Abstract
The role of an alien predator in the community depends on its interaction with native predators. The absence of apex predators may facilitate outbreaks of invasive mesopredators, but the effect of apex predators may vary between species and environments. We analysed the occurrence of a common invasive mesopredator in Europe, the raccoon dog (Nyctereutes procyonoides), and native mesopredators, the red fox and the Eurasian badger, in camera-trap data from Finland. The observations in cameras were analysed in relation to the presence of apex predators in the landscape (grey wolf and Eurasian lynx), human density, and habitat. We observed negative effect of increasing presence of wolves and lynxes on the occurrence of raccoon dogs. This effect appeared clear compared to the effects of habitat and human density. The effect of lynxes on raccoon dogs was clearer in areas with short growth season. For the occurrence of badgers, the presence of wolves had a weak negative effect and the presence of lynxes had a positive effect. For the occurrence of red foxes, wolves had a positive effect when agricultural fields were sparse in the landscape and lynxes had no effect. We also observed that the invasive raccoon dog currently appears to be the most common mesopredator within the study area. We conclude that the effect of apex predators on mesopredators depends on the environment and, in our case, was more suppressive on the alien mesopredator than on the native mesopredators. Thus, apex predators can play an important role in controlling invasive mesopredators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of an alien predator in the community depends on its interaction with native predators. The arrival of an alien mesopredator may increase competition between mesopredator species in the community (Baum and Worm 2009; Wallach et al. 2015). In addition, the lack of apex predators may facilitate invasions by alien mesopredators, as well as population outbreaks of native mesopredators (Terborgh and Estes 2010; Wallach et al. 2010; Ruscoe et al. 2011). Thus, the effect of mesopredators on their prey depend on the presence of apex predators in the community (Crooks and Soulé 1999; Salo et al. 2008; Ritchie and Johnson 2009; Prugh and Sivy 2020).
For example in Europe, increasing mesopredator numbers are suggested to be a threat for ground-nesting birds due to increased nest predation (Dahl and Åhlen 2019, Nummi et al. 2019, Koshev et al. 2020, McMahon et al. 2020). This is indicated by the more pronounced population declines in ground-nesting bird species than in other bird groups (McMahon et al. 2020; Pöysä and Linkola 2021). In large parts of Europe, apex predators have been exterminated, which may have increased mesopredator numbers, both native and invasive (Terborgh and Estes 2010). The most common invasive mesopredator in large parts of Europe is the raccoon dog (Nyctereutes procyonoides). The raccoon dog is a nest predator listed among the harmful alien species that should be controlled or eradicated within EU (Anonymous 2017). It appears to be an effective nest predator based on artificial nest studies (Krüger et al. 2018, Dahl and Åhlen 2019, Nummi et al. 2019, Holopainen et al. 2020a, b, 2021). However, the role of raccoon dog in the decline of endangered prey remains unclear (Kauhala et al. 2000; Kauhala 2004), but a negative impact of the raccoon dog has been observed in the Dalmatian pelican population in Bulgaria (Koshev et al. 2020) and common eider in Archipelago in Finland (Jaatinen et al. 2022).
In northern Europe, the raccoon dog co-occurs with similar-sized mesopredators, the red fox (Vulpes vulpes) and the Eurasian badger (Meles meles; from now on badger), and with apex predators including the grey wolf (Canis lupus; from now on wolf) and the Eurasian lynx (Lynx lynx; from now on lynx). Whether or not the raccoon dog competes with badgers and red foxes is not known, but badgers and raccoon dogs are known to partly share their home ranges and dens (Kauhala and Auttila 2010; Kauhala and Kowalczyk 2011). The role of apex predators in raccoon dog occurrence remains also unclear, but potentially they are important for the protection of native species from the invasive mesopredators (e.g. Salo et al. 2008; Wallach et al. 2010). Predator occurrence in the landscape depends on factors, such as ecosystem productivity (Elmhagen and Rushton 2007) and human presence in the landscape (Kuijper et al. 2016). For example, the effects of lynxes and wolves on red foxes may vary depending on ecosystem productivity (Elmhagen and Rushton 2007). Thus, for the management of invasive mesopredators, it is important to understand the co-occurrence of invasive and native predators in relation to habitat (Kurki et al. 1998) and human presence (Kuijper et al. 2016) in the landscape.
We analyse the occurrence of the raccoon dog, the red fox, and the badger in camera trap data from southern and central Finland. The camera trap data on these mesopredators is studied in relation to the presence of apex predators in the landscape (wolf and lynx), human population density, and habitat variables. Both the wolf and lynx populations have increased in Finland during last decades, after being eradicated to near extinction in early twentieth century. The lynx is now occupying most parts of southern and central Finland, but for wolves the population increase has been slower. A major reason for this has been widespread poaching (Suutarinen and Kojola 2017), suggesting that the recovery of the wolf population is to a large extent dependent on management decisions and the acceptance of wolves by local people. The current situation provides us an opportunity to compare areas with and without wolves.
