmarva Peninsula was implemented. Since that time the nutria population has been effectively reduced, and no nutria have been detected since May 2015. A lack of detection does not equate with complete absence. We address the following three questions. (1) What is the expected probability of nutria eradication from the Delmarva Peninsula as of the end of 2020? (2) If the probability of eradication is below the management target of 0.95, how much more surveillance is required? (3) How sensitive is the estimated probability of eradication to varying levels of public surveillance and modelled population growth rates? These questions were addressed by employing a stochastic spatially-explicit surveillance model that uses data in which no nutria were detected to quantify the probability of complete absence (PoA) over the entire Delmarva Peninsula. We applied an analytical framework that decomposes the spatial risk of survivors and data into management zones, and took advantage of low-cost public reporting of nutria sightings. Active surveillance by the eradication program included detector dog and tracker surveys, shoreline surveys, detection with ground and water platforms (with hair snares), and camera traps. Results showed that the PoA increased with time and surveillance from a beginning PoA in May 2015 of 0.01 to a mean of 0.75 at the end of 2020. This indicates that the PoA on the Delmarva was well below the target threshold of 0.95 for declaring eradication success. However, given continued surveillance without detection, a PoA of 0.95 would be achieved by June 2022. This analysis provides an objective mechanism to align the expectations of policy makers, managers and the public on when eradication of nutria from the entire Delmarva Peninsula should be declared successful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive alien species are well recognized as a threat to the integrity of natural biodiversity, ecosystem function and services, and primary productivity (Gurevitch and Padilla 2004; Julia et al. 2007; Mack et al. 2000; Pimentel et al. 2005; Vilá and Hulme 2017; Vitousek et al. 1997). Given the high financial expenditures required in perpetuity to minimize invasive species impacts (Pimentel et al. 2001), eradication is becoming an increasingly attractive strategy which potentially can deliver high ecological benefits (Cleeland et al. 2020; Jones et al. 2016; Russell and Holmes 2015). Eradication success of invasives is highest for newly founded populations with a limited spatial extent (Tobin et al. 2014; Robertson et al. 2017) or on offshore islands (Donlan et al. 2003; Russell et al. 2009; Towns and Broome 2003) where the risk of reincursion is low. However, new technologies and evidence-based strategies (Tobin et al. 2011; Liebhold et al. 2016; Nugent et al. 2018; Murphy et al. 2019) are allowing for invasives to be eradicated from increasingly large mainland areas (Robinson and Copson 2014; Martin and Richardson 2019).
The removal of nutria (Myocastor coypus) from the 1.7M ha Delmarva Peninsula, USA is a current broadscale eradication of an established mammal from a mainland area ( Pepper et al. 2017, http://www.fws.gov/chesapeakenutriaproject; Fig. 1). Nutria were successfully eradicated from a similar sized area in the U.K. (Gosling and Baker 1989). Nutria are an aquatic rodent native to South America (Ehrlich 1967) that have been introduced widely internationally for the fur trade (Carter and Leonard 2002). They were introduced to the eastern shore of the Chesapeake Bay, Maryland in the 1940s (Willner et al. 1979), reached peak densities in the late 1990s and have caused massive wetland loss by converting emergent marsh to shallow open water (Evers et al. 1998). The eradication of nutria from the Delmarva Peninsula began in 2002, and since that time the population has been effectively reduced with no nutria having been detected since May 2015 (Pepper et al. 2017). Eradication is only feasible if reinvasion can either be stopped or infrequent immigrants can be removed before reproducing (Bomford and O’Brien 1995), which is difficult for mainland populations. However, reinvasion onto the Delmarva Peninsula is unlikely because the climate of the northern part of the peninsula approaches the physiological limit of nutria (Hilts et al. 2019), and the Chesapeake Bay is a formidable barrier to the west (Fig. 1).
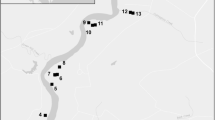
The relative risk of the center of activity of a surviving reproductive unit of nutria on the Delmarva Peninsula since 2015. The black lines demarcate the boundaries of the four management zones: ”Maryland” upper left; ”Delaware” upper right; ”Eastern shore of Virginia” bottom; and ”Blackwater” middle left. White areas within the management zones indicates zero risk. The inset map on the lower right shows the peninsula location on the east coast of the USA
The immediate management question is whether all individual nutria have been removed from the Delmarva Peninsula and eradication has been successfully achieved. Importantly, a lack of detection does not equate with complete absence. Individuals in a ’persecuted’ population change behavior and become very difficult to detect (Cruz et al. 2005). The search effort deployed since May 2015 may or may not have been adequate to detect a few remaining survivors, which if left alone for sufficient time could re-seed the population and lead to continued ecological damage.
