Abstract
Several alien predator species have spread widely in Europe during the last five decades and pose a potential enhanced risk to native nesting ducks and their eggs. Because predation is an important factor limiting Northern Hemisphere duck nest survival, we ask the question, do alien species increase the nest loss risk to ground nesting ducks? We created 418 artificial duck nests in low densities around inland waters in Finland and Denmark during 2017–2019 and monitored them for seven days after construction using wildlife cameras to record whether alien species visit and prey on the nests more often than native species. We sampled various duck breeding habitats from eutrophic agricultural lakes and wetlands to oligotrophic lakes and urban environments. The results differed between habitats and the two countries, which likely reflect the local population densities of the predator species. The raccoon dog (Nyctereutes procyonoides), an alien species, was the most common mammalian nest visitor in all habitats and its occurrence reduced nest survival. Only in wetland habitats was the native red fox (Vulpes vulpes) an equally common nest visitor, where another alien species, the American mink (Neovison vison), also occurred among nest visitors. Although cautious about concluding too much from visitations to artificial nests, these results imply that duck breeding habitats in Northern Europe already support abundant and effective alien nest predators, whose relative frequency of visitation to artificial nests suggest that they potentially add to the nest predation risk to ducks over native predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation pressure seems to be the most important factor affecting nesting success in boreal breeding ducks (Holopainen et al. 2015). Predator presence and densities vary between habitats, which affects local avian nest predation rates (Nilsson et al. 1985; Stephens et al. 2005). Populations of egg-consuming predators are considered to have increased during the last five decades in Europe (Kauhala 1996; Panek and Bresinski 2002; Roos et al. 2018), affecting nesting success of water birds (MacDonald and Bolton 2008; Brzezinski et al. 2019). In addition to native species, several invasive alien predator species have become dispersed widely in Europe, such as the raccoon dog (Nyctereutes procyonoides), American mink (Neovison vison) and raccoon (Procyon lotor, Kauhala 1996). Invasive species affect native ecosystems by complex interactions with native species (McGeoch et al. 2010) of which predation is likely among those causing the most profound direct effects (Mooney and Cleland 2001).
The effects of alien species on their prey species are considered potentially to be greater than those of native predators (Salo et al. 2007), although the true influence of alien species as nest predators remains largely unstudied and unclear. A review showed that the effect of American mink on ground nesting birds is significant (Bonesi and Palazon 2007), while for the raccoon dog (Mulder 2012) and raccoon (Salgado 2018) (although less well studied) the pattern was not so clear. However, recent studies have raised particular concerns regarding the role of the raccoon dog as a nest predator (Krüger et al. 2018; Dahl and Åhlen 2019; Nummi et al. 2019). The presence of just a few individual raccoon dogs can have major adverse impacts on inland nesting waterbird colonies (Koshev et al. 2020).
In this study, we compare the relative frequency of alien and native mammal nest predator occurrence in different duck breeding habitats in Finland and Denmark. To do so, we established large numbers of artificial duck nests monitored by wildlife cameras, to identify the visitation rate of different predator species at the nests. In addition to primary predators, we were also able to monitor secondary predators (following initial predation visits by the same or other species) to provide a broader perspective of the predator fauna associated with a given site. We fully appreciate that predator presence at artificial nests might not reflect actual nest predation rates (but see Anthony et al. 2006). However, we use this approach here to reveal the relative abundance and activity of different predator species in different habitats in response to a common food resource, which mimics genuine wild duck nests in the very early stages of egg laying. Because our nests were distributed over large areas at very low densities, we assume the predator-specific nest visitation rates reflects to some extent the species’ local density and relative nest predation pressure. We hypothesized that alien species are no more abundant visitors at the duck nests than native predators, nor are they more effective egg predators.
Material and methods
Study areas
We conducted artificial nest experiments in 2017–2019 to compare the presence and abundance of potential nest-predator species. We established artificial nests in wetland habitats along the gradient from temperate broad-leaf to boreal forest within three areas in Finland 2017–2019 (North-Savo, Häme, Uusimaa) and two areas in Denmark 2019 (east Jutland, west Jutland) (Fig. 1, Online Appendix 1 and 2), further subdivided into 12 subareas based on location and landscape (Online Appendix 3). We established artificial nests near to permanent lakes retaining water throughout the summer, but also around wetlands characterised by shallow water with varying shorelines, which included seasonal ponds, beaver ponds, man-made ponds and/or larger flooded wetland complexes with varying water levels. One subarea in Uusimaa lies within an urbanized part of the capital city area, where our nests were established along blue and green corridors (i.e. city parks with and without water elements; Online Appendix 3).
Map of the study areas in Finland (1) North-Savo, (2) Häme, (3) Uusimaa, and Denmark (4) east Jutland and (5) west Jutland (south-north transition c. 800 km) (Base map: Esri 2019)
For the purposes of this analysis, we defined two major types of water bodies: permanent lakes and shallow wetlands with varying shoreline. Studied water bodies varied from oligotrophic to eutrophic in water quality. All the studied water bodies at the Finnish study areas freeze during the winter. Seasonal ponds might not exist every year, and will dry out during the course of summer. Danish water bodies do not freeze every year, which was the case also in 2018–2019.
