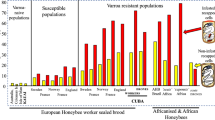
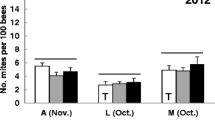
Abstract
Established invasive species can pose a continuous threat to biodiversity and food security, thereby calling for sustainable mitigation. There is a consensus that the ubiquitous ecto-parasitic mite Varroa destructor, an invasive species from Asia, is the main biological threat to global apiculture with Apis mellifera. V. destructor has almost completely wiped out wild European honey bee (Apis mellifera) populations. The only remedy for apiculture, to date, is frequent control measures against the mite throughout the season, which prevents possible adaptations. While targeted breeding efforts have, so far, not achieved the selection of tolerant or resistant bees, natural selection approaches have succeeded at least seven times. Here, we propose to take advantage of natural selection for honey bee resistance by stopping mite treatment in managed colonies. The main principles are within population mating of the colonies’ own virgin queens and drones and selection based on survival and proliferous development of colonies. Being used for 10 years, it has shown to result in grosso modo ‘normal’ colonies with a high level of resistance to V. destructor. Here, we call for local groups of beekeepers and scientists to join a novel natural selection program that has started so far on three locations. This will eventually lead to several locally adapted V. destructor resistant honey bee populations around the world, and help global apiculture becoming more sustainable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Western honey bee (Apis mellifera L.) has an almost global distribution, both as wild (endemic in Europe, Africa and the middle-East, naturalized in the Americas, Asia and Australia) and as a managed species (Moritz et al. 2005). A. mellifera is the most important managed pollinator species, thereby contributing to both food security and production of apicultural products worldwide (honey, pollen, wax and propolis, Klein et al. 2007; Aizen et al. 2008). It is therefore evident that apiculture is of significant economic and societal value.
As a consequence of the globalization of apiculture, the number of novel pathogens affecting A. mellifera has increased through host shifts from closely related species. This was the case for the ecto-parasitic mite Varroa destructor (Anderson and Trueman 2000), which has shifted hosts from the Eastern honey bee (Apis cerana) to A. mellifera. This mite has now an almost ubiquitous distribution (Ellis and Munn 2005) and is the primary biological cause of A. mellifera colony mortality worldwide (Neumann and Carreck 2010; Rosenkranz et al. 2010). V. destructor is a very efficient vector of several honey bee viruses, generating a disease epidemic within the colony, which dwindles until it dies within 2–3 years (Neumann et al. 2012).
The global spread of V. destructor has been very rapid, leaving very little time to mitigate against the biological invasion by preventing introduction, eradicating introduced mites, or finally, by inhibiting further spread (Van der Weijden et al. 2007). Interestingly, A. mellifera is the only honey bee that did not naturally carry parasitic brood mites before its exposure to V. destructor (Eickwort 1994). The apparent lack of host–parasite co-evolution history just after hosts shifts (Woolhouse et al. 2005), may explain the much higher susceptibility of A. mellifera towards V. destructor and, as a result, the rapid decline of wild and feral European (-derived) honey bee populations globally since the spread of V. destructor began (Rosenkranz et al. 2010; Villa et al. 2008; Kraus and Page 1995). Even though it is inevitable that natural selection would result in A. mellifera adapting to V. destructor if managed colonies were left untreated, this option was not considered in apiculture as the foreseen loss of colonies raised understandable concern over the sustainability of crop pollination and hence food security (Klein et al. 2007; Aizen et al. 2008). Therefore, V. destructor has been permanently controlled in managed colonies, preventing coevolution processes (as predicted by Oldroyd 1999), leading to host resistance and tolerance or lowered virulence of the parasite, to take place (Neumann and Blacquière 2017; Brosi et al. 2017).