The declaration of eradication success signals a policy change in which resources are allocated away from the program to other environmental issues. Premature declaration will cause program failure because of nutria persistence, whereas conducting excessive surveillance beyond that which is necessary will divert limited resources away from other environmental issues. Eradication programs should be declared a success once a robustly estimated probability of successful eradication exceeds an agreed target level (e.g. 0.95-0.99; Ramsey et al. 2009). Establishing a target probability of eradication and a quantitative mechanism for estimating the effort required to achieve it helps guide management decisions and resource allocation, and creates consistent goals and expectations among field staff, managers and policy makers.
In an eradication program that covers a very large area, managers cannot control and survey the entire area at one time, and the eradication process needs to be divided into two stages (Anderson et al. 2017). First, the broadscale area is divided into management zones, which consist of areas that can be individually eradicated as a unit and are sufficiently large to minimize the risk of re-invasion from neighboring zones. Stage I occurs at the zone level and consists of invasive population reduction/control and surveillance until a sufficient level of confidence in freedom is achieved within the zone. With high confidence in freedom, the zone is passed into Stage II, which triggers an operational decision to reallocate resources to other management zones. In this way, the control/surveillance operation progresses stepwise across the region of interest. In Stage II, surveillance within zones declared free at Stage I should continue so that any residual survivors are detected. Stage II surveillance is also used to estimate the probability of eradication across all zones (i.e. the entire region). Because resources have been allocated away from the zone, Stage II surveillance must be low cost, such as low intensity and frequency surveys, or public reporting.
All zones in the Delmarva eradication program are now in Stage II. Nutria have not been seen since 2005, 2007, 2012 and 2015 from the Delaware, Maryland, Eastern shore of Virginia and Blackwater zones, respectively (Fig. 1). While active and organized surveillance has been concentrated in the Blackwater zone in recent years, the public and other environmental management staff (hereafter public surveillance) had the potential to detect and report the presence of surviving nutria if they were sighted in any of the zones. The two-stage, multi-zone approach for quantifying the probability of eradication incorporates the times since last observed nutria across the four zones. The important implication is that if there was a surviving reproductive unit in one of the zones, the population would have been expected to grow and expand, making it easier to detect with the passage of time. Given that eradication is the goal, the modelled growth rate of the population should be conservative and much lower than the expected biological rate. Nevertheless, the rate will vary across species and have important implications for the sensitivity of surveillance and quantifying the probability of eradication. Fast growing species will be relatively easy to detect over time (e.g. rats), but risk growing to high density levels quickly if left undetected.
In this paper we examined the Delmarva nutria Stage II surveillance data using the broadscale eradication framework outlined in Anderson et al. (2017). We demonstrate the process over the entire peninsula using multiple sources of surveillance data. We addressed the following three questions. (1) What is the expected probability of nutria eradication from the Delmarva Peninsula as of the end of 2020? (2) If the probability of eradication is below the management target of 0.95, how much more surveillance is required? (3) How sensitive is the estimated probability of eradication to varying levels of public surveillance and modelled population growth rates? We then make some general recommendations to improve outcomes for similar broadscale eradication programs.
Methods
We superimposed a virtual grid cell system over the entire extent of interest (Anderson et al. 2013) to establish a spatial framework for analysis of the surveillance data (see detailed description in Appendix S1 in Supplemental Material). The grid cell is the fundamental surveillance unit, so that the surveillance system is attempting to detect a residual nutria that has its home range center in a particular grid cell. Surveillance data were composed of point-location and raster-grid data (see below).
Data sources
The total area of the Delmarva peninsula (17,295 \(km^2\)) was sub-divided into four management zones: ”Maryland” (upper left), ”Delaware” (upper right), ”Eastern shore of Virginia” (bottom) and ”Blackwater” (middle) (Fig. 1). Each zone was then rasterized, using a spatial resolution (cell size) of 100 m. Data on the locations of active surveillance from a variety of different sources were available such as detector-dog searches (trained to seek and respond to nutria scat), ground and shoreline surveys by trained personnel, hair snare surveys from water and land platforms (Pepper et al. 2017) and camera surveys. All surveillance data returned negative results, i.e. no nutria were ever detected.
The surveillance data were collected since the last nutria was captured in May 2015 to December 2018. Hair snare and camera data were point data with eastings, northings and deployment duration. The track data (provided as GIS shapefiles) for people and detector dogs were discretized into points along the tracks at 50-m intervals. Therefore, all of the active surveillance data were point data with deployment duration within each calendar year from June 2015 - December 2018. To quantify the probability of eradication for 2019 and subsequent years, we assumed that the surveillance effort conducted from 2016 through 2018 would apply as it was anticipated by the eradication program to apply the same effort in the coming years.
