Abstract
Global climate change could alter the range, abundance, and interactions of species, potentially favouring invasive species and harming endemics. Ship rats (Rattus rattus) are one of the world's worst invasive predators but are typically absent from Aotearoa New Zealand's native Fuscospora cliffortioides (mountain beech) forest above 1000 m. Stoats (Mustela erminea) are another damaging invasive predator in Aotearoa New Zealand and prey on ship rats. We analyse community trapping records 2007–2020 to investigate the spatial and temporal distribution of ship rats and their key predator stoats at Craigieburn Forest Park. We document an invasion of ship rats after 2010 at Craigieburn and hypothesised two drivers of the increase in rat abundance: (1) more frequent mountain beech high-seed years providing more food for rats; and (2) warming winter temperatures allowing rats to invade areas that were previously too cold. We were unable to test a third possible driver (stoat trapping resulting in top-down meso-predator release) due to the nature of the data available. Rats were more common at low altitudes near streams, and stoats were more common at higher-altitudes on forest edges. Average winter temperature, but not seedfall, increased significantly at Craigieburn mid-elevations since 1972. The best predictor of annual rat catch was higher average winter temperatures interacting with high seedfall. This shows a key interaction between two global change drivers: warming temperatures have allowed exotic ship rats to expand into areas where they were previously absent, increasing the resultant "thermal squeeze" of predation on sensitive endemic birds at higher-altitude sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, invasive species distribution patterns are changing and shifting along with the environments they inhabit (McGlone and Walker 2011; Bellard et al. 2013). At the same time, global weather patterns are shifting and temperatures are predicted to increase (McGlone and Walker 2011; Bellard et al. 2013). This raises an important question about the synergistic effect of these two drivers of global change, with warming temperatures predicted to favour invasive species (McGlone and Walker 2011; Bellard et al. 2013). Introduced predators are considered as a key threat to the survival of Aotearoa New Zealand birdlife and biodiversity (Kelly et al. 2005; Innes et al. 2010). Ship rats (Rattus rattus) and stoats (Mustela erminea) are among the most widespread of these mammalian predators and have substantial impacts on native bird numbers (Kelly et al. 2005; Innes et al. 2010; Innes and Russell 2021; King and Veale 2021). For example, in Nelson Lakes National Park southern beech (Nothofagaceae) forest, bird decline and changes in community composition are attributed to exotic species such as ship rats, stoats, possums (Trichosurus vulpecula) and wasps (Vespula vulgaris; Elliott et al. 2010, Walker et al. 2019b).
Ship rats are present throughout Aotearoa New Zealand native forests, although historically they have only been common at altitudes lower than 1000 m and are largely absent from alpine tussock (Efford et al. 2006; Christie et al. 2017; Innes and Russell 2021). Southern beech forests make up much of Aotearoa New Zealand’s remaining native forest, particularly in the cooler, drier areas of the South Island (Wardle 1984; Kelly et al. 2008; Wiser et al. 2011; Walker et al. 2019a). In higher-altitude beech forest ship rats are usually absent or rare except in some years (King 1997; Christie et al. 2017; Walker et al. 2019a), and their densities in mid- to high-altitude beech forests seem to be determined by several factors such as pulsed food sources, cold temperatures, and presence of predators (King 1997; Innes et al. 2001; Walker et al. 2019a; Innes and Russell 2021). However, it is unclear how these drivers interact to determine their distribution, range, and abundance in southern beech forests (Walker et al. 2019a; Innes and Russell 2021). Irregular large spatially-synchronous seeding events known as mast seeding lead to mice and stoat irruptions that then decrease within the following 12 months (Wilson et al. 1998; Schauber et al. 2002; Kelly et al. 2008). Mice are particularly responsive to these resource pulses and start increasing in numbers in early summer by feeding on caterpillars which eat beech flowers and then on ripe seeds (Fitzgerald et al. 1996; Ruscoe et al. 2006; Murphy and Nathan 2021). Stoats in turn increase in response to the mice (Kelly et al. 2008; Jones et al. 2011; Veale et al. 2015). The relationships between mast seeding, ship rat densities, and stoat responses are less well known; however, there is some evidence of bottom-up effects of ship rat abundance on stoat abundance (Jones et al. 2011; Norbury 2017).
To combat the effects of invasive mammalian predators on native wildlife, Aotearoa New Zealand has a history of predator removal and control through widespread trapping and poisoning and the creation of island sanctuaries where native species are free from predation pressure (Butler et al. 2014; Innes et al. 2019). Most trapping networks primarily target stoats, as trapping is not effective in controlling ship rat populations (Langham and Kelly 2011; Innes et al. 2019) except in small or predator-fenced patches. Careful placement of traps contributes to more efficient predator control in community trapping efforts, to maximise the control possible with limited volunteer hours and trap resources (Ruffell et al. 2015). Features of trap location and the surrounding area can affect trap catch rates (King et al. 1996; Christie et al. 2009; Ruffell et al. 2015). These trapping networks play an important role in protecting native biodiversity (Butler et al. 2014; Innes et al. 2019) and allow insights into how populations change in space and time by analysis of catch rates and by necropsy of trapped animals.