There is clear evidence that wild populations of A. mellifera can survive V. destructor infestations by means of natural selection (Seeley 2007; Le Conte et al. 2007; Fries et al. 2006; see Locke 2016 for a review). This also holds true for managed populations, which have not been treated against V. destructor (Oddie et al. 2017, 2018; Kruitwagen et al. 2017; Panziera et al. 2017; McMullan 2018). Therefore, sustainable beekeeping without the need to treat V. destructor is clearly feasible. Even though the mechanisms underpinning resistance to V. destructor infestation without treatment are not yet fully understood, we propose to take advantage of Darwinian natural selection for adaptation of bees to this novel parasite as an integrated part of local beekeeping practice. We present an outline for a (natural) selection route that can be incorporated in any local beekeeping, which includes methods to limit collateral damage, such as reinvasion in neighboring apiaries. The protocol [named ‘Darwin’s Black Bee Box’ (DBBB)] has been derived from 10 years of earlier work (see Panziera et al. 2017; Kruitwagen et al. 2017), and is going to be further tested in a new project that has just started at three locations (i.e. with three local groups of colonies). We here call for beekeepers and bee researchers to join this project and follow simultaneously the scheme outlined below at their own locations.
Darwin’s Black Bee Box
The scheme is based on the principles of evolution by natural selection: colonies able to survive and reproduce successfully pass on their genes to the next generation. Alleles of genes coding for traits contributing to survival will gradually increase in frequency, while harmful or neutral alleles will become less frequent. Like Darwin’s observations, it is possible to observe adaptations without understanding the underlying mechanisms. Here, we use the analogy of a black box from which the content remains hidden while the obvious effects of this content are nonetheless clear and visible. Moreover, the route does not beforehand qualify certain traits thought to be of advantage, but just follows nature to the outcome of survival and reproduction. Inside the black box, alleles associated with a successful phenotype are conserved and will persist in the next generation. Natural selection is therefore ‘inclusive’ as it maintains genetic diversity by keeping all surviving phenotypes in the black box, including possibly rare alleles beneficial for resistance to parasites and pathogens (Delaplane et al. 2015). Targeted selective breeding programs, on the contrary, are by definition reducing genetic diversity by selecting from the surviving phenotypes only those of their preference of chosen traits, thereby potentially excluding many of the phenotypes despite their shown capability of survival (Uzunov et al. 2017). DBBB selection follows the natural seasonal reproduction cycle of honey bee colonies, mimicking swarming by splitting colonies. Consequently, the population not only preserves its genetic diversity, but also its associated diverse biome (both favourable and unfavourable associated organisms; bacteria, yeasts, fungi, viruses, mites, etc.,) which fosters development of less virulent host–parasite relationships (Neumann and Blacquière 2017; Blacquière and Panziera 2018). This also precludes a next generation queen being confronted with a completely new biotic environment (colony plus biota). Vice versa it prevents colony offspring (plus biota) being exposed to a foreign young queen (queen + possible biota). Below we describe the practical principles and methods used in the approach, which we actively advocate to use wherever possible in a well-structured manner.
Preconditions
-
1.
25–30 colonies
The DBBB program can be started in spring with a population of 25–30 genetically diverse local colonies (from different beekeepers) from one region. This enables ample variation and conserves local/regional adaptation in the population (genotype–environment interactions Büchler et al. 2014). After splitting the successfully developing colonies into four nucleus colonies (see Procedure below), in the first year these colonies must be treated against V. destructor once, when the young queens have started laying eggs and prior to brood capping, by spraying with oxalic acid. The colonies that survive winter into the next spring, and which show good spring development (growth of the colony and drone production), will be the parent colonies to produce the next generation. In mid-winter, when sampling bees for mite infestation occurs, a quarter of the nucleus colonies can be chosen to serve as a control group. The presence of a control/reference group of colonies is not necessary for the selection procedure, but it can be very helpful in order to compare the selected and non-selected groups in the future to reveal the local mechanisms enabling colony survival despite V. destructor infestations.
-
2.
Remote area
A remote area is needed in the beginning of the summer to mate queens and drones within the population. If a control group of colonies is part of the set-up, a separate remote area will be required. We consider an area with no or hardly beekeeping in a radius of 3 km as being adequately isolated. The work of Jaffé et al. (2009) and Moritz et al. (2007) concluded that in a radius of 1200 m, 60–75% of the matings were with drones from within. In a trial of Jensen et al. (2005) the mating distance (flight radius of queens + flight radius of drones) was in 50% less than 2.5 km, and for 90% within 7.5 km. The higher latter distance can probably be explained by an increase in the flight range of queens, presumably caused by a lack of drones in the area (as reflected by a reduced number of matings per queen, Neumann et al. 1999a, b).