For each cell, a relative risk value was calculated, representing the likely occurrence of a single small surviving reproductive unit of nutria, relative to other cells (e.g. one male and female; Fig. 1). Relative risk values were based on expert opinion, quantitative estimates of habitat suitability (Hilts et al. 2019) or occurrence records with values of zero indicating non-habitat (i.e. no risk). Nutria can be in or pass through, but cannot persist in no risk areas, such as roads, buildings, industrial areas and water bodies. Areas categorized with a relative risk value of 1 were very unlikely habitat, such as high density residential, commercial and industrial areas. The areas categorized as 5 could potentially support nutria, but none have been found in these areas on the Delmarva (Pepper, Pers. comm.). The relative risk areas categorized as 8 were estuarine and marine wetland and/or freshwater emergent habitats occurring outside the Blackwater National Wildlife Refuge. The relative risk area with a value of 50 were the estuarine and marine wetland and/or freshwater emergent habitats occurring within the Blackwater National Wildlife Refuge but not near known capture locations. The high relative risk value of 500 was in estuarine and marine wetland and/or freshwater emergent areas in proximity to captures within the last 10 years. Each capture and sign point was given a buffer of 1980 ft (or 604 meters) where habitat within the buffer was categorized as 500. This distance was based on an enlargement of a 16 ha nutria home range in Maryland (Pepper, pers. comm.).
If we were to find a surviving nutria, the relative risk map suggests that it would be 500 times more likely to be found in the 500-risk area than in the 1-risk area (Fig. 1). It would be 10 times more likely to be in the 500-risk area than in the 50-risk area. This increase from a relative risk value of 50 to 500 is justified by the expertise of the management team and the habitats from which nearly 14,000 nutria were removed since 2002 (Pepper et al. 2017).
In addition to the sources of active surveillance, passive surveillance was also accounted for by acknowledging that residual surviving nutria could potentially be detected by the public or environmental managers not associated with the eradication program. Public surveillance was included using a spatial raster (binary 1 and 0) indicating areas where detection of nutria by the public was possible. Due to a lack of information on the spatial variation in public surveillance effort, all pixels had the same probability of detection. Given the high level of hiking, hunting/fishing and recreational use of this area (Pepper pers. comm.), the public surveillance raster was assigned a value of 1 where the relative risk map was \(> 0\). That is, all areas considered to be habitat were surveyed by the public.
Model description
We modified a spatially-explicit surveillance model (Anderson et al. 2013) that used surveillance data in which no nutria were detected to quantify the probability of absence PoA over the entire Delmarva Peninsula (Appendix S1). The fundamental surveillance unit is the grid cell, and the model uses the surveillance data to quantify the probability of detecting a nutria that has its home range center in given grid cell (unit sensitivity). This approach accounts for the home range movement behavior of nutria (Ramsey et al. 2015; Anderson et al. 2013), and allows for surveillance points outside the cell containing a home range centre to detect the nutria from that cell. Model results are independent of the grid cell size, which represents the area of surveillance unit and should be smaller than the expected home range size so that an individual effectively utilises multiple grid cells.
The model used an annual time step so that surveillance data were partitioned into the corresponding calendar years. Multiple detection methods deployed in the same year have an additive effect on the unit sensitivity of a given grid cell. Unit sensitivity values are quantified with model parameters, which are input as distributions (Table 1). To account for uncertainty, 200 random variates for each parameter were drawn and used in calculations of the PoA.
Unit sensitivity values across all grid cells in a zone were used to quantify the probability of detecting a surviving nutria given an occupied grid cell in the zone (zone sensitivity). Combining the four zone sensitivities, the model estimated the probability of detecting a nutria anywhere on the Delmarva Peninsula (system sensitivity), assuming that at least one grid cell was occupied by a nutria in one of the zones. System sensitivity and PoA were calculated annually.
The model used surveillance data and Bayes theorem to calculate the PoA, which incorporated a Prior, or the starting PoA (Gelman et al. 2004). The prior was then updated with the surveillance data to give the posterior PoA. We used a PERT distribution with a most likely prior value of 0.01, and lower and upper bounds of 0.001 and 0.1, respectively (Table 1). That is, there was most likely a 1% chance that eradication was achieved in May 2015 after the last known nutria was removed. This informed and pessimistic prior was obtained by questioning the eradication program staff on their thoughts on the probability that nutria had been eradicated in May 2015. All staff replied that there was a very low to zero chance that eradication had been achieved at that time.
In addition to a residual viable population of nutria persisting undetected in this landscape, there was also the potential for a new incursion to occur. In this analysis we assumed that this risk was very low (effectively zero) due to the Chesapeake Bay to the west and the nutria physiological limits to the north (Hilts et al. 2019) inhibiting re-invasion. We set the PERT distribution minimum, most likely, and maximum to 0.00005, 0.0001 and 0.0002, respectively (Table 1).