While there is considerable effort placed in protecting native species in Aotearoa New Zealand (Innes et al. 2019), warming temperatures are predicted to increase risks to native species due to the extension of predator range into cooler areas (Elliott et al. 2010; Walker et al. 2019b). For example, currently ship rat range is thought to be limited by cold temperatures, and they are at low densities in mountain beech forest except in mast years (Walker 2019a; Innes and Russell 2021). If so, ship rats may be able to increase their range if environmental conditions become more favourable with climate change. However, there is little evidence of climate-change driven range increases for mammalian predators in Aotearoa New Zealand.
This paper aims to use community trapping data from a mid-altitude beech forest in two ways. First, to measure individual trap catch rates as a function of local site features and measure the spatial distribution of rats and stoats through trap-catches, to increase efficiency of trapping efforts. Second, it aims to document the expansion of the ship rat population in Craigieburn Forest since sustained trapping began in 2007 and to consider potential environmental drivers of the expansion.
For the first aim, we investigated the distribution of stoat and ship rat catches in Craigieburn Forest Park. We tested factors such as trap altitude, slope, proximity to water, and proximity to forest margins as ship rat and stoat distributions may be affected by these (King et al. 1996; Harper et al. 2005; Christie et al. 2006, 2009, 2017).
For the second aim, we tested two non-exclusive hypotheses for why there may be an increase in ship rat abundance: (1) more frequent beech high-seed years providing more food for rats; and (2) warming winter temperatures allowing rats to invade areas that were previously too cold. We were unable to test a third possible factor (stoat trapping releasing rats at Craigieburn from predation pressure) because our mammal data were from kill-traps, so we had no non-treatment data. However, we tested for any decrease in stoat catches over time to check for one possible mechanism of meso-predator release. Based on previous research, we also hypothesised that stoat catch would increase after a large seedfall, but one year later due to the time lag between when the mast causes an increase in mice abundance, and when stoats next breed after feeding on the more abundant mice.
Methods
Study site
Craigieburn Forest Park (43° 09′ S, 171° 43′ E) is situated in inland Canterbury, Aotearoa New Zealand, near the main divide of the Southern Alps. The altitude of our study area in Craigieburn Forest Park ranges from 800 to 1300 m; below the treeline at 1300 m the park is almost entirely old-growth forest of mountain beech (Fuscospora cliffortioides, previously Nothofagus solandri var. cliffortioides). Mountain beech is a mast-seeding tree that creates a pulse of resources in the form of intermittent heavy seed crops (Schauber et al. 2002; Allen et al. 2012; Kelly et al. 2013). The park has had control of some mammalian pests undertaken since 2007 by the community group Canterbury Environmental Trust (CET), with prior mammal monitoring for scientific studies 1973–79 and 1999–2004 (King 1983; Kelly et al 2005). Land use surrounding the study site is primarily unmanaged grassland, with some exotic conifers on the eastern edge of the study area facing the highway from Helicopter Hill to Bridge Hill (Fig. 1; Ledgard and Paul 2008). Since 2016 these conifers have been gradually removed to reduce conifer invasion to the east of our study site.
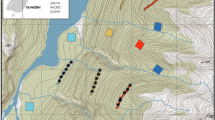
Map of Craigieburn Forest Park network of 263 traps with total stoat catch per trap 2007–2020 (blue, maximum = 15) and rat catch per trap (pink, maximum = 8). Open circles indicate traps with no recorded catches (n = 10). Seedfall trap location marked with red star (1050 m, 8 traps). Stoats were slightly (but significantly) more common at higher altitudes, while rats were much more common at lower altitudes
Trapping records
The trap lines set up by the Canterbury Environmental Trust (CET) used in this study run along six walking tracks and narrow gravel roads on the eastern side of the Craigieburn range (Fig. 1). Since 2007 volunteers from CET and latterly on two lines the New Zealand Conservation Trust (NZCT) have been checking the lines. Each time a trap was found with a catch, volunteers recorded the date, trap ID, and the species caught. The NZCT also provided GPS points for many of the traps on the lines for which they became responsible.
All the 263 traps analysed here were DOC 200 kill traps, which were designed to target mustelids (stoats, weasels Mustela nivalis and ferrets M. furo) but catch other species as bycatch (Warburton et al. 2008). The traps are "double sets", i.e. a trap is a single box with two trap mechanisms inside. Hence the traps have the capacity to catch two animals in them at a time, however this did not occur frequently. Weasels were recorded separately in the trapping data. Weasels are typically less common than stoats in beech forest (King and Murphy 2021). Correct identification of weasels vs stoats is sometimes problematic, which we consider further in results. Rats were caught frequently and were recorded. While the trap counts list them only as rats, all rat records are very likely to be ship rats, as kiore (Rattus exulans) are not present in Canterbury and Norway rats (R. norvegicus) are thought to be absent from South Island beech forest (Russell and Innes 2021). Mice (Mus musculus) are usually too light to set the mechanism off, so although some were caught, they were not tallied. Hedgehogs (Erinaceus europaeus) were caught but were not recorded after 2010. Feral cats (Felis catus) and brushtail possums are too big to be effectively targeted by these traps as the box is designed to prevent access by larger animals, however smaller individuals are sometimes caught and have been recorded.