In this study, we focus only upon the effect of mammalian predators, which can potentially result in loss of eggs, but also threaten the survival of duck females. The mammalian predator species differ naturally between the study habitats and areas. In both Finland and Denmark red fox (Vulpes vulpes), pine marten (Martes martes), European badger (Meles meles), stoat (Mustela erminea), European polecat (Mustela putorius), Eurasian otter (Lutra lutra), brown rat (Rattus norvegicus) and European hedgehog (Erinaceus europaeus) are widespread (Lindén et al. 1996; Baagøe and Jensen 2007). In addition, Finland has Eurasian lynx (Lynx lynx) (Lindén et al. 1996) and Denmark has stone marten (Martes foina) as native species (Baagøe and Jensen 2007). Alien mammals include raccoon dog (Nyctereutes procyonoides), American mink (Neovison vison), domestic cat (Felis catus), and domestic dog (Canis lupus familiaris) in both countries as well as raccoon (Procyon lotor) very locally in Denmark (Kauhala 1996; Salgado 2018). All these mammals were classified as potential predators of adult female ducks or eggs. In addition, Finland and Denmark support a range of avian nest predators that do not threat duck females (Holopainen et al. 2020a).
While ducks breed in all the study areas, we are well aware that the areas differ from each other in ways that are highly likely to affect local predator density and occurrence (Online Appendix 4). In this analysis, for instance, we have not controlled for the hunting effort on native and/or alien predators within the areas. However, in Europe, ducks breed very widely (in terms of geography and habitat exploitation) and are exposed to differing diversity and densities of potential predator species. To account for this variation, it is important to recognize the effects of potential predator species on the breeding ducks throughout that range.
Nest experiment
Artificial nests were placed where a dabbling duck hen could potentially lay a clutch, based on our own experience (although nest site selection of boreal ducks remains poorly studied; Holopainen et al. 2015, Online Appendix 5). Some dabbling duck species nest along shorelines, while others can place nests in the forest far from wetlands, so our artificial nest sites reflected this distribution. Our forest nests were established inside forests, at least 70 m from the shoreline to avoid the edge effect (Paton 1994). We classified every nest site to one of the three habitat type categories to capture the habitat-level variance in local predator community: (1) forest, (2) shorelines of permanent lakes, and (3) wetlands (seasonal pond, beaver pond, man-made pond, wetland complex; also nests situated on the floating vegetation).
Each artificial nest contained two farmed mallard Anas platyrhynchos eggs and some down from shot mallard females (in Finland) or down from eider Somateria mollissima nests (Denmark), mimicking the situation in the early stage of egg laying, as far as possible, by wild mallards. This is the stage where females only visit the nest when laying an egg and the nest is not fully covered by down. Nests were constructed to resemble real ones: natural nest material from the nest surroundings was collected to form ac. 20 cm wide nest cup and used to cover the eggs slightly. Nests were established under small trees or bushes where available and within tussocks in open wetlands. Light-triggered passive wildlife cameras were mounted ca. 1–1.5 m from nests, attached on trees or 1 m stakes. Cameras responded to movement and were adjusted to take three pictures at row, followed by a one-minute pause.
We started nest experiments when ducks initiate egg laying locally: April in Denmark and late April-June in Finland following the natural nesting phenology of ducks (in Finland defined by ice-out phenology, Oja and Pöysä 2007). Nests were left for seven days without visits. All nests were established and deconstructed between 09:00 and 16:00 local time. Artificial nest density was kept low, around one nest km−2 to avoid any density effects caused by artificial nests.
In total, we established 418 nests, but four were discarded because of camera failure or excavation activities at the nest site and seven because of memory card overflow. In total, we had data from 156 forest nests, 127 shoreline nests and 124 wetland nests (see Online Appendix 3 for divisions between subareas). We counted visits made to nests by all mammalian species that represented a potential mortality risk to duck females or eggs. Croston et al. (2018) observed that depredation events at the duck nests always lasted less than half an hour, so in this study, visits made by the same species were counted as independent if the time lag between visits exceeded half an hour (i.e. we assumed that the predator was a new individual establishing a new visiting event). While we acknowledge that this threshold is rather subjective, it provides a cut-off to reflect the degree of visits by potential egg or hen predators to duck nests. We divided the visits to the nests into three categories: (1) primary predation events, (2) visits before depredation (i.e. early visits: observation at nests before a depredation event, including at nests that were not depredated at all) and (3) after depredation (secondary predation; i.e. all the visits after the primary depredation event). We defined a nest depredated if at least one egg was broken or removed. After the depredation event the initial predator is aware of the nest and egg-breakage can potentially leave cues for the other predators too (Holopainen et al. 2020a). Our focus was on this break point, to compare secondary predation events with the circumstances of the initial predation.
Predation risk posed by alien and native species
In this study, we compared the country-specific nest visiting activity of mammalian predators to find out whether alien species were visiting the nests as often as native species. We expect that the overall visitation rate reflects the potential threat posed by the species to duck hens. In addition, we assume that the relationship between initial predation events and other observations reflects the effectiveness of the species as egg predators: for instance, a species having high primary predation rates compared to early and secondary visit rates does prey on nests effectively, and vice versa. Accordingly, we calculated a species-specific predation rate for all the mammals by comparing the number of nests they depredated with all the observations made of the species, either as a secondary predator or at nests that had yet to be preyed upon. Furthermore, we compared the visitation ratios (early, primary, secondary) with those of the most common native species, the red fox.