Each surveillance data point has the potential to detect a nutria that has a home range center in grid cells that surround the point. The daily maximum probability of detection occurs in the grid cell in which the point is located. This maximum probability is referred to as \(g_0\) (see values in Table 1). The probability of detection decreases with distance from the device or point as a function of the home range-decay parameter \(\sigma\) (Ball et al. 2005), which we set at 100 m with a standard deviation of 40 m. The mean of 100 m corresponds to a home range size of \(\approx 20\) ha. We increased the estimated home range size for Maryland nutria from 16 ha (Pepper unpub. data) to 20 ha because animals at low density exhibit larger home ranges than those at high density (Efford et al. 2016).
Data were not available to empirically estimate some parameters. For these, we queried the eradication program staff who had years of experience in this system with the detection methods. We also used high variance values to account for parameter uncertainty. The \(g_0\) estimates for the 50-m interval detector dog and people surveillance points along tracks were based on a desktop elicitation exercise with program staff. The elicitation exercise involved program staff identifying likely values for the probability of a detector dog search and person search detecting a nutria given four different survey intensities in the vicinity of a nutria home range center (Fig. 2). In Fig. 2 the red paths were the hypothetical movement paths of a detector dog or a person. The black stars represented the home range centers of four different nutria (numbered 1–4). The black circle around nutria 2 represents its home range size (95% probability isopleth), which provides perspective for the potential detection zone of a surveillance point (home range circles are not provided in the figure for the other nutria). For detector dogs we used a mean (sd) of 0.14 (0.06), and for people 0.02 (0.01) (Table 1), which gave probabilities of detection varying from 0.16 to 0.95 for dogs, and 0.02 to 0.34 for a person (Table 2). The probability of detection was highest for home range 1 because a substantial portion of the home range was covered by the search tracks, and the search effort was concentrated in the high-use central area near the home range center.
\(g_0\) parameter test for detector dogs and people survey points along tracks. Red paths are the hypothetical movement paths of a detector dog or a person. The black stars represent the home range centers of four different nutria (numbered 1–4). The black circle around nutria 2 represents its home range size
The annual probability of detection by public surveillance of a nutria with its home range center in a particular grid cell had a mean (sd) of 0.03 (0.015) (Table 1). Hence, if there was a single reproductive unit in one grid cell, the public would have a 3% chance of detecting it over the course of a year. This was based on expert elicitation, public awareness and reporting, and public use of the landscape.
Given that each zone had been declared nominally ’free’ in 2015, the surveillance model was used to estimate the probability of detecting a nutria with the Stage II surveillance effort (quantified by the system sensitivity) for each year since May 2015. Practically and intuitively it is easier to detect a nutria when many grid cells are occupied (i.e. high population density) than when very few are occupied. The model requires the specification of the number of grid cells potentially occupied (”Occupied cells”; Table 1), which is not related to the actual number occupied but is a statistical design parameter in the model. This parameter consists of the target number of occupied grid cells potentially available for detection by the surveillance model and is similar to the ”design prevalence” used in disease surveillance (Cameron and Baldock 1998; Martin et al. 2007; Martin 2008). Because the objective is eradication, this number is set very low. In the present analysis the Occupied cells was set to 1 in the Blackwater zone (0.002% of zone; Table 1). The 1 occupied cell represents the minimum prevalence level for a viable population from which a thriving population could potentially grow if left un-controlled. The starting number of Occupied cells was set higher for the other three zones to account for the longer period since nutria have been detected, and the potential for population growth and spread in the intervening years. The Eastern shore of Virginia, Maryland and Delaware zones had starting numbers of occupied cells equal to 4, 9 and 11, respectively. To derive these numbers we set the number of occupied cells equal to 1 for the last year in which a nutria was detected in each zone, and then added 1 cell for each year (Annual increase occupied cells; Table 1) up to and including 2015. The number of Occupied cells in each zone continues to increase annually by the model parameter Annual increase occupied cells. This corresponds to the potential and conservative population growth and expansion referred to in the Introduction. Therefore, if there was a surviving reproductive unit on the peninsula, nutria would become easier to detect over time.
Similar to the Occupied cells parameter, there is an Occupied zones parameter that is used in the calculation of the zone sensitivity (AppendixS1). This was set to the minimum value of 1 (Table 1).
We assessed the sensitivity of the calculated time to obtain a 0.95 probability of eradication success by varying Public detection from 0.02-0.2 and the Annual increase occupied cells from 0.25-3.0 \(cells \cdot year^{-1}\). All other parameters values were those in Table 1. When testing the sensitivity of Public detection, the Annual increase occupied cells was fixed at 0.25. Similarly, the Public detection was fixed at 0.02 when testing the Annual increase occupied cells. Other parameters in the model were not subject to sensitivity testing as these have been previously investigated (see Anderson et al. 2013).