We predict that apex predator presence has negative effects on mesopredator occurrence. In particular, we are interested in comparing this effect between the raccoon dog (invasive mesopredator) and the red fox and the badger (native mesopredators). We predict positive relationships between the raccoon dog and the badger, because they may live in the same areas. For the red fox and raccoon dog, we do not know whether the relationship is positive or negative due to possible similarities in the habitat preferences or due to competition for the best habitat patches between the species. For habitat variables and human population density we are simply interested on controlling their role for occurrence of studied mesopredators. However, we also test whether environmental variables have interactive effects with apex predator presence on the occurrence of mesopredators.
Material and methods
Study species
The raccoon dog is an omnivorous canid predator that weighs typically 5 kg in early summer and about 9 kg in autumn before hibernation (Kauhala 1993). It is monogamous and uses dens especially for breeding and winter sleep in areas where winters are harsh (Kauhala and Kowalczyk 2011). It invaded Finland from Soviet Union in the 1950s (Kauhala and Kowalczyk 2011). The red fox is a native species in Finland weighing typically 5 − 8 kg. It dens solitarily, in pairs or in family groups, depending on food resources (Lindström 1989). Although the red fox is omnivorous, it is more carnivorous than the raccoon dog, preying more often on birds and mammals (Kauhala et al. 1998). The badger is also an omnivorous predator feeding e.g. on invertebrates, amphibians and small rodents (Kauhala et al. 1998). Its mean weight is from 9 to 12 kg (according to the season; Macdonald and Barrett 1993). It dens in large burrows, which it digs itself and uses the year round. Both the red fox and the badger are able to kill raccoon dogs, especially pups (Kauhala and Kowalczyk 2011), but the badger also shares its burrows with raccoon dogs especially in winter (Kowalczyk et al. 2008). Thus, the presence of badgers may benefit raccoon dogs, but due to similarities in omnivorous feeding habitats of all the studied mesopredators (Elmeros et al. 2018), they may also compete with each other for food resources.
In Finland, the apex predators include the wolf, the lynx, the brown bear (Ursus arctos) and the wolverine (Gulo gulo). However, the latter two were not included in the current analysis, as wolverines are rare in southern Finland and brown bears are not considered very important predators of raccoon dogs. Lynx numbers have been steadily increasing in Finland after restrictions for hunting in 1960s. The increase has been strongest during the last two decades with about 2000 lynx individuals in year 2020 distributed to whole area of southern and central Finland (Natural Resources Institute Finland: http://riistahavainnot.fi/suurpedot/kannanarviointi/). The population increase of wolf has been slower than that of lynx, but the species has occupied new territories in particular in south-western parts of the country, in addition to territories in eastern Finland. It is estimated that there were 250 wolf individuals and 46 permanent wolf territories in Finland year 2020, whereas the population remained for a long time below 100 individuals before year 2000, being near extinction in beginning of twentieth century (Kojola et al. 2018; Natural Resources Institute Finland: http://riistahavainnot.fi/suurpedot/kannanarviointi/). Based on earlier studies, the wolf can be expected to depredate raccoon dogs and badgers (Olsson et al. 1997, Heptner and Naumov 1998, Kowalczyk et al. 2009). The lynx may again be an important predator of red foxes (Elmhagen et al. 2010, Pasanen-Mortensen et al. 2013, Pasanen-Mortensen and Elmhagen 2015, but see Wikenros et al. 2017). Raccoon dogs were, however, found more often than foxes in the diet of lynxes in Belarus (Sidorovich 2006).
Study areas and data
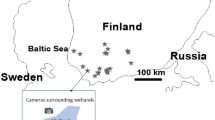
The study areas are located in southern and central Finland (Fig. 1.). The landscape in these areas is dominated by bodies of water and managed coniferous and mixed forests, with scots pine (Pinus sylvestris), Norway spruce (Picea abies), and birch (Betula sp). Agricultural areas and denser human populations are found mainly in southwestern and southern Finland, but occur sparsely within the whole study area. The data were collected from 37 different study areas where cameras (Uovision, NITEforce or Burrel) were typically set at least 300–500 m from each other (N = 580 cameras; 17 ± 13 (average ± SD) per area; in total 5996 camera days). Many of the camera sites were forest areas near lakes or other waterbodies. Data was gathered in years 2015 (2 study areas), 2016 (5 study areas), 2017 (6 study areas), 2018 (1 study areas), and 2020 (30 study areas). Four areas included data from more than one year, but cameras were in different locations each year.
A majority of the camera sites (333) included scent lure to attract predators to the site. We are not aware of any differences in the responses of different predator species to this scent lure (Gray ambush, gray fox, Urocyon cinereoargenteus, gland lure). In addition, many of the cameras were without any lures (222; Table 1), these were placed to openings in forest cover or at edge of forest and shore meadows. Finally, a small proportion of the cameras were placed near wildlife feeding stations, offering mainly cereals, apples, carrots and sugar beet (25; Table 1; these sites are known to attract different kind of animals including mesopredators). The cameras were set to take photos or in some cases videos, which were treated as a single photo, and only photos separated by 30 min were included in the data (that is, multiple photos within this time period were treated as one), except for the feeding-station data for which the time interval between photos was 10 min. Thus, the feeding-station data may include more observations than other cameras trap types in our data. However, the camera trap type (scent lure, feeding-station, no lure) was controlled for in the analysis and no significant differences were observed (see results).