We attempted to evaluate whether the risks of mammalian species are potentially additional or compensatory within the predator community. To do this, we (1) calculated the ratio of the number of nests visited by the species alone to the number of nests also visited by some other predator species, and (2) analyzed whether the number and composition of the predator species observed at the nest sites affected the nest survival. In the case of (1), we assume and in (2) we test a very conservative approach that the effect of one species is related to the predator community, and additive effects emerge especially in species-poor communities, although this is of course not necessarily the case (Sih et al. 1998). We acknowledge that the visits made by the predators might not be independent, but predators may use spatial memory to improve searching efficiency (Phillips et al. 2004) or utilize cues after egg-breakage (Holopainen et al. 2020a) and that these qualities may differ between the species. While we argue that every visit is potential threat, in the second analysis we take a conservative approach and consider only the occurrence of the species in the predator community. For these analyses, we included observations of all the predator species, including avian predators, to form the community variable. As our observation period was restricted to seven days, we are aware that with a longer study period, the nests potentially could have been visited by more predators. However, we argue that our results offer at least a theoretical view of predator community assembly based on relative frequency of visits.
Predation rates of artificial nests provide an uncertain reflection of those at real nests (Wilson et al. 1998). It is essential to understand that there are important differences between real and artificial nests, meaning that observations at artificial nests are unlikely to correspond with reality at genuine nests. For instance, the predator species may differ because of differing cues to nest detection; resident ducks may successfully defend the nests; human scent might attract or repel mammals that primarily use their olfactory sense to detect nests; artificial nests do not correspond the real ones; observations might be non-independent; the wrong kinds of eggs are used. All these factors might contribute bias to the range of predator species detected at artificial nests relative to genuine wild duck nests (Whelan et al. 1994; Butler and Rotella 1998; Wilson et al. 1998; Pärt and Wretenberg 2002). Effort was invested to reduce these uncertainties: real mallard eggs were used, and the species observed in the camera pictures are known predators of real duck nests (Pöysä et al. 1997; Opermanis et al. 2001). We therefore assumed that the observed species do not differ from the actual nest predator assemblage, an assumption confirmed by Anthony et al. (2006), who found the relative frequency of predator species was similar at artificial and real goose nests followed by the cameras. Our nest density was low ensuring that observations were independent. We circumvented the problem of the absence of an associated hen by focusing only on the early egg-laying stage when females are not on their nests, so the experimental set-up resembled the actual situation.
Statistics
Predator abundance
To compare the total abundance of mammalian predators with each other, we included only those nests that were visited as a primary or secondary predator(s) by the five main predator species with sufficient data. We used the number of visits made by the five mammalian species to these nests as the dependent variable: every nest thus gave five values, one per each predator species. Mammalian species formed a five level factor variable. We also added a categorical country variable to account for the potential species-specific density difference between the two countries and formed an interaction term with the country and species variable. Nest visiting data were zero-inflated and thus zero-inflated negative binomial models were used (glmmTMB, Brooks et al. 2017). We used nest ID as a random factor, as we had five values for every nest. However, as the variance parameter was estimated as zero, indicating that the nest had a limited effect on the nest visits, we excluded the random effect and used a simplified model instead. We used the most common alien species, the raccoon dog, as the baseline and included it into the intercept.
We used the G-test of goodness-of-fit to compare the observed relative abundance of red fox and raccoon dog during the one experiment week to the overall relative abundance generated from the cumulative data gathered from cameras during the entire year from another study (Online Appendix 6).
Nest predation rates
To analyse the effect of the predator community composition and richness to the nest survival, we used a generalized linear mixed model (GLMM) framework to calculate daily nest survival probability. We applied a modified logistic regression which incorporated the number of exposure days into the link function (i.e. link function includes an exponent 1/t indicating the observation time, Shaffer 2004), based on the entire data set for seven days, each beginning at 12 pm. The logistic exposure method is a modification of logistic regression and maximizes the use of nest survival data by treating each measurement day as a discrete trial. Daily nest fate was analysed as a binary response variable (1 = survived, 0 = depredated). The explanatory variables were “Day” (1,…7), “Ntot” (the number of all the species which visited the nest site, including mammalian and avian predators) and occurrence of the raccoon dog, red fox, pine/stone marten, American mink and European badger (binomial distribution). To account for the species richness dependent effects, we applied an interaction term for the species occurrence and Ntot. Nest_ID was incorporated as a random factor. We used the package lme4 (Bates et al. 2015). All analyses were carried out using R 4.0.3 (R Development Core Team 2020).
Results
Predator abundance
In total, mammals preyed on 63 nests, and visited 37 nests before the actual predation event (early visits: including those nests that survived the week) and 82 nests as secondary predators. The most common mammalian nest predators in Finland and Denmark were the raccoon dog and the red fox, respectively (Online Appendix 7). Based on all nest visits by the five most common mammalian predators, raccoon dogs visited nests significantly more often than the other four other species (Table 1). However, this pattern differed between the countries: in Denmark, the native red fox, European badger and martens (because differentiation of pine from stone martens was often impossible, we combined these species) visited the nests more often than raccoon dogs. American mink visitation rates were low and did not differ between countries (Table 2). Overall, visitation rates were higher in Finland than in Denmark (Table 1). Raccoon dog activity was also reflected in the number of nests visited: raccoon dogs visited numerically more nests than any other mammal species (Table 2). After raccoon dog, the most common nest visitors were red foxes and martens.
Nest predation rates and habitats
The most common primary predator was the raccoon dog, which was responsible for 44% of all the initial predation events made by mammals. The relative abundance of the most common predators differed between habitats, but the raccoon dog was the most common primary and secondary predator species in every habitat (Fig. 2). This species proved to be present in every kind of landscape, including within the urban areas.