Results
To estimate the current probability that eradication has been achieved (PoA), we analyzed the surveillance data from May 2015 through December 2020 using the parameters in Table 1. Results show that the PoA increased with time and surveillance from the mean Prior of \(\approx 0.01\) to a mean of 0.75 (95% CI: 0.30 – 0.93) in December 2020 (Fig. 3), indicating that the PoA on the Delmarva was well below the target threshold of 0.95 for declaring eradication success.
Estimated PoA from May 2015 to December 2024 (left panel). The mean and 95% confidence interval shown at 5/2015 represent the prior PoA. Subsequent values are the PoA at the end of the calendar year following the year’s surveillance effort with no detections. The horizontal dashed line marks the target PoA of 0.95. The mean and 95% confidence intervals of the annual system sensitivity are shown in the right panel
Forcasting forward, results show that a 0.95 PoA can be achieved by mid 2022 (Fig. 3). Zone sensitivities (Table 3) were influenced by the amount of active and public surveillance, and the parameter Number of occupied cells. Blackwater had a lot of active surveillance but a low Number of occupied cells (Table 1). The other three zones relied on public surveillance but had relatively high Number of occupied cells, because several years had passed since nutria were seen in those zones. At the end of 2022 there were 8, 11, 16 and 18 Occupied cells in Blackwater, Eastern shore of Virginia, Maryland and Delaware zones, respectively. While the unit sensitivity in some areas of the Blackwater zone exceeded 0.99, surveillance for most of the peninsula depended on low sensitivity public reporting (Fig. 4). The on-the-ground surveillance effort did not change much over the years 2016–2018, yet the zone sensitivity for each zone increased over time due to the increasing number of Occupied cells (Table 3).
The parameter sensitivity analysis showed that variation in the Annual increase occupied cells had a stronger effect on time to confirming eradication success than public surveillance detection probability (Fig. 5). Time to eradication decreased with increases in both parameters. The Annual increase occupied cells is based on the species biology, whereas the Public detection is a management effort parameter and can be varied by allocation of resources. Therefore, we analyzed the surveillance data with no public surveillance (i.e. Public detection = 0.0 for all years), and results showed the time to eradication increased by six months compared to when Public detection = 0.03. We found that the target probability would have been achieved at the end of 2020 had the Public detection been increased to 0.1 for the entire program. Forecasting ahead from the end of 2020, eradication could be declared by the end of 2021 if Public detection could be increased to 0.1 starting in January 2021. An important caveat in these analyses in which the Public detection is increased is that the active surveillance by the eradication team is not decreased, which would require a significant increase in budget to handle the increased work load associated with public surveillance.
Results of sensitivity analysis of the probability of detection by public surveillance (solid line) and the Annual increase occupied cells (dashed line) on the years to confirming eradication success. The proportional increase in the parameters is plotted against the years of surveillance required to confirm eradication with a 0.95 probability. Public detection probability ranged from 0.02 to 0.2, and Annual increase occupied cells ranged from 0.25-3.0 \(cells \cdot year^{-1}\)
Discussion
Analysis of surveillance data provided an objective mechanism to align the expectations of funders, managers and the public on when eradication of nutria from the Delmarva Peninsula should be declared successful. Despite no sign of nutria since May 2015, evidence indicates that there remains a high level of uncertainty as to whether eradication has been achieved. With the data and parameters used here, at the end of 2020 the modelling estimated a 0.75 PoA with 95% confidence intervals ranging from 0.30 to 0.93. This is not to say that eradication has not occurred, but only that our confidence in successful eradication remains insufficient to pull financial and logistical resources away from this project. Forecasting forward and using the surveillance effort from 2016-2018, high confidence in eradication should be achieved by June 2022.
If successful, the eradication of nutria from the 1.7M ha Delmarva Peninsula, U.S.A. will be the largest area from which a mammal has been eradicated. This case study demonstrates the utility of the two-staged and multi-zoned approach to conducting and confirming eradication success over very large areas (Anderson et al. 2017). Accounting for the varying time since nutria were last found in the different zones was particularly important for model results. If surviving nutria were present, the population would grow, which would result in an increasing zone sensitivity with increasing population expansion. The sensitivity analysis showed that the estimated time to confirming eradication decreases dramatically with increasing Annual increase occupied cells (Fig. 5). We reiterate the importance of assigning minimum expected values for Occupied cells and Annual increase occupied cells. Nutria are considered a highly fecund species, with early maturation, multiple litters per year and litter sizes of 4–6.5 offspring (Willner et al. 1979; Runami et al. 2013; Brown 1975). In the Delaware management zone (Fig. 1), for example, the last nutria seen was in 2005, and the Occupied cells in 2022 had grown to 18 cells. This is equivalent to 0.006% of the zone area, which could be considered well below the expected minimum. This is appropriate given that nutria is a cold sensitive species (Gosling and Baker 1989; Runami et al. 2013), and this area is at their northern physiological limit (Hilts et al. 2019), which could result in a low population growth rate. Individuals in this population have been observed to huddle in large groups during cold weather periods to conserve heat among conspecifics (Pepper pers. comm.). When at low population density, few individuals with which to aggregate could result in increased over-winter mortality and low population growth approaching an Allee effect (Courchamp et al. 1999). Therefore, a Annual increase occupied cells of 1 \(cell \cdot year^{-1}\) was an appropriate value for confirming eradication success.