The DBBB selection scheme that we propose is based on negative selection (non-survival of non-adapted phenotypes) and does not aim for strong increases of specific (chosen) traits/phenotypes. Therefore, if a few foreign alleles enter the genepool this will probably not interfere with the natural selection process. Moreover, the DBBB procedure (see below) produces an excess of mature local own drones at the right mating time, which will most likely outcompete far flying foreigners by far.
-
3.
No Varroa destructor control
No V. destructor control is applied to the DBBB selection colonies throughout the duration of the program, apart from the one oxalic acid treatment in the first summer. Conversely, V. destructor is controlled in the control group in order to keep the population of mites at an acceptable level to avoid damage and to avoid selection pressure by V. destructor infestation taking place in control colonies too. In our case, the use of oxalic acid in the absence of capped brood twice a year proved to be a sufficient control treatment (Panziera et al. 2017).
-
4.
Feeding of the colonies
Feeding colonies is obviously not natural. We know from the work of Seeley (1995, 2017) that in the wild (here Arnot Forest), only 23% of founder colonies from primary swarms that successfully occupy a nest cavity are able to survive the first winter. In the case of established colonies, the winter survival rate increases to 84% (Seeley 2017). Starvation over winter may be the prime cause for not surviving of colonies in the wild. In comparison, > 95% of well managed bee colonies (= well fed and treated against V. destructor) in Germany survive winter (Genersch et al. 2010). In our scheme, we avoid an extra selection pressure due to the lack of food by feeding the young colonies with sugar dough. In periods of poor forage, established colonies should also be fed.
-
5.
Minimum duration of 4 years
The program should run for at least 4 years to see the first effects, and preferably be continued. The reason being that in a previous trial in the Netherlands we saw changes in V. destructor reproduction success in the non-treated colonies after 4 years (Panziera et al. 2017; Kruitwagen et al. 2017). Recent research of Avalos et al. (2017) showed that evolution by passage through a selective bottleneck can be very fast indeed (only ten generations/years) in the highly polyandrous honey bee.
Procedure and principles
No control of V. destructor: in the DBBB selected colonies, the control of V. destructor will be ceased after the first summer. Ultimately, only adaptation of the bees to the novel parasite is a vital option (possibly including mite adaptation, see Seeley 2007), every other being a dead end.
Reproductive capacity: in temperate regions, a wild A. mellifera colony will, if circumstances allow, reproduce in spring or summer by producing a prime swarm and rear new queens in the remaining part of the colony (some of which will leave in after swarms). We follow this natural development of colonies during the season: our colonies have the opportunity to reproduce (produce drones and swarm cells) when the colonies choose to. However, because we aim to derive four offspring colonies (each with a virgin queen from the mother colony) we avoid losing swarms by making an artificial swarm just before natural swarming would commence. By removing the queen (in an artificial swarm) the production of new queen cells is synchronized. This nevertheless implies that only colonies engaging timely in the reproduction process (by producing a frame with drone brood) will contribute to the next generation. Selection will therefore also be on reproductive abilities (drone and queen production).
Growth capacity: Selecting for the ability to reproduce (described above) indirectly selects for the ability of the colony to grow in spring, as only colonies that grow and develop well will initiate behaviour associated with swarming. This selection for the capacity to grow is also strong during summer: colonies made in early summer only have 3 months to grow to a sufficient size to survive the subsequent winter.
Survival: The first milestone for survival is the successful mating of the queens. Those colonies that consecutively produce a strong and healthy population of winter bees will have a good chance to survive the winter, and develop further the next spring.