The percentage of primary predation events (indicated by black bars) and secondary predation visits (grey bars) of mammalian predators in relation to visits made by all mammalian predators (avian visits excluded) at forest, shoreline and wetland habitat nests. Mammal observations for the forest nests 22 primary/44 secondary; shore nests 25/86 and wetland nests 16/64
Red fox was responsible for 22% and martens 19% of all the mammalian depredation events. Red foxes were especially common predators in wetland and martens in forest habitats (Fig. 2). Some of the secondary visits made by mustelid species remained unidentified to species level (3%).
Other mammals were rarer nest predators. European badgers were initial predators at 10% of the nests depredated by mammals. American minks preyed on relatively few nests (2%), but were more slightly more common as secondary visitors (Fig. 2). American minks were observed visiting wetland nests, but were totally absent from the forest sites. Eurasian otters visited several nests during the course of the study, but did not prey on any of them, as did the European polecats, stoats, brown rats and domestic cats (Table 2). A domestic dog destroyed one nest. Domestic cats and dogs appeared mainly at forest nests, especially in urban environments. By definition, by removing eggs from a nest, Eurasian hedgehog was responsible for one of the depredation events, although it did not break any eggs. Eurasian lynx was observed at one of the nests as secondary predator.
The two mammalian species most often witnessed as the sole species at nests were European polecats and martens (Table 2, Fig. 3). While European polecats did not prey on any of the nests, martens had the highest predation rate, as predation events were involved in almost half of their nest visits (Table 2). The third most common lone visitor was the raccoon dog, which also occurred at the nests with high predator richness (Table 2, Fig. 3). Raccoon dogs had also a high predation rate and its nest visits led to predation more often than red foxes (Table 2, Online Appendix 6). European badger and red fox were lone visitors to about one third of the nests, but both were observed also in nests with higher predator richness. For these two species, predation rates were also rather similar, showing that while these species often visited the nests, they did not prey upon them as often as martens and raccoon dogs. American mink was rarely the only predator observed at the nests (Table 2).
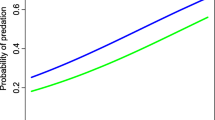
Nest survival increased significantly during the experimental period, but the predator species richness at the nest site elevated the nest depredation risk significantly (Table 3). The results shows that the pure occurrence of the martens and raccoon dogs decreased the nest survival rate. The effect for both of the species was community dependent: adding species made the species-specific effect weaker. Other species had no effect on nest survival rates.
Discussion
Our results showed that among the variety of contrasting northern European duck breeding habitats investigated in this study, the raccoon dog proved to be ubiquitous in a way not reflected among the native predator species, contrary to our hypotheses. Raccoon dog seemed to be the most common primary and secondary mammalian nest predator at monitored artificial nests in all the studied habitats, especially in forests and along the shores of permanent lakes. In wetland habitats, raccoon dog was accompanied by another alien species, the American mink. Raccoons were not observed at all during this study, although they remain relatively rare in Denmark and absent from Finland (Salgado 2018). We fully accept the limitations imposed by the fact that our experiments were carried out using artificial nests and are very aware of the uncertainties related to the method. However, as Anthony et al. (2006) showed with dusky Canada geese (Branta canadensis occidentalis), predator species ratios at artificial nests can correspond those of the real nests.
The raccoon dog was often the sole predator visiting the nests during the week of monitoring. The only native nest predators visiting nests more often alone were martens. This is logical, as martens were often observed in forests with low predator species richness (Holopainen et al. 2020b). Both raccoon dogs and martens also had high predation rates, indicating that they effectively prey on those nests that they find and not just visit them. Presence of martens and raccoon dogs in the community (including both mammalian and avian predators) significantly decreased nest survival, while the occurrence of the other species was not reflected in the nest survival rates. However, the pattern for both species was diminished in a multi-predator community. These results imply that the nest predation caused by martens and raccoon dogs is less likely to be compensatory, and more likely additive compared to predation by other mammalian species, but that the pattern seems to be dependent on predator species richness.
The broad habitat use of the raccoon dog can be explained by its highly opportunistic feeding habits. The diet of the raccoon dog has been shown to be wider than that of both the European badger and red fox (Elmeros et al. 2018) despite overlapping strongly with both these species and with the pine marten (Baltrûnaitë 2002; Elmeros et al. 2018). Across all studies, it seems that avian prey contributes a large amount to the raccoon dog diet in northern Europe (Sutor et al. 2010). Diet studies show that raccoon dogs exploit the most abundant locally available food resource (Kauhala and Kowalczyk 2011) and, for example, wetland availability increases consumption of avian prey (Sidorovich et al. 2008). Although reviews of raccoon dog diet suggest a minor impact on game birds or their eggs (Kauhala and Kowalczyk 2011), most of the earlier studies on raccoon dog diet were based on feces or stomach samples. Such studies likely underestimate egg consumption, since the species does not always consume eggshells, as confirmed by Dahl and Åhlen (2019) from natural nests and experimentally by Eronen (2007). While we accept that our study does not constitute solid proof that raccoon dogs are harmful to duck populations, the results show that it is a numerous, widely spread and frequent potential egg predator, at least at artificial nests. Nevertheless, the long-term duck breeding pair surveys show declining pair trends since 1980s for two of our study areas (Häme and North Savo), indicating changes in the some feature of their breeding habitats and possibly in their predator pressure (Pöysä et al. 2017; Pöysä et al. 2019; see also Pöysä and Linkola 2021 for the pair trends since 1950s for Häme).