Another novel feature of this eradication program and analysis was the use of public surveillance to detect survivors. Generally, the value of public reporting and our ability to make inference on it across biosecurity surveillance systems is increasing (Pawson et al. 2020; Caley et al. 2020). The sensitivity of public surveillance for nutria is very low, but critical because it covers most of the peninsula increasing the spatial coverage of surveillance. Broadscale eradication programs are unlikely to be successful without ancillary low-cost surveillance with wide spatial coverage. Another example of ancillary surveillance is the use of livestock as sentinels for detecting bovine tuberculosis (caused by Mycobacterium bovis) in a population of brushtail possums (Trichosurus vulpecula; Nugent et al. 2018). Management zones in this broadscale eradication effort of bovine tuberculosis will receive little to no Stage II active surveillance, however ongoing slaughterhouse carcass inspection of cattle can detect the disease, which could signal its presence in the surrounding possum population. While public reporting for nutria on the Delmarva Peninsula is relatively inexpensive, it is not free. Public reporting of sightings is contingent on an awareness campaign, and the capacity to screen, visit and verify reports. Our analysis showed that an increased investment in public surveillance could decrease the time to eradication. An increased investment must come with either an augmented budget or reallocation of resources away from active surveillance in the high risk areas. Our modelling did not reduce active surveillance to counter the increase in public surveillance.
How an eradication program strategically distributes resources between active surveillance in high risk areas and surveillance coverage of lower risk areas requires further investigation. A critical factor in determining how to balance the allocation of resources in Stage II of a broadscale eradication are the financial and logistical consequences of a surviving population going undetected for an extended period of time (Anderson et al. 2017). If the population grows rapidly and the costs of recontrol (i.e. reducing the population to zero), following the eventual detection of the surviving population, are high relative to the costs of surveillance, then the allocation of resources should be shifted towards increasing coverage with more indirect surveillance, such as public reporting. However, the optimal allocation in other scenarios requires further investigation.
In summary, this study has demonstrated the utility of the broadscale eradication framework (Anderson et al. 2017) for making inference on the probability of eradication over large areas where control and surveillance activities are required to be conducted over several years in order to completely treat the region of interest. A critical component of this framework is the ability to use information on the likely rates of population increase and spread in the event that undetected survivors persist. Using this information along with the time since last detection of an invasive is critical for making more informed inference from the available surveillance data. Hence, the broadscale eradication framework provides a flexible tool for managers to make quantitative estimates of the probability of absence that makes allowances for possibilities for persistence or re-invasion, which become more likely as invasive species eradication programs are applied to increasingly large areas.
Supplemental material
Detailed description of the PoA model and Python scripts for running the model are available at https://doi.org/10.7931/54ye-7v13
Data availability
Due to sensitivity of the eradication management program, the USDA Wildlife Services elects to not make the data openly available.
Code availability
Python scripts for running the model are available at https://doi.org/10.7931/54ye-7v13.