Explanatory variables
Apex predator data
Data for wolf and lynx presence in Finland is based on data on yearly observations of these species, collected by the Natural Resources Institute Finland (Luke). The data includes all visual and track observations, hunting statistics, genetic analysis of non-invasive scat collection and GPS-data of apex predators in Finland (available in http://riistahavainnot.fi/suurpedot/kannanarviointi/lausunnot). Trained volunteer citizens and government officials from Luke and Parks and Wildlife Finland (Metsähallitus) put yearly a huge effort in collecting data on apex predator presence in Finland. The data we used included all wolf observations and the territories for wolf packs and pairs determined based on the observations, except for years 2015 and 2016, for which we had information only for the location of the pack and pair territory boundaries. The majority of the wolf observations were made within the determined territory boundaries (69%; Kojola et al. 2018). In the current study, we used all observations available, including those outside the territory boundaries, to include also the presence of wolves that may not yet have established a permanent territory. For lynx, the determination of the territory boundaries was based on average territory size of the lynx, thus we were less confident on territory boundaries for lynx than for the wolf.
For all cameras that were within wolf territories, we gave index value “1 “ (n = 52 cameras; average size of wolf territories in Finland is 1000 km2, Kojola et al. 2018). Outside the known territories, the index value gradually decreased to zero, based on the distance from the territory. The distance was calculated as average distance from the edge of territory and from the three closest wolf observations. Cut-off distance where the index dropped to zero was 20 km. That is, the index value was zero if there was no single wolf observation or territory edge within 20 km (n = 110 cameras with index = 0) and the index value was 0.5 if there were wolf observations and an estimated territory edge within 10 km from the camera trap (n = 418 cameras with index > 0 and < 1).
For the lynx, we gave an index value “1”, if the camera was within the territory (n = 328 cameras); at the edge and outside the territory we followed a similar approach as for the wolf, except that the cut of distance for index value “0” was in 10 km (n = 10). It should be noted, that our index is not an exact indication of the apex predator location, but an index of their presence in the landscape. In addition, the territory boundaries were not exact.
Habitat data
We counted the area in hectares for “forest” (combination of coniferous, mixed and deciduous forest), “agriculture” (including all agricultural area classes), “water” (combination of wetlands and water bodies), and “urban” (all artificial surfaces) from CORINE land cover map data (resolution 20 m × 20 m; years 2012–2018 data available by Finnish Environment Institute, SYKE) within a 500 m buffer from each camera location. The 500 m buffer was selected to roughly correspond the central part of the home range of the mesopredators (see below). In addition, we classified each camera location based on the observations made in the field to four categories: “mixed spruce and deciduous forests” (herb-rich forests and herb-rich heath forests), “pine and spruce forest” (heath forests), “agriculture” (including the edge area of agriculture and forest), and “wetland meadows” (open grassland at shore) (Table 1).
Human presence and other variables used in the models
We used data by Statistics Finland that provide the number of people living within each 1 km2 quadrat in Finland (hereafter human population density). We used the average value of squares that were within 500 m from the camera location. Other variables that we used were season, camera trap type, survey duration for each camera, and the length of growth season (Table 1). The length of growth season (days; data by Statistics Finland) describes how the location of the sites moves northeast from the south-west coastal areas of Finland. In northeast the climate becomes colder with longer winters, deeper snow cover and a shorter growing season.
Analysis
Several methods have been developed for analyzing animal densities from wildlife camera data, and many of these depend on estimates of movement or space use of the animals (Foster and Harmsen 2012; Burton et al. 2015). Our data was quite heterogeneous, including cameras with scent lures, at wildlife-feeding stations, or cameras without any attractants. Most of the cameras were also located quite far away from each other, complicating the use of the cameras for estimating movements within home range. Thus, methods such as spatial capture analysis or estimation of density based on movement activity did not seem best suited for our data. Because our intention was not to estimate absolute densities but relative occurrences and the factors affecting them, we followed many earlier studies that have used the number of photos (from now on called observations) during a survey period as the variable to be analyzed (for a review see Burton et al. 2015). We note that some of the cameras located close to each other could be within a territory of the same focal individual. Based on radiotelemetry studies in Finland, the home-range sizes are around 390 , 660 , and 1470 ha, for the raccoon dog, the badger and the red fox, respectively (Kauhala et al. 2006). Thus, it was possible that some spatial autocorrelation occurs in our data and we considered this in the analyses.
We built three generalized linear mixed-effects models (glmm) with the dependent variable being the number of observations of (1) raccoon dog, (2) red fox or (3) badger within each camera trap. In the models, the study area was set as a random effect using Kenward-Roger degrees of freedom approximation in Glimmix SAS 9.4. In addition, spatial correlations in the residuals were accounted for by adding easting and northing of each camera location as a random variable in the model to account that residuals of the model were independent and identically distributed (random_residual_ statement in Glimmix, SAS). We used Poisson distribution for the red fox and badger model that were not overdispersed (chi2/df around 1), but the raccoon dog model showed signs of overdispersion (chi2/df = 1.8), for which we used a negative binomial distribution instead. Figures were produced by taking predicted values of the full model with output statement in SAS Glimmix with Ilink command that computes the statistic on the scale of the data.