There were 215 individual dates where traps were recorded with a catch, though not all traps were checked on every date. There was inconsistent frequency of trap-checking across years; however, this was not adjusted for in the analysis as catches were grouped into annual totals for the analysis. Furthermore, there was very low catch frequency. Check frequency would become an issue where traps were consistently filled with animals, as was the case for the Rotoiti Nature Recovery Project (RNRP) during a mast year. There, 34 checks were completed from June 2004-December 2004 (more than one check per week, with 30 weeks in the period) with up to 200 rats caught in a month and 93% of traps filled with rats between checks (Paton et al. 2005). Hence, at RNRP the rate of clearing and resetting traps strongly affected the total number of rats caught. In Craigieburn, there was always a large proportion of traps available at a given time due to the much lower abundance of animals. The maximum number of traps filled on one date was 52, or 20%, of the total traps. On average 9% of traps had a catch on a given date.
The CET and the NZCT provided trapping records. CET records started on 14 May 2007 and records up to 11 January 2020 were used in this study, and NZCT records started on 18 February 2014 and ended on 23 June 2019. The data did not have the information necessary to calculate the usual standard index of abundance of catches per 100 trap-nights, so total catches over each period were used instead. All the lines analysed were present for the whole study period, so trapping effort remained constant. Because winter temperature and seedfall (which is available to rodents from March to September) were used as predictor variables, and rats can breed in winter following a mast seeding event (Innes and Russell 2021), annual rat catches were tallied over 12-month periods from winter to the following autumn (June to May) rather than calendar years. Since stoats only breed once a year with young emerging in December, annual stoat catches were tallied by calendar years. Kill-trapping has been widely used to monitor ship rat abundance (e.g. King et al. 1996), and trapping and footprint tracking data have been shown to be closely correlated (Blackwell et al. 2003), as are tracking data and absolute density (Brown et al. 1996). Therefore, total catch was considered as a reasonable proxy for species abundance in this study.
Environmental data
For spatial analysis, GPS waypoints were used to determine trap elevation. Additional information about trap features was recorded on-site as the waypoints were marked. Water presence was recorded as ‘yes’ if a trap was within 80 m of a water body (using ArcGIS software and trap waypoints). Vegetation within 10 m of traps was classified as ‘Shrub or tussock’ (no forest canopy cover), ‘Forest edge’ (trees bordering tussock clearings), or ‘Forest’ (mature forest away from an edge). Finally, slope was recorded using an inclinometer and classified as ‘steep’ (> 25°), ‘moderate’ (10°–25°) and ‘flat’ (< 10°).
Seedfall was recorded from 1965 as average annual viable mountain beech seedfall in Craigieburn collected in eight seedfall traps, each 0.28 m2 catch area at 1050 m (for sampling details see Allen et al. 2014). Seedfall rates have been shown to vary with elevation (Allen et al. 2014) and although there were also seedfall traps at 1340 m and 1150 m, only the seedfall traps at 1050 m were used as this was considered the closest elevation to most of the trapping operation. Viable rather than total seed counts were used because because viable seeds have a nutritious endosperm eaten by rodents which drives their population dynamics (Beggs 1999; Ruscoe et al. 2005). As seedfall was strongly right-skewed it was log(10) transformed before analysis to improve normality. We analysed seedfall for the period with any rat data at Craigieburn (1972–2020). We also checked trends in seedfall over time using untransformed viable seedfall, to ensure that the log transformation was not obscuring any trends, but this did not change the conclusions so is not presented here.
We sought weather information to relate to rat catch patterns. Air temperatures were measured on-site at Craigieburn (43° 9.1′ S, 171° 42.8′ E, 914 m) from 1964 until 2013. For data to 2020 we checked three other weather stations for correlation with the Craigieburn data: physical weather stations at Arthur's Pass (National Institute of Water and Atmospheric Research, 42° 56.5′ S, 171° 33.8′ E, 745 m) and Cass (University of Canterbury, 43° 2.1′ S, 171° 45.6′ E, 580 m), and the nearest grid point in NIWA's Virtual Climate Station Network (VCSN; 43° 7.3′ S, 171° 43.3′ E, 940 m), which uses nearby physical stations to estimate weather at grid points for each day. The highest correlation with Craigieburn winter temperatures was for Arthur's Pass, so this site was used for 2007–2020 analyses. Since Arthur's Pass temperatures were only available from 2006, for analysis of temperature trends 1972–2020 we combined data from Craigieburn (up till 2005) and Arthur’s Pass (2006 onwards). The mean difference in monthly temperatures at the two sites in winter over the period of overlap (n = 23 months) was less than 0.05 °C. Average winter temperature (mean of the daily mean air temperatures during June–August) was used as the predictor, as there is evidence that the low temperatures may limit rat abundance (Ruffell et al. 2015; Walker et al. 2019a, b).