Despite the role of American mink implied by other studies of freshwater ducks (Brzezinski et al. 2012, 2019; Zschille et al. 2014), especially on maritime islands (Nordström et al. 2002), in this study, the species was rarely encountered and very rarely as an active nest predator. The species occurred in wetland and lake habitats, but was not observed at all in forest habitat, confirming other studies that have shown the species habitat use is closely associated with wetland environments (Bonesi and Palazon 2007). American mink was almost never the only predator observed at the nests, which might reflect its occurrence in predator species rich wetland habitats. Nevertheless, the species seems to be rather good at finding nests that other predators have depredated, indicated by the relatively high secondary predator detection rate of the species. Therefore, while American minks did not prey on many nests, their nest visits still present a risk to duck hens. The same possible risk applies to the domestic cats and dogs observed by the cameras.
Some of the potential native nest predator species had low nest predation rates. While Eurasian otters and European polecats visited several nests, they did not consume any eggs, supporting earlier observations that these species do not prefer eggs compared to other constituents in their diet (Hammershøj et al. 2004; Malecha and Antczak 2013; Krawczyk et al. 2016). Red fox showed a low predation rate especially in Finland, suggesting that the species is not particularly effective egg predator. Alternatively, it could indicate that red fox is wary in the vicinity of newly established artificial nests and cameras or that the competition caused by raccoon dog has resulted in the diet of the Finnish red fox diet becoming more centered on live prey (Virranta and Kauhala 2011).
Predator densities vary greatly between different European countries (Roos et al. 2002) and this is also the case for invasive alien species that are in the process of dispersing into new areas (Kauhala and Kowalczyk 2011), which might explain higher raccoon dog nest depredation rates in Finland than in Denmark. In Finland, the effect of the raccoon dog as a nest visitor predominated over that of the native species, while in Denmark nest visits by native species were more common. Kill rates by hunters per unit area for the raccoon dog were higher than for red fox in Finland (the two most abundant predators), and vice versa in Denmark (Online Appendix 4), possibly reflecting the differing population levels, which were reflected in our results. While raccoon dogs are still establishing in Denmark, Finland has experienced a longer period during which the raccoon dog has become established within its national boundaries, hence local densities can be higher than those of the sympatric red fox and European badger there (Kauhala et al. 2006). As shown by the results from the urban area subject to long-term camera monitoring, the relative frequency of red fox and raccoon dog observations throughout the whole year was not different to that during the one-week nest study at the landscape scale (Online Appendix 6). Our results indicate that in the Finnish urban landscape, raccoon dogs are more common than red foxes and that the difference can be observed even during the course of a one-week study. The raccoon dog has also a smaller territory size (3.9 km−2) than red fox (6.6 km−2) and badger (14.7 km−2) allowing for a greater potential for the species to attain higher densities than those of the native mesopredators (Kauhala et al. 2006). An experiment to remove raccoon dog in southern Finland generated 8.6–20 animals shot km−2 per hunting season (Nummi et al. 2019), indicating high raccoon dog densities and immigration rates, likely associated with wetland density. Hence, as well as being an effective nest predator, raccoon dog populations can potentially achieve higher densities than those of native species, underlining the potential for increasing predator pressure on breeding ducks.
Increased predation rates are possibly limiting the numbers of ground-nesting birds, such as gamebirds and waders in Europe (MacDonald and Bolton 2008; Roos et al. 2018). Annual Finnish breeding duck pair surveys show drastic declining trends for several duck species over the last 30 years, but those breeding in eutrophic lakes have declined more than in oligotrophic lakes (Pöysä et al. 2013; Lehikoinen et al. 2016; Pöysä and Linkola 2021). Habitat-related differences in generally increasing predator pressure is one of the suspected reasons for differences in population trajectories between habitats and between species within habitats (see Pöysä et al. 2019; Pöysä and Linkola 2021).
Alien predators may also affect duck nesting success through complex species interactions. For instance, loss of black-headed gull (Chroicocephalus ridibundus) colonies removes a local protective “umbrella” of mobbing gulls, in a way that is thought to expose the nests of associated waterbird to greater predation threat (Pöysä et al. 2019). Pöysä et al. (2019) speculate that the presence of two alien species, American mink and raccoon dog, might be causing serious reductions in breeding numbers and the abandonment of inland gull colonies. Both species are known to prey on gull colonies effectively on the Finnish archipelago (Kilpi 1995).
The raccoon is the dominant avian nest predator among North American mesopredators (DeGregorio et al. 2016), being a major predator in prairie waterfowl nesting habitats (Fritzell 1978). The raccoon population is still rather small and restricted in Denmark and we did not observe any individuals of this invasive alien species in the cameras. However, because of its rapid population growth rate and range expansion in central Europe, we can also expect this population to develop rapidly in Denmark (Salgado 2018). Raccoon and raccoon dog are known opportunistic omnivores, but in Japan their habitats overlap little, as raccoon dogs (native) are more common in forested landscape and raccoons (alien) in agricultural land: competition between these two species is low (Osaki et al. 2019). Further expansion of the current distribution of the raccoon in northern Europe would enlarge the potential of alien predators to function as major duck nest predators. Raccoon dogs seem to be already common throughout most duck breeding habitats in Finland and Denmark where they occur, perhaps especially so in mixed forest-agricultural landscapes. Hence, as in Japan (Osaki et al. 2019), it is possible that raccoons occupying open agricultural areas could occur without risking high competition with raccoon dogs.