For the raccoon dog model, we added the number of red fox and badger observations as explanatory variables in the model, but added no other mesopredators for the red fox and badger models to avoid repetition. The explanatory variables used in all the three models were wolf and lynx presence indices, camera trap type (scent lure, wildlife-feeding station, no lure), survey duration (days; camera-specific), season (spring, early summer, late summer), length of growth season (days), and human population density (ind. /km2). To simplify the models, we did not include all habitat variables in each model. Instead, we did preliminary model selection with Akaike’s Information Criterion (AIC) and selected the best-fitted habitat variables to each model (the raccoon dog, the red fox and the badger model). This was done with a model using Laplace estimation method that allows AIC comparison. Habitat variables within ΔAIC two were selected to final models. To further simplify the models, we tested the effect of interactions between habitat variables (including length of growth season) and the apex predator (lynx and wolf) presence indexes, but these were removed from the models if found nonsignificant (the results for the removed variables are in Supplement Table 1). These interactions were tested, because it is known that apex predator effect on mesopredators may depend on productivity of the landscape (Elmhagen and Rushton 2007). The effect of year was tested (as a categorical variable and a continuous variable), but it had no effect (p > 0.05) in any models and was dropped out from the final models, to simplify the analysis.
Results
The raccoon dog and the red fox were both present in 36 out of the 37 study areas, but the badger was not observed in cameras of 11 study areas. In total, there were 442, 214, and 114 observations in 214, 139, and 70 cameras (out of the 580 cameras), for the raccoon dog, the red fox and the badger, respectively.
The raccoon dog model
The number of raccoon dog observations increased with the number of badger observations in the data and decreased with increasing wolf presence index in the landscape (Fig. 2a; Table 2). The presence index of the lynx and the length of growth season had an interactive effect: the negative effect of lynx was clearer in areas with short growing season (Fig. 2b; Table 2). Raccoon dogs were observed more often in herb-rich forests than in heath forests, when the area of agricultural fields increased and in late summer than in other seasons (Table 2).
The model predicted number of raccoon dog observations in camera traps versus a Wolf presence index and b Lynx presence index with data divided to two parts based on average value for length of growth season (growth season*lynx interaction in Table 2). c The red fox observations versus wolf index with data divided to two parts based on average value for area of agricultural fields (agriculture*wolf interaction in Table 3) and d The red fox versus lynx index. The badger observations versus e Wolf index and f Lynx index
The red fox model
The number of red fox observations in the camera trap data had no negative association with either wolf or lynx presence (Fig. 2c, d; Table 3), but the wolf presence had an interactive effect with area of agriculture (within 500 m from a camera): the wolf presence had a stronger positive effect on red fox observations in areas with less agriculture than in areas with more agriculture (Fig. 2d). The red fox observations were also positively associated with human population density (Table 3).
The badger model
The number of badger observations in the camera trap data slightly decreased with increasing wolf presence and increased with increasing lynx presence (Table 4, Fig. 2e, f). For the badger, we did not observe significant interaction terms between the habitat variables and the apex predator indices. Badgers were less often observed in areas with short growth season, in early summer than in other seasons, and in wetland meadows than in other habitats (Table 4).
Discussion
We observed a negative association between the presence of apex predators, the wolf and the lynx, and the occurrence of the invasive raccoon dog in our wildlife camera trap data. The wolf had only a weak negative effect on the occurrence of native badgers but a positive effect on that of red foxes especially in landscapes with less agricultural fields than forest. The lynx was not observed to affect the red fox occurrence and had positive effect on the badger occurrence. We did not observe negative relationships between the mesopredators. On the contrary, as predicted, raccoon dogs and badgers were often observed in the photos of the same camera.
The effect of apex predators on mesopredators
Our study supports the view that a release from predation by the apex predators affects the occurrence of invasive mesopredators, possibly increasing their negative influence on the native fauna (Mooney and Cleland 2001; McGeoch et al. 2010). In our case, it is even more interesting that apex predators had a clear negative effect on the invasive raccoon dog, but only a minor negative or positive effect on the native mesopredators, the badger and the red fox. This result is in line with the recent observations by Cunningham et al. (2020) who also found that an apex predator (Tasmanian devil, Sarcophilus harrisii) had a stronger suppressive effect on an invasive mesopredator (feral cat, Felis catus) than on native mesopredators. In such a situation the apex predator can confer resistance to the impacts of invasive mesopredator populations. Examples of the invasive mammalian mesopredators that have severe consequences on native fauna include the red fox in Australia (Ruscoe et al. 2011; Wallach et al. 2015), American mink (Neovison vison) in Europe (Salo et al. 2008) and feral cat worldwide (Doherty et al. 2016). For example, the dingo (Canis dingo) is observed to be central in decreasing numbers of invasive red foxes in Australia, a severe threat for native small marsupials (Wallach et al. 2010).