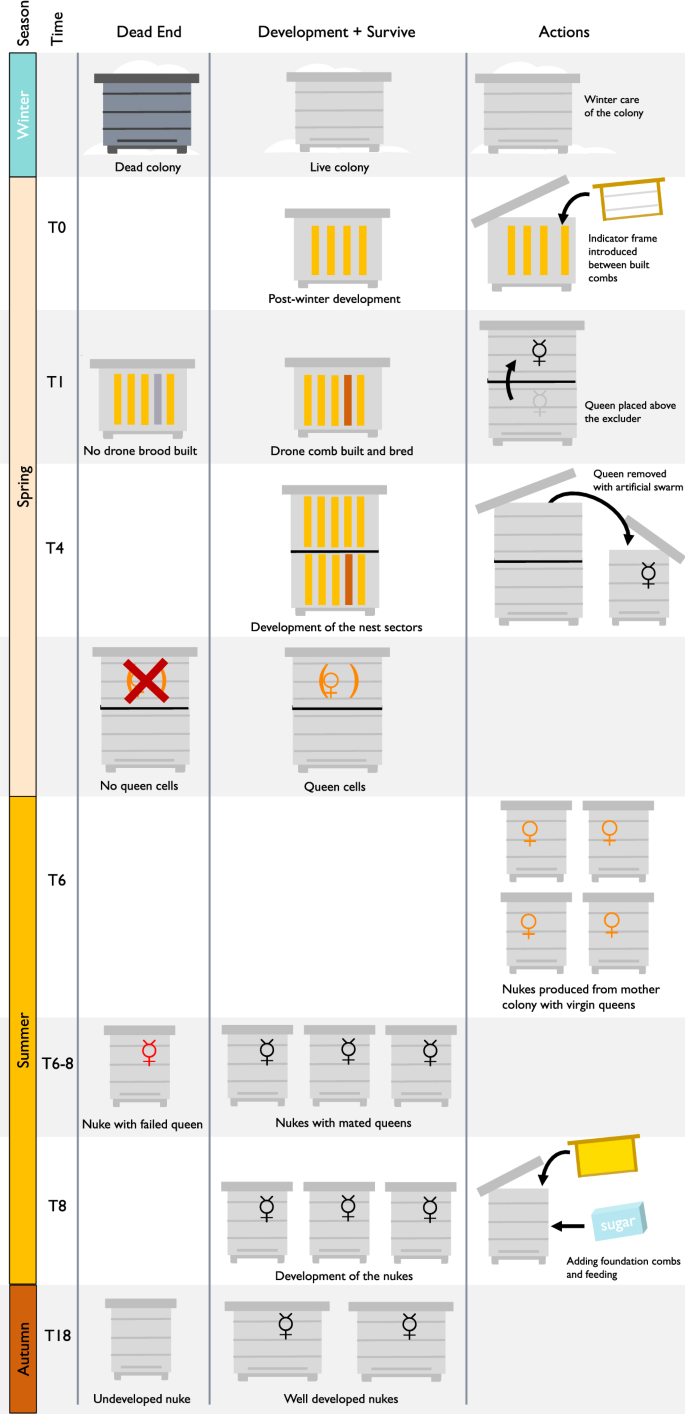
Protocol through the season
Procedural steps taken during the year (Fig. 1), follow the natural development of colonies as much as possible. However they simultaneously ascertain that enough new colonies get raised every season to allow losses through natural selection pressure, and that the most important factor driving selection is the ability of colonies to survive V. destructor mite infestations. The steps are not prescriptive, but aim to illustrate the room the protocol leaves to nature to take the selective lead: elimination of non-vital phenotypes after mating, before and during winter and in spring; following natural development as much as possible during the season; only offspring queens from the colony in which they were raised. In the control group of colonies, the possible adaptation of the bees to the V. destructor mite is interrupted twice a year (in summer, 2 weeks after making the nucleus colonies, and in winter when no brood is present) by treating the colonies with oxalic acid to kill the phoretic mites. Depending on local conditions and practice, other known efficient treatments may be used (reviewed by Rosenkranz et al. 2010).