Analysis
All analyses were conducted using R version 4.1.0 (R Core Team 2018). The relationship between elevation and catch per mammal kill-trap (total over the study period) was investigated using a generalised linear mixed model (GLMM) with a Poisson distribution. The elevation factor had to be log transformed to fit on the same scale as the other model parameters. Catch per mammal kill-trap (over total study period) was also tested by proximity to water, slope, and other environmental features as potential predictors, using GLMMs with Poisson distribution. Trends in temperature and seedfall over time were investigated using generalised linear models (GLMs). Stoat and rat abundance over time (catch per trap per year) used a GLMM with a Poisson distribution. The influence of seedfall on stoat abundance was tested using GLMM with a Poisson distribution, as was the influence of temperature and seedfall on rats. Random factors of the trap line and individual trap ID were used to account for the spatial non-independence and overdispersion (Zuur 2009).
Results
Factors affecting rat and stoat catches per trap
From the 263 individual traps recorded in the 2007–2020 period, there were nine mammal species caught. Stoats (901 catches) made up 64% of the total recorded catches in the traps over the trapping period, and ship rats (317) were 24% of the catches. Mice and hedgehogs were caught but not counted; other species caught were 91 weasels, 11 cats, 8 possums, 2 ferrets and one rabbit. There were no records of any birds caught in the traps as bycatch over the 13 years. Ten traps had no recorded animal catches over the whole study period (Fig. 1).
Stoat catches per trap (total 2007–2020) varied significantly with altitude (ANOVA on Poisson GLMM, Chisq = 8.424, p = 0.004, df = 1) with fitted values of 3.0 per trap at 800 m rising to 4.4 per trap at 1250 m. The effect of trap placement on stoat catches was significant (Chisq = 24.40, df = 2, p < 0.001) with 1.75 ± 0.59 stoats caught per trap in the open, compared to 5.8 ± 1.17 on the forest edge (Fig. 2). Stoats were also caught significantly more often on moderate and steep ground than flat ground when adjusted for altitude (Chisq = 9.48, df = 2, p < 0.05). Proximity to water had no effect on stoat catches (Chisq = 1.61, df = 1, p = 0.20).
There was a significant negative relationship between total rat catch per trap and altitude (ANOVA on Poisson GLMM, Chisq = 81.67, df = 1, p < 0.001, Fig. 3). Rats were caught more on flat ground than moderate terrain, however this became non-significant when corrected for altitude, presumably because steeper sites tended to be at higher altitudes. Rats were also caught significantly more frequently at traps near water, even when this was adjusted for altitude (ANOVA on Poisson GLMM, Chisq = 6.45, df = 1, p < 0.05). The average number of rats caught per trap was 1.98 ± 0.26 (± S.E.) in proximity to water, but 0.89 ± 0.12 away from water (Fig. 2). This shows that rat catches per trap varied with altitude and proximity to water.
Factors affecting annual stoat and rat catches
There was a significant positive linear trend in average winter (June–August) daily mean temperature, both from 2007 to 2019 (spanning our trapping data; F = 15.07, DF = 1, 11, p = 0.003), and from 1972 (the earliest years with rat information at Craigieburn) to 2020, (Fig. 4). However, there were no significant trends in log(10) transformed viable seedfall (F = 0.66, df = 1, 47, p = 0.42) nor total viable seedfall values (F = 1.43, df = 1, 47, p = 0.24) at mid-elevations 1972–2019.
Environmental trends over time. A Trend in mean winter (June–August) daily mean air temperatures (°C) in the Craigieburn area 1972–2020 (linear regression: F = 9.654, df = 1,47, p < 0.01). The blue line indicates the fitted regression (with confidence interval). B Log (10) Viable seedfall (per calendar year) over the same period. There was no significant trend in log seedfall over time
Stoat abundance had a significant positive relationship with seedfall from the previous year (z = 6.49, df = 1, p < 0.001). There was no trend in stoat abundance over time 2007–2020, either when considering time alone (z = − 1.51, df = 1, p = 0.13) or in a model controlling for seedfall (time effect: z = 0.001, df = 1, p = 0.88). Temperature was non-significant as a predictor of stoat numbers (z = − 1.325, df = 1, p = 0.185). The best model only had one fixed effect, previous seedfall, confirming previous research on drivers of stoat abundance in southern beech forests.
There were 91 weasels trapped along with 901 stoats. Although identification of weasels vs stoats is sometimes inaccurate, two analyses suggest that weasel records were plausible. Firstly, weasel and stoat numbers across calendar years were not significantly correlated (r = 0.45, df = 12, p = 0.10). Secondly, unlike stoats, annual weasel totals were not significantly related to the previous year’s seedfall (z = − 0.081, df = 1, p = 0.24) but were significantly related to the current year’s seedfall (z = 4.60, df = 1, p < 0.001). These results are consistent with weasels having a faster reproductive cycle than stoats (King and Murphy 2021).