Earlier studies on wolf predation on raccoon dogs are scarce, but raccoon dogs have been observed in the diet of wolves (e.g. Kowalczyk et al. 2009) and observations from Russia suggest that wolf predation on raccoon dogs can locally be substantial (Heptner and Naumov 1998). Indeed, it has been suggested that the lack of apex predators in large parts of Europe may allow the spread of the raccoon dog (Sutor et al. 2014). There is also a wide debate on the role of the wolf in shaping communities through top-down cascading effects (Ripple et al. 2014, Martin et al. 2020, but see Mech 2012, Barber-Meyer 2015). To this debate, we suggest that the wolf might have an important role in the control of invasive raccoon dogs. We also suggest that the lynx may have the same role. Lynxes are known to kill raccoon dogs (Sidorovich 2006) and also red foxes (Sunde et al. 1999; Helldin et al. 2006), with potential effects at the population level for the latter (Elmhagen et al. 2010; Pasanen-Mortensen et al. 2013). However, locally the red fox may actually benefit from carcasses left by the lynx (Wikenros et al. 2017). In our study, there was a positive correlation between badger occurrence (badger is also consuming carrion) and lynx presence, but for the red foxes the correlation remained statistically not significant.
The observed negative association between the presence of the wolf and the occurrence of the badger was very weak (see Fig. 2e), but could be expected, because the badger is mentioned to be on the kill list of the wolf (Olsson et al. 1997). Wolves also kill red foxes (Palomares et al. 1996; Wikenros et al. 2017), but earlier studies indicate that red foxes rather benefit, through carcass availability, from wolf presence than suffer much from predation by the wolf (Jędrzejewski and Jędrzejewska 1992; Wikenros et al. 2013; Ferretti et al. 2021). Interestingly, in the current study the wolf had a positive effect on red fox presence, especially in forest dominated landscapes. Perhaps red foxes are less dependent on carcasses in landscapes dominated by agriculture, where food availability likely is more diverse than in forests (Jahren et al. 2020). Similar observations that the effect of the wolf (and the lynx) on red foxes depends on productivity of environment were made by Elmhagen and Rusthton (2007). That the effect of apex predators is environment-dependent was also supported for raccoon dogs in our study, because the lynx had negative effect on raccoon dogs mainly in areas with short growing season. In practice, these were the study sites outside coastal area of our study area. The coastal area is more productive with long growth season and higher human population density but appear also to have fewer lynxes than the inland area of our study area (Fig. 2b). In any case, our results for raccoon dogs and lynxes are in line with earlier results by Elmhagen et al. (2010) who observed that the impact of the lynx on red foxes increased when productivity of landscape decreased.
The use of wildlife cameras to estimate mesopredator occurrence
We used observations in wildlife camera data, which may not be directly related to the density of the species. However, the number of observations in cameras correlated with the snow-track index for the red fox in the Finnish wildlife triangle scheme (see supplement). This is an important result supporting that camera trap data correlates with local animal abundance. Cameras were not placed randomly, but mostly to forests surrounding lakes. An earlier study from Finland indicated that the raccoon dog is a generalist in its habitat use (Holopainen et al. 2020a), although it favours habitats which are mosaics of deciduous or mixed forests, damp meadows, fields and gardens (Kauhala and Auttila 2010; Kauhala et al. 2010). Habitat was controlled for in the analysis and the main results of the study should not be affected by the spatial design of the camera traps. In any case, we note that our data on mesopredator occurrence is not a random sample of their occurrence in Finland. In addition, we cannot rule out the possibility that movement behaviour of individual mesopredators, and thus likelihood to be detected in wildlife camera photos, varied within our study area. For example, the effect of apex predators on raccoon dog occurrence in the cameras could be, in addition to mortality, partly behavioural, i.e. individuals becoming more vary and moving shorter distances within apex predator territories. In this case, individuals might be less often observed in wildlife camera photos within apex predator territories than elsewhere. Unfortunately, we lack data for evaluating this possibility. For example, we cannot use the majority of our cameras to estimate speed of individuals in the camera view. It, however, seems unlikely that the speed detected in camera view would much reflect the possible changes in space use of mesopredators within or outside apex predator territories. Individuals might rather avoid habitats with high risk when apex predators are present.
We did not detect any negative associations between the occurrences of the mesopredators at the same site. Instead, there was a clear positive association between badger and raccoon dog observations in a camera site. Raccoon dogs and badgers often share their home ranges and the dens, both in summer and during hibernation, and the raccoon dog may benefit from the good burrow digging abilities of the badger (Kowalczyk et al. 2008; Kauhala and Auttila 2010), which may partly explain their co-occurrence. There may also be preferences for similar habitats, although all the studied three mesopredators are quite flexible in their habitat use and differences do occur in their habitat preferences (Kauhala and Auttila 2010; Drygala and Zoller 2013). Despite being located within the same area, the mesopredator species may use the best habitat patches at different times.