Management implications
Although our study period lasted only one week, the results show that the raccoon dogs are effective nest predators, preying on artificial nests in habitats where other predator species were scarce, but also in species rich habitats. The presence of raccoon dogs in the predator community significantly decreased nest survival. Our study suggests that the raccoon dog is a more widely distributed and common predator of artificial (and potentially therefore of wild duck) nests than any of the natural native mammalian predators in Northern Europe. In a multi-predator community, it is possible that the removal of one predator has a compensatory effect from the increase in predation risk from other predators in complex ecosystems (Ellis-Felege et al. 2012; Beggs et al. 2019). Although we cannot know that native predators might have found all the depredated nests later in the absence of raccoon dogs, our results provide support for the hypothesis of additive nest predation in the presence of this species.
The European landscape seems beneficial to the spread of the raccoon dog (Sutor and Schwarz 2013) and while already widely dispersed in Europe, an ongoing successful eradication program has been established to prevent the species establishing in Sweden (Dahl and Åhlen 2019). Based on the results from this observational study, we urge more controlled removal experiments to determine whether the eradication of alien invasive predators has a beneficial effect on duck reproductive success. If so, controlling predators, especially alien species like raccoon dog, could be an important conservation action to improve duck breeding success with potentially wider ecological benefits.
Data availability
Data will be managed by the Wetland Ecology Group, University of Helsinki and available if requested.
References
Anthony RM, Grand JB, Fondell TF, Miller DA (2006) Techniques for identifying predators of goose nests. Wildlife Biol 12:249–256. https://doi.org/10.2981/0909-6396(2006)12[249:TFIPOG]2.0.CO;2
Baagøe HJ, Jensen TS (2007) Dansk pattedyratlas. Gyldendal, Denmark
Baltrûnaitë L (2002) Diet Composition of the Red Fox (Vulpes Vulpes L.), Pine Marten (Martes Martes L.) and Raccoon Dog (Nyctereutes Procyonoides Gray) in Clay Plain Landscape, Lithuania. Acta Zool Lituanica 12(4): 362–368
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beggs R, Pierson J, Tulloch AIT, Blanchard W, Westgate MJ, Lindenmayer D (2019) An experimental test of a compensatory nest predation model following lethal control of an overabundant native species. Biol Conserv 231:122–132. https://doi.org/10.1016/j.biocon.2019.01.003
Bonesi L, Palazon S (2007) The American mink in Europe: status, impacts and control. Biol Conserv 134:470–483. https://doi.org/10.1016/j.biocon.2006.09.006
Brooks ME, Kristensen K, van Benthem KJ et al (2017) GlmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9(2):378–400. https://doi.org/10.3929/ethz-b-000240890
Brzezinski M, Natorff M, Zalewski A, Zmihorski M (2012) Numerical and behavioral responses of waterfowl to the invasive American mink: a conservation paradox. Biol Conserv 147(1):68–78. https://doi.org/10.1016/j.biocon.2011.11.012
Brzezinski M, Żmihorski M, Nieoczym M, Wilniewczyc P, Zalewski A (2019) The expansion wave of an invasive predator leaves declining waterbird populations behind. Divers Distrib 26:138–150. https://doi.org/10.1111/ddi.13003
Butler MA, Rotella JJ (1998) Validity of using artificial nests to assess duck-nest success. J Wildl Manag 62(1):163–171. https://doi.org/10.2307/3802274
Croston R, Ackerman JT, Herzog MP, Kohl JD, Hartman CA, Peterson SH et al (2018) Duck nest depredation, predator behavior, and female response using video. J Wildlife Manage 82:1014–1025. https://doi.org/10.1002/jwmg.21444
Dahl F, Åhlén PA (2019) Nest predation by raccoon dog Nyctereutes procyonoides in the archipelago of northern Sweden. Biol Invasions 21:743–755. https://doi.org/10.1007/s10530-018-1855-4
DeGregorio BA, Chiavacci SJ, Benson TJ, Sperry JH, Weatherhead PJ (2016) Nest predators of North American birds: continental patterns and implications. Bioscience 66:655–665. https://doi.org/10.1093/biosci/biw071
Ellis-Felege SN, Conroy MJ, Palmer WE, Carroll JP (2012) Predator reduction results in compensatory shifts in losses of avian ground nests. J Appl Ecol 49:661–669. https://doi.org/10.1111/j.1365-2664.2012.02126.x
Elmeros M, Mikkelsen DMG, Norgaard LS, Pertoldi C, Jensen TH, Chriel M (2018) The diet of feral raccoon dog (Nyctereutes procyonoides) and native badger (Meles meles) and red fox (Vulpes vulpes) in Denmark. Mammal Res 63(4):405–413. https://doi.org/10.1007/s13364-018-0372-2
Eronen V (2007) Supikoiran ravinnon koostumus uusmaalaisilla lintuvesillä. Masters thesis, University of Helsinki
Esri (2019) "Light grey canvas" [basemap]. Scale Not Given. "Light grey canvas map". Aug 30, 2019. http://www.arcgis.com/home/item.html?id=ed712cb1db3e4bae9e85329040fb9a49 Accessed 1 November 2019
Fritzell EK (1978) Habitat use by prairie raccoons during the waterfowl breeding season. J Wildl Manage 42(1):118–127
Hammershøj M, Thomsen EA, Madsen AB (2004) Diet of free-ranging American mink and European polecat in Denmark. Acta Theriol 49(3):337–347