Rats, unlike stoats, had a significant positive trend over time, from when the trapping began in 2007 to 2020 (Fig. 5; slope ± SE = 0.13 ± 0.017, z = 7.67, p < 0.001). This increase over time was not explained by the clearance of conifers on the eastern edge of the study area from 2016, as that land use change adjoined only a small part of the study area and came after the major increase in rats. For predicting annual rat catch (June–May years), average winter temperature, and viable seedfall as single predictors were both significant (p < 0.001). The best model (lowest AIC) was a biologically relevant one including both viable seedfall and temperature and their interaction (Table 1, Fig. 6). There was a clear peak of predicted rat abundance only when both seed and temperature were high (Fig. 6). Since temperature is the only environmental driver that changed significantly from 2007 to 2020 (Fig. 4), this suggests that increasing temperature is the main reason for the observed increase in rats at Craigieburn (Fig. 5).
Craigieburn fitted rat catch per trap per June-May year 2007–2020 predicted by mean winter air temperature (June–August) at Arthurs Pass and Log(10) Seedfall (m2) and their interaction, from the Poisson GLMM shown in Table 1
Discussion
We found both local and large-scale effects on mammal catch rates at Craigieburn. At a local scale, the significant effects of trap placement on trapping rate presumably indicate where each species was more common. Conservation action is often costly in time and money, and therefore maximising efficiency of kills through trap placement is of interest to conservation groups (Ruffell et al. 2015). Traps that were in open habitat caught fewer stoats than those in forest, and stoats were caught most frequently on the forest edge. Stoats in prior studies have been found more frequently at forest or trapping margins, although in the past this has been ascribed to the effect of the trap margin (Christie et al. 2006, 2009). King (1996) observed that mice and weasels were found more frequently on forest edges and considered the weasels were responding to the increased presence of food (mice). Rob Allen (RBA unpubl. data) has found higher mountain beech seedfall in seed traps at the forest edge than in the adjacent forest at Craigieburn, suggesting an increased food source at the forest margin. Thus, in Craigieburn Forest there may be a similar effect of increased prey leading to increased predators on the forest margin. Ship rats were caught much more frequently in traps near water, a phenomenon not observed for ship rats before. However, Christie et al. 2009 found an increase in rats in poor drainage areas, and other rat species such as Norway rats (Rattus norvegicus) are well known to be more abundant near bodies of water (King et al. 1996). Finally, we found much higher rat catch rates at lower altitudes, which supports conclusions that ship rats are limited by cold temperatures. In contrast, stoats were significantly more common at higher altitudes.
Climate-driven range expansion
Rats have effectively colonised mid-altitudes in Craigieburn Forest Park during this study. Rats were never found in the study area over five years in the 1970s (King 1983), and only a single rat was recorded during a second five-year study in 1999–2003 (Kelly et al 2005). In our trapping data no rats were seen in 2007, only three were caught in 2008–09, but 314 have been caught in the 10 years since. This documents an upslope expansion of rats from lower altitudes where they are ubiquitous in New Zealand.
At a large scale, rats apparently responded to temperature, while stoats responded to prey abundance. The pattern of stoat abundance increasing one year after high seedfall is consistent with previous studies at Craigieburn (King and Powell 2011) showing stoats respond to increased prey availability (mice and rats). But our evidence for climate-driven range expansion in ship rats in mountain beech forest is novel. We showed that there has been a large increase in mean winter temperature over the period when the observed expansion in ship rat abundance occurred. We also showed that the temperatures had increased significantly since the 1970s, the period with the first data on rat abundance (King 1983). It is interesting to note that our results suggest that even in low seedfall years, when there are high temperatures ship rats will still be present in low densities. Furthermore, we found no significant increase in seedfall over our study period at 1050 m, matching the finding of Allen et al (2014) over the period 1965–2009 in Craigieburn Forest Park. At higher altitudes seedfall did increase; Allen et al (2014) found rising seedfall over time above 1150 m. However, ship rats were caught primarily below 1050 m, with only 12% of all rats caught above this altitude and only 3% caught above 1150 m. So, we consider it unlikely the rats in our study were affected by the increase in seedfall over time at the highest altitudes. Our results indicated that ship rats need both high seedfall and warm temperatures to be present in large numbers. Land-use change can be a driver of changes in invasive species abundance. However, most of our study site had no change in land use, and we found no evidence that the clearing of exotic conifers on the eastern edge of the study site from 2016 increased rat abundance. Thus, the increase in ship rat abundance in Craigieburn is likely to have been driven largely by the observed temperature increase over the past 13 years.
As our study was observational it cannot prove causation, but these data are useful to generate hypotheses for experimental tests. We hypothesise that warm temperatures increase rat abundance, either directly through lower winter mortality from hypothermia, and/or indirectly through increased food supply such as invertebrates over the winter months. Studholme (2000) considered rats to be limited to areas with a winter monthly average temperature above 2 °C. The extent to which various environmental drivers limit ship rat densities is little understood and apparently varies with forest type and other factors (Innes and Russell 2021). Food resources for ship rats in southern beech forests are primarily driven by beech masting which is temporally highly variable (Kelly et al. 2013). Alternative foods besides beech mast may explain the less predictable relationship between mast events and rats, compared to mast events and mice and stoats (Elliott and Kemp 2016; Walker et al. 2019a).