The invasive raccoon dog in Finland and the effects of habitat and human density on mesopredator occurrence
Our results indicate that the invasive raccoon dog is currently the most common mesopredator in southern and central Finland. That is, the raccoon dog was the most frequently observed mesopredator in our data, red fox the second, and badger the rarest of the studied three species. The relative abundance of these species is of interest, because we lack the abundance data for raccoon dog and badger in Finland, whereas the red fox abundance index can be estimated annually with snow-track data (snow tracts/10 km/24 h; the Finnish wildlife triangle scheme, Pellikka et al. 2005). We used so called ‘naïve’ occupancy in camera trap data (number of detection events per sampling effort; MacKenzie et al. 2018), which underestimates the true occurrence and does not take into account differences in movement activity or cryptic behaviours between the species (see above). However, it is notable that the raccoon dog has smaller home ranges than badgers and red foxes (Kauhala et al. 2006; Kauhala and Kowalczyk 2011). Thus, the occurrence of the raccoon dog should be underestimated compared to the badger and red fox in our data. This further supports the conclusion that the invasive raccoon dog is currently the most abundant mesopredator within our study area. This highlights the importance of active management of this invasive species.
Habitat variables had only modest effects in raccoon dog model emphasizing the generalist nature of the species. However, the observed relationships were as expected (Kauhala et al. 2010) i.e. occurrence increasing with increasing agricultural areas and more productive land. For the red fox, our results were also in accordance with earlier results showing that in forest-dominated landscapes the species favors areas near agriculture (Kurki et al. 1998; Holopainen et al. 2020a). The result that badgers did not use wetland meadows was also expected for this forest species (Kauhala and Auttila 2010). Presence of humans as measured by population density of inhabitants did not affect the mesopredators in our study in the countryside, expect having a positive effect on the red fox occurrence. All three species, the raccoon dog, the badger and the red fox, are found close or within urban areas in Finland, but the red fox is the most clearly urbanized mesopredator, being located within cities in Finland and elsewhere (Kauhala et al. 2016).
To conclude, apex predators have both desirable and undesirable impacts depending on ecosystem contexts (Ritchie et al. 2012). It has been suggested that apex predators may be important in what is called predator-driven ecological restoration, but this approach includes economic, environmental and social considerations that need to be accounted for (Ritchie et al. 2012). Our study supports the view that apex predators can play an important role in controlling invasive alien mesopredators (Ritchie and Johnson 2009; Wallach et al. 2010). That is, native apex predators can promote resilience against introduced mesopredators and ecosystem change.
Data availability
The datasets generated during and/or analysed during the current study are available from the authors and Natural Resources Institute Finland Luke and will be made available on request from the corresponding author.
References
Anonymous (2017) Comission implementing regulation (EU) 2017/1263. Official Journal of the European Union: L 182/37.
Barber-Meyer SM (2015) Trophic cascades from wolves to grizzly bears or changing abundance of bears and alternate foods? J Anim Ecol 84:647–651
Baum JK, Worm B (2009) Cascading top-down effects of changing oceanic predator abundances. J Anim Ecol 78:699–714
Burton AC, Neilson E, Moreira D, Ladle A, Steenweg R, Fisher JT, Bayne E, Boutin S (2015) Review: wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J Appl Ecol 52:675–685
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566
Cunningham CX, Johnson CN, Jones ME (2020) A native apex predator limits an invasive mesopredator and protects native prey: tasmanian devils protecting bandicoots from cats. Ecol Lett 23:711–721
Dahl F, Åhlén PA (2019) Nest predation by raccoon dog Nyctereutes procyonoides in the archipelago of northern Sweden. Biol Invasions 21:743–755
Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR (2016) Invasive predators and global biodiversity loss. PNAS 113:11261–11265
Drygala F, Zoller H (2013) Spatial use and interaction of the invasive raccoon dog and the native red fox in Central Europe: competition or coexistence? Eur J Wildl Res 59:683–691
Elmeros M, Mikkelsen DMG, Norgaard LS, Pertoldi C, Jensen TH, Chriel M (2018) The diet of feral raccoon dog (Nyctereutes procyonoides) and native badger (Meles meles) and red fox (Vulpes vulpes) in Denmark. Mammal Res 63:405–413
Elmhagen B, Rushton SP (2007) Trophic control of mesopredators in terrestrial ecosystems: top-down or bottom-up? Ecol Lett 10:197–206
Elmhagen B, Ludwig G, Rushton SP, Helle P, Lindén H (2010) Top predators, mesopredators and their prey: interference ecosystems along bioclimatic productivity gradients. J Anim Ecol 79:785–794
Ferretti F, Pacini G, Belardi I, ten Cate B, Sensi M, Oliveira R, Rossa M, Burrini L, Lovari S (2021) Recolonizing wolves and opportunistic foxes: interference or facilitation? Biol J Linn Soc 132:196–210
Foster RJ, Harmsen BJ (2012) A critique of density estimation from camera-trap data. J Wildl Manage 76:224–236
Helldin JO, Liberg O, Glöersen G (2006) Lynx (Lynx lynx) killing red foxes (Vulpes vulpes) in boreal Sweden – frequency and population effects. J Zool 270:657–663
Heptner VG and Naumov NP, eds. (1998) "Sirenia and Carnivora (Sea cows; Wolves, and Bears)". Mammals of the Soviet Union. II. USA: Science Publishers, Inc. Part 1a.