Holopainen S, Arzel C, Dessborn L et al (2015) Habitat use in ducks breeding in boreal freshwater wetlands: a review. Eur J Wildl Res 61:339–363. https://doi.org/10.1007/s10344-015-0921-9
Holopainen S, Väänänen V-M, Fox AD (2020a) Artificial nest experiment reveals inter-guild facilitation in duck nest predation. Glob Ecol Conserv 24:1–7. https://doi.org/10.1016/j.gecco.2020.e01305
Holopainen S, Väänänen V-M, Fox AD (2020b) Landscape and habitat affect frequency of artificial duck nest predation by native species, but not by an alien predator. Basic Appl Ecol 48:52–60. https://doi.org/10.1016/j.baae.2020.07.004
Kauhala K (1996) Introduced carnivores in Europe with special reference to central and northern Europe. Wildlife Biol 2:197–204. https://doi.org/10.2981/wlb.1996.019
Kauhala K, Holmala K, Lammers W, Schregel J (2006) Home ranges and densities of medium-sized carnivores in south-east Finland, with special reference to rabies spread. Acta Theriol 51(1):1–13. https://doi.org/10.1007/BF03192650
Kauhala K, Kowalczyk R (2011) Invasion of the raccoon dog Nyctereutes procyonoides in Europe: history of colonization, features behind its success, and threats to native fauna. Curr Zool 57(5):584–598. https://doi.org/10.1093/czoolo/57.5.584
Kilpi M (1995) Breeding success, predation and local dynamics of colonial common gulls Larus canus. Ann Zool Fennici 32(2):175–182
Koshev YS, Petrov MM, Nedyalkov NP, Raykov IA (2020) Invasive raccoon dog depredation on nests can have strong negative impact on the dalmatian pelican’s breeding population in Bulgaria. Eur J Wildl Res 66:85. https://doi.org/10.1007/s10344-020-01423-9
Krawczyk AJ, Bogdziewicz M, Majkowska K, Glazaczow A (2016) Diet composition of the eurasian otter Lutra lutra in different freshwater habitats of temperate Europe: a review and meta-analysis. Mammal Rev 46:106–113
Krüger H, Väänänen V-M, Holopainen S, Nummi P (2018) New faces for nest predation in agricultural landscapes – a wildlife camera survey with experimental nests. Eur J Wildl Res 64:76. https://doi.org/10.1007/s10344-018-1233-7
Lehikoinen A, Rintala J, Lammi E, Pöysä H (2016) Habitat-specific population trajectories in boreal waterbirds: alarming trends and bioindicators for wetlands. Anim Conserv 19:88–95. https://doi.org/10.1111/acv.12226
Lindén H, Hario M, Wikman M (1996) Riistan jäljille. Riista- ja kalatalouden tutkimuslaitos. Edita, Helsinki
MacDonald MA, Bolton M (2008) Predation on wader nests in Europe. Ibis 150:54–73. https://doi.org/10.1111/j.1474-919X.2008.00869.x
Malecha AW, Antczak M (2013) Diet of the European polecat Mustela putorius in an agricultural area in Poland. Folia Zool 62:48–53
McGeoch M, Butchart SHM, Spear D et al (2010) Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Divers Distrib 16:95–108. https://doi.org/10.1111/j.1472-4642.2009.00633.x
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. PNAS USA 98:5446–5451. https://doi.org/10.1073/pnas.091093398
Mulder JL (2012) A review of the ecology of the raccoon dog (Nyctereutes procyonoides) in Europe. Lutra 55:101–127
Nilsson SG, Björkman C, Forslund P, Höglund J (1985) Egg predation in forest bird communities on islands and mainland. Oecologia 66:511–515
Nordström M, Högmander J, Nummelin J, Laine J, Laanetu N, Korpimäki E (2002) Variable responses of waterfowl breeding populations to long-term removal of introduced American mink. Ecography 25:385–394. https://doi.org/10.1034/j.1600-0587.2002.250401.x
Nummi P, Väänänen V-M, Pekkarinen A-J et al (2019) Alien predation in wetlands – raccoon dog and waterbird breeding success. Balt For 25(2):228–237
Oja H, Pöysä H (2007) Spring phenology, latitude and the timing of breeding in two migratory ducks: implications of climate change impacts. Ann Zool Fennici 44:475–485
Opermanis O, Mednis A, Bauga I (2001) Duck nests and predators: interaction, specialisation and possible management. Wildl Biology 7(2):87–96. https://doi.org/10.2981/wlb.2001.012
Osaki A, Sashika M, Abe G et al (2019) Comparison of feeding habits and habitat use between invasive raccoons and native raccoon dogs in Hokkaido Japan. BMC Ecol 19:35. https://doi.org/10.1186/s12898-019-0249-5
Panek M, Bresinski W (2002) Red fox Vulpes vulpes density and habitat use in a rural area of western Poland in the end of the 1990s, compared with the turn of the 1970s. Acta Theriol 47:433–442
Paton PW (1994) The effect of edge on avian nest success how strong is the evidence. Conserv Biol 8: 17–26. http://www.jstor.org/stable/2386717
Phillips ML, Clark WR, Nusser SM, Sovada MA, Greenwood RJ (2004) Analysis of predator movement in prairie landscapes with contrasting grassland composition. J Mammal 85:187–195. https://doi.org/10.1644/1545-1542(2004)085%3c0187:AOPMIP%3e2.0.CO;2
Pärt T, Wretenberg J (2002) Do artificial nests reveal relative nest predation risk for real nests? J Avian Biol 33(1):39–46. https://doi.org/10.1034/j.1600-048X.2002.330107.x
Pöysä H, Rintala J, Lehikoinen A, Väisänen RA (2013) The importance of hunting pressure, habitat preference and life history for population trends of breeding waterbirds in Finland. Eur J Wildl Res 59:245–256. https://doi.org/10.1007/s10344-012-0673-8