Meso-predator release
There has been some discussion in the literature about whether predator control allows ship rats to increase in abundance through predator release (Blackwell et al. 2003; Innes and Russell 2021). Although stoats in our study showed no decreasing trend in catch rates over time, the extent to which stoat control allows ship rats to increase could not be tested as the data came from kill traps so there were no data from a non-treatment area. This means that it may be the compounding effect of stoat control and temperature increase that has led to the increase in ship rat abundance. Several papers have found little evidence for stoats limiting rat populations (Blackwell et al. 2003; Ruscoe et al. 2011). However, some studies in southern beech forest have suggested stoat trapping may lead to an increase in rats (Efford et al. 2006; Robertson and de Monchy 2012, Whitau 2017). The question of when and to what extent certain predators may suppress ship rat densities is thus unresolved (Walker et al. 2019a). An experimental manipulation would be necessary to test this further. However, it is likely that in Craigieburn the stoat trapping is not the primary driver of rat abundance. Stoat trapping for five years at this site in the 1970s, when winters were colder, did not result in any records of ship rats (King 1983), and we found a significant relationship between average winter temperature and ship rat abundance.
Conclusions
Global warming-driven range expansion of invasive species is a widespread concern, with average global temperatures already up by more than 1 °C and expected to increase by at least 1.5 °C by 2040 (McGlone and Walker 2011; Millar et al. 2017). There have been numerous global scale predictions showing the range expansion of invasive species across small and large scales, with the primary predictions of a range shift towards the poles as some areas become more habitable and some less (McGlone and Walker 2011; Bellard et al. 2013). Invasive plants and predators are of particular concern in Aotearoa New Zealand, as there is the potential for the squeezing out of native species from previous climate-based refugia such as mountain habitat (McGlone and Walker 2011; Walker et al. 2019b). Climate-driven range expansion of rodents and potentially an increase in the presence of other predator species like stoats is of significant concern in many alpine and subalpine areas. Such areas across Aotearoa New Zealand are home to many endangered bird species such as the rock wren (Xenicus gilviventris) and Hutton’s shearwater (Puffinus huttoni), as well as wētā, and lizard species (O’Donnell et al. 2017). Ship rats are known to prey on wētā and lizards, as well as small bird species and eggs (Innes et al. 2010; O’Donnell et al. 2017; Clapperton et al. 2019). Therefore, temperature-driven ship rat expansion may lead to increased predation pressure in Aotearoa New Zealand on species that are currently partially protected by the altitudinal restrictions on ship rat abundance (Christie et al. 2017; Walker et al. 2019b). Globally, understanding how species will change in range and abundance in response to changing climate is clearly very important.
Warming temperatures are a significant global threat as exotic species are predicted to have an advantage in range expansion and invasion (Bellard et al. 2013). This study provides evidence of climate-driven range expansion of ship rats in mid-altitude mountain beech forests, over a period of 42 years, and thus emphasises the potential synergistic effects of multiple drivers of global change. The observed rat increase in the last 13 years is a relatively short timeframe, as mountain beech forests are predicted to move upslope very slowly with increased warming (Walker et al. 2019b). Therefore, endemic species that are more abundant at higher altitudes may be exposed to higher predation pressure (Elliott et al. 2010; O’Donnell et al. 2017). The knowledge that ship rats are increasing in Craigieburn, apparently due to climate warming, lets conservation managers take appropriate action, and contributes to better predictions of possible ship rat range shifts around the world.
Data availability
The datasets analysed for this study are available from the Environmental data initiative (EDI) or from the corresponding author on request.
References
Allen RB, Mason NWH, Richardson SJ, Platt KH (2012) Synchronicity, periodicity and bimodality in inter-annual tree seed production along an elevation gradient. Oikos 121:367–376
Allen RB, Hurst JM, Portier J, Richardson SJ (2014) Elevation-dependent responses of tree mast seeding to climate change over 45 years. Ecol Evol 4(18):3525–3537
Beggs R (1999) Comparison of the quality of red and silver beech (Nothofagus) seeds in Nelson Lakes National Park, New Zealand. N Z J Bot 37:495–501
Bellard C, Thuiller W, Leroy B, Genovesi P, Bakkenes M, Courchamp F (2013) Will climate change promote future invasions? Glob Change Biol 19:3740–3748