Holopainen S, Väänänen V-M, Fox AD (2020a) Landscape and habitat affect frequency of artificial duck nest predation by native species, but not by an alien predator. Basic Appl Ecol 48:52–60
Holopainen S, Väänänen V-M, Fox A (2020b) Artificial nest experiment reveals inter-guild facilitation in duck nest predation. Global Ecol Cons 24:e01305
Holopainen S, Väänänen V-M, Vehkaoja M, Fox A (2021) Do alien predators pose a particular risk to duck nests in Northern Europe? Results from an artificial nest experiment. Biol Invasions 23:3795–3807
Jaatinen K, Hermansson I, Mohring B, Steele BB and Öst M (2022) Mitigating impacts of invasive alien predators on an endangered sea duck amidst high native predation pressure. Oecologia, available online.
Jahren T, Odden M, Linnell JDC, Panzacchi M (2020) The impact of human land use and landscape productivity on population dynamics of red fox in southeastern Norway. Mamm Res 65:503–516
Jędrzejewski W, Jędrzejewska B (1992) Foraging and diet of the red fox Vulpes vulpes in relation to variable food resources in Biatowieza National Park, Poland. Ecography 15:212–220
Kauhala K (1993) Growth, size, and fat reserves of the raccoon dog in Finland. Acta Theriol 38:139–150
Kauhala K (2004) Removal of medium-sized predators and the breeding success of ducks in Finland. Folia Zool 53:367–378
Kauhala K, Auttila M (2010) Habitat preferences of the native badger and the invasive raccoon dog in Finland. Acta Theriol 55:231–240
Kauhala K, Kowalczyk R (2011) Invasion of the raccoon dog Nyctereutes procyonoides in Europe: history of colonization, features behind its success, and threats to native fauna. Current Zool 57:584–598
Kauhala K, Laukkanen P, von Rége I (1998) Summer food composition and food niche overlap of the raccoon dog, red fox and badger in Finland. Ecography 21:457–463
Kauhala K, Helle P, Helle E (2000) Predator control and the density and reproductive success of grouse populations in Finland. Ecography 23:161–168
Kauhala K, Holmala K, Lammers W, Schregel J (2006) Home ranges and densities of medium-sized carnivores in south-east Finland, with special reference to rabies spread. Acta Theriol 51:1–13
Kauhala K, Schregel J, Auttila M (2010) Habitat impact on raccoon dog Nyctereutes procyonoides home range size in southern Finland. Acta Theriol 55:371–380
Kauhala K, Talvitie K, Vuorisalo T (2016) Encounters between medium-sized carnivores and humans in the city of Turku, SW Finland, with special reference to the red fox. Mamm Res 61:25–33
Kojola I, Heikkinen S, Holmala K (2018) Balancing costs and confidence: volunteer-provided point observations, GPS telemetry and the genetic monitoring of Finland’s wolves. Mamm Res 63:415–423
Koshev YS, Petrov MM, Nedyalkov NP, Raykov IA (2020) Invasive raccoon dog depredation on nests can have strong negative impact on the Dalmatian pelican’s breeding population in Bulgaria. European J Wildl Res 66:85
Kowalczyk R, Jędrzejewska B, Zalewski A, Jędrzejewski W (2008) Facilitative interactions between the Eurasian badger (Meles meles), the red fox (Vulpes vulpes), and the invasive raccoon dog (Nyctereutes procyonoides) in Białowieża Primeval Forest, Poland. Can J Zool 86:1389–1396
Kowalczyk R, Zalewski A, Jędrzejewska B, Ansorge H, Bunevich AN (2009) Reproduction and mortality of invasive raccoon dogs Nyctereutes procyonoides in the Białowieża Primeval Forest (eastern Poland). Ann Zool Fenn 46:291–301
Krüger H, Väänänen V-M, Holopainen S, Nummi P (2018) The new faces of nest predation on agricultural landscapes – a wildlife camera survey with artificial nests. Eur J Wildl Res 64:76
Kuijper DPJ, Sahlén E, Elmhagen B, Chamaillé-Jammes S, Sand H, Lone K, Cromsigt JPGM (2016) Paws without claws? Ecological effects of large carnivores in anthropogenic landscapes. Proc R Soc B 283:20161625
Kurki S, Nikula A, Helle P, Linden H (1998) Abundances of red fox and pine marten in relation to the composition of boreal forest landscapes. J Anim Ecol 67:874–886
Lindström E (1989) Food limitation and social regulation in a red fox population. Ecography 12:70–79
Macdonald D, Barrett P (1993) Mammals of Britain and Europe. Harper Collins Publishers
MacKenzie D, Royle JN, Pollock K, Bailey L, Hines J (2018) Occupancy estimation and modelling inferring patterns and dynamics of species occurrence. Academic Press
Martin J, Chamaillé-Jammes S, Waller DM (2020) Deer, wolves, and people: costs, benefits and challenges of living together. Biol Rev 95:782–801
McGeoch MA, Butchart SHM, Spear D, Marais E, Kleynhans EJ, Symes A, Chanson J, Hoffmann M (2010) Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Divers Distrib 16:95–108