Pöysä H, Elmberg J, Gunnarsson G, Holopainen S, Nummi P, Sjöberg K (2017) Habitat associations and habitat change: seeking explanation for population decline in breed-ing wigeon Anas penelope. Hydrobiologia 785:207–217
Pöysä H, Lammi E, Pöysä S, Väänänen V-M (2019) Collapse of a protector species drives secondary endangerment in waterbird communities. Biol Conserv 230:75–81. https://doi.org/10.1016/j.biocon.2018.12.016
Pöysä H, Linkola P (2021) Extending temporal baseline increases understanding of biodiversity change in European boreal waterbird communities. Biol Conserv 257:109139. https://doi.org/10.1016/j.biocon.2021.109139
Pöysä H, Milonoff M, Virtanen J (1997) Nest predation in hole-nesting birds in relation to habitat edge: an experiment. Ecography 20(4):329–335. https://doi.org/10.1111/j.1600-0587.1997.tb00377.x
R Development Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Roos S (2002) Functional response, seasonal decline and landscape differences in nest predation risk. Oecologia 133(4):608–615. https://doi.org/10.1007/s00442-002-1056-8
Roos S, Smart J, Gibbons DW, Wilson JD (2018) A review of predation as a limiting factor for bird populations in mesopredator-rich landscapes: a case study of the UK. Biol Rev 93:1915–1937. https://doi.org/10.1111/brv.12426
Salgado I (2018) Is the raccoon (Procyon lotor) out of control in Europe? Biod Conserv 27:2243–2256. https://doi.org/10.1007/s10531-018-1535-9
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. P Roy Soc B-Biol Sci 274:1237–1243. https://doi.org/10.1098/rspb.2006.0444
Shaffer TL (2004) A unified approach to analyzing nest success. Auk 121(2):526–540. https://doi.org/10.1642/0004-8038(2004)121[0526:AUATAN]2.0.CO;2
Sidorovich VE, Solovej IA, Sidorovich AA, Dyman AA (2008) Seasonal and annual variation in the diet of the raccoon dog Nyctereutes procyonoides in northern Belarus: the role of habitat type and family group. Acta Theriol 53:27–38. https://doi.org/10.1007/BF03194276
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355. https://doi.org/10.1016/s0169-5347(98)01437-2 (PMID: 21238339)
Stephens SE, Rotella JJ, Lindberg MS, Taper ML, Ringelman JK (2005) Duck nest survival in the missouri coteau of North Dakota: landscape effects at multiple spatial scales. Ecol Appl 15:2137–2149. https://doi.org/10.1890/04-1162
Sutor A, Kauhala K, Ansorge H (2010) Diet of the raccoon dog Nyctereutes procyonoides — a canid with an opportunistic foraging strategy. Acta Theriol 55:165. https://doi.org/10.4098/j.at.0001-7051.035.2009
Sutor A, Schwarz S (2013) Seasonal habitat selection of raccoon dogs (Nyctereutes procyonoides) in Southern Brandenburg. Germany Folia Zool 62(3):235–243
Viranta S, Kauhaka K (2011) Increased carnivory in finnish red fox females — adaptation to a new competitor? Ann Zool Fennici 48(1):17–28. www.jstor.org/stable/23737051
Whelan CJ, Dilger ML, Robson D, Hallyn N, Dilger S (1994) Effects of olfactory cues on artificial-nest experiments. Auk 111(4):945–952
Wilson GR, Brittingham MC, Goodrich LJ (1998) How well do artificial nests estimate success of real nests? Condor 100(2):357–364. https://doi.org/10.2307/1370277
Zschille J, Stier N, Roth M, Mayer R (2014) Feeding habits of invasive American mink (Neovison vison) in northern Germany-potential implications for fishery and waterfowl. Acta Theriol 59(1):25–34. https://doi.org/10.1007/s13364-012-0126-5
Acknowledgements
We wish to thank all the landowners (people and organizations) who allowed the use of their wetlands and provided help for this study: Metsähallitus, Aage V. Jensen Naturfond, Naturstyrelsen, National Park Thy, Fugleværnsfonden, Aarhus Kommune, Løvenholm, Klosterhede Plantage, the cities of Helsinki, Vantaa and Espoo, private landowners and the duck farmers for providing the eggs. Preben Clausen and Claus Lunde Petersen made significant contributions to the Danish experiments and the contributions of Elmo Miettinen and Minna Hakala were vital during the Finnish experiments: we warmly thank you all.
Funding
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. Post doc grants by Osk. Huttunen foundation in addition to Maj and Tor Nessling Foundation for SH and post doc grants by Haavikko Foundation and Maj and Tor Nessling Foundation for MV. Kuopion Luonnon Ystäväin yhdistys supported V-MV with a camera grant.
Author information
Authors and Affiliations
Contributions
SH, VMV and ADF planned the study, SH, VMV, MV and ADF did field work, SH analyzed the data and SH, VMV, MV and ADF wrote the text.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Consent to participate
All authors consent to participate.
Consent for publication
All authors consent to publish.
Ethical approval
There are no ethical, welfare or environmental implications of the experimental design and procedures of this study. The unfertilized mallard eggs used in this study were obtained from mallard farmers. Down was from legally shot mallards in Finland and from abandoned eider nests in Denmark.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holopainen, S., Väänänen, VM., Vehkaoja, M. et al. Do alien predators pose a particular risk to duck nests in Northern Europe? Results from an artificial nest experiment. Biol Invasions 23, 3795–3807 (2021). https://doi.org/10.1007/s10530-021-02608-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02608-2