References
Anderson D, Ramsey D, Nugent G, Bosson M, Livingstone P, Martin P, Sergeant E, Gormley A, Warburton B (2013) A novel approach to assessing the probability of disease eradication from a wild-animal-reservoir host. Epidemiol Infect 141:1509–1521. https://doi.org/10.1017/S095026881200310X
Anderson D, Gormley A, Ramsey D, Baxter P, Nugent G, Martin P, Bosson M, Livingstone P, Byrom A (2017) A bio-economic decision process for in broadscale eradications of invasive pests and disease. Biol Invas 19:2869–2884. https://doi.org/10.1007/s10530-017-1490-5
Ball S, Ramsey D, Nugent G, Warburton B, Efford M (2005) A method for estimating wildlife detection probabilities in relation to home range use: insights from a field study on the common brushtail possum, Trichosurus vulpecula. Wildl Resear 32:217–227
Bomford M, O’Brien P (1995) Eradication or control for vertebrate pests? Wildl Soc Bullet 23(2):249–255. https://doi.org/10.2307/3782799
Brown LN (1975) Ecological relationships and breeding biology of the nutria (myocastor coypus) in the tampa, florida, area. J Mammal 56(4):928–930
Caley P, Welvaert M, Barry SC (2020) Crowd surveillance: estimating citizen science reporting probabilities for insects of biosecurity concern. J Pest Sci 93(1):543–550
Cameron A, Baldock F (1998) A new probability formula for surveys to substantiate freedom from disease. Prevent Veter Med 34:1–17
Carter J, Leonard BP (2002) A review of the literature on the worldwide distribution, spread of, and efforts to eradicate the coypu (myocastor coypus). Wildlife Society Bulletin (1973-2006) 30(1):162–175
Cleeland JB, Pardo D, Raymond B, Terauds A, Alderman R, Mcmahon CR, Phillips RA, Lea MA, Hindell MA (2020) Introduced species and extreme weather as key drivers of reproductive output in three sympatric albatrosses. Scient Report 10(1):8199. https://doi.org/10.1038/s41598-020-64662-5
Courchamp F, Clutton-Brock T, Grenfell B (1999) Inverse density dependence and the allee effect. Trend Ecol & Evolut 14:405–410
Cruz F, Donlan C, Campbell K, Carrion V (2005) Conservation action in the galàpagos: feral pig (sus scrofa) eradication from santiago island. Biol Conservat 121:473–478
Donlan C, Tershy B, Campbell K, Cruz F (2003) Research for requiems: the need for more collaborative action in eradication of invasive species. Conservat Biol 17:1–2
Efford MG, Dawson DK, Jhala YV, Qureshi Q (2016) Density-dependent home-range size revealed by spatially explicit capture-recapture. Ecography 39(7):676–688
Ehrlich S (1967) Field studies in the adaptation of nutria to seasonal variations. Mammalia 31(3):347–360. https://doi.org/10.1515/mamm.1967.31.3.347
Evers DE, Sasser CE, Gosselink JG, Fuller DA, Visser JM (1998) The impact of vertebrate herbivores on wetland vegetation in atchafalaya bay, louisiana. Estuaries, 21(1):1–13, http://www.jstor.org/stable/1352543
Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Bayesian Data Analysis. Chapman & Hall/CRC, Boca Raton
Gosling LM, Baker SJ (1989) The eradication of muskrats and coypus from Britain. Biol J Linn Soci, 38(1):39–51, https://doi.org/10.1111/j.1095-8312.1989.tb01561.x, https://doi.org/10.1111/j.1095-8312.1989.tb01561.x, https://academic.oup.com/biolinnean/article-pdf/38/1/39/14071694/j.1095-8312.1989.tb01561.x.pdf
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trend Ecol & Evolut 19(9):470–474. https://doi.org/10.1016/j.tree.2004.07.005
Hilts DJ, Belitz MW, Gehring TM, Pangle KL, Uzarski DG (2019) Climate change and nutria range expansion in the eastern united states. J Wildl Manage 83(3):591–598, https://doi.org/10.1002/jwmg.21629, https://wildlife.onlinelibrary.wiley.com/doi/pdf/10.1002/jwmg.21629
Jones HP, Holmes ND, Butchart SHM, Tershy BR, Kappes PJ, Corkery I, Aguirre-Muñoz A, Armstrong DP, Bonnaud E, Burbidge AA, Campbell K, Courchamp F, Cowan PE, Cuthbert RJ, Ebbert S, Genovesi P, Howald GR, Keitt BS, Kress SW, Miskelly CM, Oppel S, Poncet S, Rauzon MJ, Rocamora G, Russell JC, Samaniego-Herrera A, Seddon PJ, Spatz DR, Towns DR, Croll DA (2016) Invasive mammal eradication on islands results in substantial conservation gains. Proceed Nation Acad Sci 113(15):4033–4038. https://doi.org/10.1073/pnas.1521179113
Julia R, Holland D, Guenthner J (2007) Assessing the economic impact of invasive species: the case of yellow starthistle (centaurea solsitialia l.) in the rangelands of idaho, usa. J Environ Manag 85:876–882
Liebhold AM, Berec L, Brockerhoff EG, Epanchin-Niell RS, Hastings A, Herms DA, Kean JM, McCullough DG, Suckling DM, Tobin PC, Yamanaka T (2016) Eradication of invading insect populations: from concepts to applications. Ann Rev Entomol 61:335–52
Mack R, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz F (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Martin AR, Richardson MG (2019) Rodent eradication scaled up: clearing rats and mice from south georgia. Oryx 53(1):27–35. https://doi.org/10.1017/S003060531700028X