McMahon BJ, Doyle S, Gray A, Kelly SBA, Redpath SM (2020) European bird declines: do we need to rethink approaches to the management of abundant generalist predators? J Appl Ecol 57:1885–1890
Mech LD (2012) Is science in danger of sanctifying the wolf? Biol Cons 150:143–149
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. PNAS 98:5446–5451
Nummi P, Väänänen V-M, Pekkarinen A-J, Eronen V, Mikkola-Roos M, Nurmi J, Rautiainen A, Rusanen P (2019) Alien predation in wetlands - raccoon dog and waterbird breeding success. Balt for 25:228–237
Olsson O, Wirtberg J, Andersson M, Wirtberg I (1997) Wolf Canis lupus predation on moose Alces alces and roe deer Capreolus capreolus in south-central Scandinavia. Wildl Biol 3:13–25
Palomares F, Ferreras P, Fedriani JM, Delibes M (1996) Spatial relationships between Iberian lynx and other carnivores in an area of south-western Spain. J Appl Ecol 33:5–13
Pasanen-Mortensen M, Elmhagen B (2015) Land cover effects on mesopredator abundance in the presence and absence of apex predators. Acta Oecol 67:40–48
Pasanen-Mortensen M, Pyykönen M, Elmhagen B (2013) Where lynx prevail, foxes will fail - limitation of a mesopredator in Eurasia. Glob Ecol Biogeogr 22:868–877
Pellikka J, Rita H, Lindén H (2005) Monitoring wildlife richness—Finnish applications based on wildlife triangle censuses. Ann Zool Fenn 42:123–134
Pöysä H, Linkola P (2021) Extending temporal baseline increases understanding of biodiversity change in European boreal waterbird communities. Biol Conserv 257:109139
Prugh LR, Sivy KJ (2020) Enemies with benefits: integrating positive and negative interactions among terrestrial carnivores. Ecol Lett 23:902–918
Ripple WJ, Beschta RL, Fortin JK, Robbins CT (2014) Trophic cascades from wolves to grizzly bears in Yellowstone. J Anim Ecol 83:223–233
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998
Ritchie EG, Elmhagen B, Glen AS, Letnic M, Ludwig G, McDonald RA (2012) Ecosystem restoration with teeth: what role for predators? Trends Ecol Evol 27:265–271
Ruscoe WA, Ramsey DSL, Pech RP, Sweetapple PJ, Yockney I, Barron MC, Perry M, Nugent G, Carran R, Warne R, Brausch C, Duncan RP (2011) Unexpected consequences of control: competitive vs. predator release in a four-species assemblage of invasive mammals. Ecol Lett 14:1035–1042
Salo P, Nordström M, Thomson RL, Korpimäki E (2008) Risk induced by a native top predator reduces alien mink movements. J Anim Ecol 77:1092–1098
Sidorovich VE (2006) Relationship between prey availability and population dynamics of the Eurasian lynx and its diet in northern Belarus. Acta Theriol 51:265–274
Sunde P, Overskaug K, Kvam T (1999) Intraguild predation of lynxes on foxes: evidence of interference competition? Ecography 22:521–523
Sutor A, Schwarz S, Conraths FJ (2014) The biological potential of the raccoon dog (Nyctereutes procyonoides, Gray 1834) as an invasive species in Europe—new risks for disease spread? Acta Theriol 59:49–59
Suutarinen J, Kojola I (2017) Poaching regulates the legally hunted wolf population in Finland. Biol Cons 215:11–18
Terborgh J and Estes JA (2010) Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature. Washington, DC, Island Press, 488pp. ISBN: 978–1–59726–486–0.
Wallach AD, Johnson CN, Ritchie EG, O’Neill AJ (2010) Predator control promotes invasive dominated ecological states. Ecol Lett 13:1008–1018
Wallach AD, Ripple WJ, Carroll SP (2015) Novel trophic cascades: apex predators enable coexistence. Trends Ecol Evol 30:146–153
Wikenros C, Sand H, Ahlqvist P, Liberg O (2013) Biomass flow and scavengers use of carcasses after re-colonization of an apex predator. PLoS ONE 8:e77373
Wikenros C, Aronsson M, Liberg O, Jarnemo A, Hansson J, Wallgren M, Sand H, Bergström R (2017) Fear or food – abundance of red fox in relation to occurrence of lynx and wolf. Sci Rep 7:9059
Acknowledgements
We thank all the field assistants participating in the data collection and the landowners who gave the permission to put wildlife cameras to their property. Finnish Ministry of Agriculture and Forestry has supported data collection in the majority of the study areas.
Funding
Open Access funding provided by University of Turku (UTU) including Turku University Central Hospital. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Selonen, V., Brommer, J.E., Holopainen, S. et al. Invasive species control with apex predators: increasing presence of wolves is associated with reduced occurrence of the alien raccoon dog. Biol Invasions 24, 3461–3474 (2022). https://doi.org/10.1007/s10530-022-02850-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02850-2