Blackwell GL, Potter MA, McLennan JA, Minot EO (2003) The role of predators in ship rat and house mouse population eruptions: drivers or passengers? Oikos 100:601–613
Brown KP, Moller H, Innes J, Alterio N (1996) Calibration of tunnel tracking rates to estimate relative abundance of ship rats (Rattus rattus) and mice (Mus musculus) in a New Zealand forest. N Z J Ecol 20:271–275
Butler D, Lindsay T, Hunt J (2014) Paradise saved: the remarkable story of New Zealand’s wildlife sanctuaries and how they are stemming the tide of extinction. Random House Auckland, New Zealand
Christie J, Kemp J, Rickard C, Murphy E (2006) Measuring stoat (Mustela erminea) and ship rat (Rattus rattus) capture success against micro-habitat factors. N Z J Ecol 30:43–51
Christie JE, Wilson PR, Taylor RH, Elliott G (2017) How elevation affects ship rat (Rattus rattus) capture patterns, Mt Misery, New Zealand. N Z J Ecol 41:113–119
Christie J, Brown D, Westbrooke I, and Murphy E (2009) Environmental predictors of stoat (Mustela erminea) and ship rat (Rattus rattus) capture success. DOC Res & Dev Ser
Clapperton BK, Maddigan F, Chinn W, Murphy EC (2019) Diet, population structure and breeding of Rattus rattus L. in South Island beech forest. N Z J Ecol 43(2):1–8
Efford MG, Fitzgerald BM, Karl BJ, Berben PH (2006) Population dynamics of the ship rat Rattus rattus L. in the Orongorongo Valley, New Zealand. N Z J Zool 33:273–297
Elliott G, Kemp J (2016) Large-scale pest control in New Zealand beech forests. Ecol Manag Restor 17:200–209
Elliott GP, Wilson PR, Taylor RH, Beggs JR (2010) Declines in common, widespread native birds in a mature temperate forest. Biol Cons 143:2119–2126
Fitzgerald BM, Daniel MJ, Fitzgerald AE, Karl BJ, Meads MJ, Notman PR (1996) Factors affecting the numbers of house mice (Mus musculus) in hard beech (Nothofagus truncata) forest. J R Soc N Z 26:237–249
Harper GA, Dickinson KJM, Seddon PJ (2005) Habitat use by three rat species (Rattus spp.) on Stewart Island/Rakiura, New Zealand. N Z J Ecol 29:251–260
Innes JG, Russell JC (2021) Ship rat Rattus rattus. In: King CM, Forsyth DM (eds) The handbook of New Zealand mammals, 3rd edn. CSIRO Publishing, Melbourne, pp 161–240
Innes JG, King CM, Flux M, Kimberley MO (2001) Population biology of the ship rat and Norway rat in Pureora Forest Park, 1983–87. N Z J Ecol 28:57–78
Innes J, Kelly D, Overton JM, Gillies C (2010) Predation and other factors currently limiting New Zealand forest birds. N Z J Ecol 34:86–114
Innes J, Fitzgerald N, Binny R, Byrom A, Pech R, Watts C, Gillies C, Maitland M, Campbell-Hunt C, Burns B (2019) New Zealand ecosanctuaries: types, attributes and outcomes. J R Soc N Z 49:370–393
Jones C, Pech R, Forrester G, King CM, Murphy EC (2011) Functional responses of an invasive top predator Mustela erminea to invasive meso-predators Rattus rattus and Mus musculus, in New Zealand forests. Wildl Res 38:131–140
Kelly D, Brindle C, Ladley JJ, Robertson AW, Maddigan FW, Butler J, Ward-Smith T, Murphy DJ, Sessions LA (2005) Can stoat (Mustela erminea) trapping increase bellbird (Anthornis melanura) populations and benefit mistletoe (Peraxilla tetrapetala) pollination? N Z J Ecol 29:69–82
Kelly D, Koenig WD, Liebhold AM (2008) An intercontinental comparison of the dynamic behavior of mast seeding communities. Popul Ecol 50:329–342
Kelly D, Geldenhuis A, James A, Holland EP, Plank MJ, Brockie RE, Cowan PE, Harper GA, Lee WG, Maitland MJ, Mark AF, Mills JA, Wilson PR, Byrom AE (2013) Of mast and mean: differential-temperature cue makes mast seeding insensitive to climate change. Ecol Lett 16:90–98
King CM (1983) The relationships between beech (Nothofagus sp.) seedfall and populations of mice (Mus musculus), and the demographic and dietary responses of stoats (Mustela erminea), in three New Zealand forests. J Anim Ecol 52:141–166
King CM (1997) Distribution and response of rats Rattus rattus, R. exulans to seedfall in New Zealand beech forests. Pac Conserv Biol 3:143–155
King CM, Murphy EC (2021) Mustela nivalis vulgaris. In: King CM, Forsyth DM (eds) The handbook of New Zealand mammals, 3rd edn. CSIRO publishing, Melbourne, pp 309–316
King CM, Powell RA (2011) Managing an invasive predator pre-adapted to a pulsed resource: a model of stoat (Mustela erminea) irruptions in New Zealand beech forests. Biol Invasions 13:3039–3055
King CM, Veale AJ (2021) Mustela erminea. In: King CM, Forsyth DM (eds) The handbook of New Zealand mammals, 3rd edn. CSIRO publishing, Melbourne, pp 285–309
King CM, Innes JG, Flux M, Kimberley MO, Leathwick JR, Williams DS (1996) Distribution and abundance of small mammals in relation to habitat in Pureora Forest Park. N Z J Ecol 20:215–240
Langham S, and Kelly D (2011) Stoat and rat effects on five-minute bird counts in Nelson Lakes National Park. Nelson-Marlborough Conservancy, Department of Conservation
Ledgard NJ, Paul TSH (2008) Vegetation successions over 30 years of high country grassland invasion by pinus contorta. NZ Plant Prot 61:98–104