Martin P (2008) Current value of historical and ongoing surveillance for disease freedom: Surveillance for bovine johne’s disease in western australia. Prevent Veter Med 84:291–309
Martin P, Cameron A, Greiner M (2007) Demonstrating freedom from disease using multiple complex data sources 1: a new methodology based on scenario trees. Prevent Veter Med 79:71–97
Murphy EC, Russell JC, Broome KG, Ryan GJ, Dowding JE (2019) Conserving new zealand’s native fauna: a review of tools being developed for the predator free 2050 programme. J Ornithol 160(3):883–892
Nugent G, Gormley AM, Anderson DP, Crews K (2018) Roll-back eradication of bovine tuberculosis (tb) from wildlife in new zealand: Concepts, evolving approaches, and progress. Front Veter Sci 5:277–277
Pawson SM, Sullivan JJ, Grant A (2020) Expanding general surveillance of invasive species by integrating citizens as both observers and identifiers. J Pest Sci 93:1155–1166
Pepper MA, Herrmann V, Hines JE, Nichols JD, Kendrot SR (2017) Evaluation of nutria (myocastor coypus) detection methods in maryland, USA. Biol Invas 19(3):831–841
Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O’Connell C, Wong E, Russel L, Zern J, Aquino T, Tsomondo T (2001) Economic and environmental threats of alien plant, animal, and microbe invasions. Agricult, Ecosys & Environ 84(1):1–20. https://doi.org/10.1016/S0167-8809(00)00178-X
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the united states. Ecol Econ 52(3):273–288. https://doi.org/10.1016/j.ecolecon.2004.10.002
Ramsey DSL, Parkes J, Morrison SA (2009) Quantifying eradication success: the removal of feral pigs from Santa Cruz Island, California. Conservat Biol 23(2):449–459
Ramsey DSL, Caley PA, Robley A (2015) Estimating population density from presence-absence data using a spatially explicit model. J Wildl Manage 79:491–499. https://doi.org/10.1002/jwmg.851
Robertson PA, Adriaens T, Lambin X, Mill A, Roy S, Shuttleworth CM, Sutton-Croft M (2017) The large-scale removal of mammalian invasive alien species in northern Europe. Pest Manage Sci 73:273–279
Robinson SA, Copson GR (2014) Eradication of cats (felis catus) from subantarctic macquarie island. Ecological Management & Restoration 15(1):34–40, https://doi.org/10.1111/emr.12073, https://onlinelibrary.wiley.com/doi/pdf/10.1111/emr.12073
Runami I, Gunji Y, Hishinuma M, Nagano M, Takada T, Higaki S (2013) Reproductive biology of the coypu, myocastor coypus (rodentia: Myocastoridae) in western japan. Zoologia (Curitiba) 30(2):130–134. https://doi.org/10.1590/s1984-46702013000200002
Russell J, Lecomte V, Dumont Y, Le Corre M (2009) Intraguild predation and mesopredator release effect on long-lived prey. Ecol Modell 220:1098–1104
Russell JC, Holmes ND (2015) Tropical island conservation: Rat eradication for species recovery. Biological Conservation 185:1 – 7, https://doi.org/10.1016/j.biocon.2015.01.009, special Issue on Tropical Island Conservation: Rat Eradication for Species Recovery
Tobin P, Berec L, Liebhold A (2011) Exploiting allee effects for managing biological invasions. Ecol Lett 14:615–624
Tobin PC, Kean JM, Suckling DM, McCullough DG, Herms DA, Stringer LD (2014) Determinants of successful arthropod eradication programs. Biol Invas 16(2):401–414. https://doi.org/10.1007/s10530-013-0529-5
Towns D, Broome K (2003) From small maria to massive campbell: forty years of rat eradications from new zealand islands, New Zealand. J Zool 30:377–398
Vilá M, Hulme P (eds) (2017) Impact of biological invasions on ecosystem services. Springer, Cham, Switzerland
Vitousek PM, D’Antonio CM, Loope L, Rejmanek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change, New Zealand. J Ecol 21:1–16
Willner GR, Chapman JA, Pursley D (1979) Reproduction, physiological responses, food habits, and abundance of nutria on maryland marshes. Wildl Monogr 65:3–43
Acknowledgements
Sam Gillingham assisted with the computer code. Graham Hickling, Cecilia Latham and two anonymous reviewers provided helpful comments on early versions of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding for this research was provided by the United States Fish and Wildlife Service and the Australian Centre for Invasive Species Solutions Project No. P01-I-005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The data analyzed for this paper were collected as a part of an on-going management plan to eradicate nutria, and not a research program. Therefore, the data were not subject to animal ethics approval.
Consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anderson, D.P., Pepper, M.A., Travers, S. et al. Confirming the broadscale eradication success of nutria (Myocastor coypus) from the Delmarva Peninsula, USA. Biol Invasions 24, 3509–3521 (2022). https://doi.org/10.1007/s10530-022-02855-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02855-x