McGlone M, Walker S (2011) Potential effects of climate change on New Zealand’s terrestrial biodiversity and policy recommendations for mitigation, adaptation and research. Department of Conservation, Science for Conservation 312:1–77
Millar RJ, Fuglestvedt JS, Friedlingstein P, Rogelj J, Grubb MJ, Matthews HD, Skeie RB, Forster PM, Frame DJ, Allen MR (2017) Emission budgets and pathways consistent with limiting warming to 1.5 ° C. Nat Geosci 10:741–747
Murphy EC, Nathan HW (2021) House mouse Mus musculus. In: King CM, Forsyth DM (eds) The handbook of New Zealand mammals, 3rd edn. CSIRO Publishing, Melbourne, pp 161–240
Norbury G (2017) The case for ‘bottom-up’pest management. N Z J Ecol 41(2):271–277
O’Donnell CFJ, Weston KA, Monks JM (2017) Impacts of introduced mammalian predators on New Zealand’s alpine fauna. N Z J Ecol 41:1–22
Paton BR, Maitland MJ, Taylor GE, Wotherspoon JA, Gasson PA, Leggett SA (2005) Rotoiti Nature Recovery Project annual report July 2004–June 2005. Department of Conservation. pp. 165
R Core Team (2018) R: a language and environment for statistical computing. In: R foundation for statistical computing, R Core Team Vienna, Austria
Robertson HA, De Monchy PJM (2012) Varied success from the landscape-scale management of kiwi Apteryx spp. in five sanctuaries in New Zealand. Bird Conservation International 22:429–444
Ruffell J, Innes J, Bishop C, Landers T, Khin J, Didham RK (2015) Using pest monitoring data to inform the location and intensity of invasive-species control in New Zealand. Biol Cons 191:640–649
Ruscoe WA, Elkinton JS, Choquenot D, Allen RB (2005) Predation of beech seed by mice: effects of numerical and functional responses. J Anim Ecol 74(6):1005–1019
Ruscoe WA, Norbury G, Choquenot D (2006) Trophic interactions among native and introduced species. In: Allen R, Lee WG (eds) Biological invasions in New Zealand. Springer, Berlin, pp 247–263
Ruscoe WA, Ramsey DSL, Pech RP, Sweetapple PJ, Yockney I, Barron MC, Perry M, Nugent G, Carran R, Warne R, Brausch C, Duncan RP (2011) Unexpected consequences of control: competitive vs. predator release in a four-species assemblage of invasive mammals. Ecol Lett 14:1035–1042
Russell JC, Innes JG (2021) Norway rat Rattus norvegicus. In: King CM, Forsyth DM (eds) The handbook of New Zealand mammals, 3rd edn. CSIRO Publishing, Melbourne, pp 161–240
Schauber EM, Kelly D, Turchin P, Simon C, Lee WG, Allen RB, Payton IJ, Wilson PT, Cowan PE, Brockie RE (2002) Masting by eighteen New Zealand plant species: the role of temperature as a synchronizing cue. Ecology 83:1214–1225
Studholme B (2000) Ship rat (Rattus rattus) irruptions in South Island beech (Nothofagus) forest. Conservation Advisory Science Notes No. 318
Veale AJ, McMurtrie P, Edge KA, Clout MN (2015) The effects of mice on stoats in southern beech forests. Austral Ecol 40:32–39
Walker S, Kemp JR, Elliott GP, Mosen CC, Innes JG (2019a) Spatial patterns and drivers of invasive rodent dynamics in New Zealand forests. Biol Invasions 21:1627–1642
Walker S, Monks A, Innes J (2019b) Thermal squeeze will exacerbate declines in New Zealand’s endemic forest birds. Biol Cons 237:166–174
Warburton B, Poutu N, Peters D, Waddington P (2008) Traps for killing stoats (Mustela erminea): improving welfare performance. Anim Welf 17:111–116
Wardle J (1984) The New Zealand beeches: ecology, utilisation and management. New Zealand Forest Service, Wellington
Whitau KR (2017) Responses to pest control in Nelson beech forest. Master’s thesis, University of Canterbury
Wilson PR, Karl BJ, Toft RJ, Beggs JR, Taylor RH (1998) The role of introduced predators and competitors in the decline of kaka (Nestor meridionalis) populations in New Zealand. Biol Cons 83:175–185
Wiser SK, Hurst JM, Allen RB, Wright EF (2011) New Zealand’s forest and shrubland communities: a quantitative classification based on a nationally representative plot network. Appl Veg Sci 14:505–523
Zuur AF (2009) Mixed effects models and extensions in ecology with R. Springer, London
Acknowledgements
We thank Ray and Marie Goldring of Canterbury Environmental Trust, and New Zealand Conservation Trust, Nicci Dillon, Allan Palmer, Troy Forsyth and Jay Harris for access to trapping data and help with field work.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the completion of this study. Data was collected by Rob Allen, volunteer trappers, and Holly Harris. Material preparation, analysis and first draft were completed by Holly Harris and Dave Kelly and advice from John Innes. All authors comments on previous versions of the manuscript and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harris, H.A.L., Kelly, D., Innes, J. et al. Invasive species and thermal squeeze: distribution of two invasive predators and drivers of ship rat (Rattus rattus) invasion in mid-elevation Fuscospora forest. Biol Invasions 24, 2547–2559 (2022). https://doi.org/10.1007/s10530-022-02789-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02789-4