Perspective
DBBB is fast, easy, and may uncover traits involved in resistance
Colonies of European A. mellifera subspecies are able to survive V. destructor infestations by means of natural selection, which has been shown in several instances (Seeley 2007; Le Conte et al. 2007; Fries et al. 2006, reviewed by Locke 2016; Oddie et al. 2017). Since then, several examples of resilience to this mite acquired within an apicultural setting have been published (Panziera et al. 2017; Kruitwagen et al. 2017; Kefuss et al. 2016; Oddie et al. 2017; McMullan 2018). All examples used selection on outcome (survival, vitality (which means well developing colonies), or on (slow) mite population growth), and not on chosen traits such as hygienic behaviour or active grooming behaviour, including the study by Panziera et al. (2017), who used exactly the approach outlined in this article.
Populations of honey bee colonies resulting from these selection approaches apparently have acquired some resistance and/or tolerance to V. destructor and can offer a good opportunity to unveil phenotypic traits underlying adaptation to this novel parasite at bee and colony level. Some traits effective at the level of the individual bee may easily be tracked through effects on drones, since drones are haploid. More importantly, such knowledge may help us to understand how parasite resistance in general evolves in honey bees.
Using the proposed approach, we may expect to see significant effects soon (4–10 years), as illustrated by the earlier examples (Panziera et al. 2017; Kefuss et al. 2016; Oddie et al. 2017, 2018; McMullan 2018; Guarna et al. 2017). By allowing selection on drones as well, which is the rule in this approach, selection may proceed even faster, both because drones with susceptible alleles may not be vital and may not mate, and because successful colonies produce a higher number of drones (Jandricic and Otis 2003). An example of fast ‘negative’ selection (elimination of non-fitting phenotypes, in this case over-aggressive colonies) within a decade has been reported from Africanised honey bees in Puerto Rico, which became as docile as European ones (Avalos et al. 2017).
For hobby/non-professional beekeepers, the approach has a low threshold: it is straightforward and does not need specific equipment. Therefore, it can be done by a single beekeeper or by a local group of (small) hobby beekeepers. According to Jandricic and Otis (2003), it is also cheaper than any targeted selection program. Randy Oliver (2018), a commercial beekeeper in California just started a comparable program including a pre-selection and concluded similarly. The Dutch commercial beekeeping company Inbuzz v.o.f. was able to fulfil such a program together with ten hobby beekeepers for at least 8 years (Van Stratum 2016).
DBBB potential limitations
In order to limit economic losses in the initial years, only a small part of a local population needs to be subjected to this natural selection approach. Beekeepers can continue to manage the majority of their colonies as before. They can, however, raise new queens from the population under selection and thus gradually change the whole population. Since we take advantage of natural selection, there will be no fitness costs for adapted colonies.
Commercial queen breeding as it is applied now does not fit with the suggested approach, because the entire colony is the basis of selection. However, beekeepers using our approach could additionally produce extra queens (in most cases colonies produce > 4 queens), add those to very small mating nukes (e.g. Apidea with a few hundred bees) and let those queens mate at the very same remote location with the rest of the population.
There is a potential for a considerable genetic bottleneck if very few colonies survive from the initial cohort. However, we do not advise total isolation. Therefore, some foreign genes are likely to enter the population. If inbreeding will nevertheless occur, adding colonies might be considered to maintain genetic diversity.
There are fundamental biological differences between Eastern and Western honey bees with respect to V. destructor infestations (reviewed by Rosenkranz et al. 2010). For example, successful mite reproduction in A. cerana is restricted to drone brood, which is not a foreseeable scenario for A. mellifera. It is therefore apparent that the novel host A. mellifera is very unlikely to reach exactly the same level of mite resistance/tolerance as the original one. Nevertheless, the reported levels of naturally selected mite tolerance in populations of European honey bee subspecies (see above) combined with the apparent tolerance of wild and managed Africanized and African honey bee populations to this mite (reviewed by Locke 2016), strongly suggest that the actual level of tolerance which can be reached by A. mellifera is apparently sufficient to maintain stable populations.
DBBB works on a local, ‘natural’ scale and will help to avoid the spread of non-native diseases and parasites
Although drones and virgin queens can fly several kilometres for mating, especially at low drone densities (Neumann et al. 1999a, b; Jensen et al. 2005), more than 75% of the matings take place with drones from within the 4.5 km2 area around the queen’s hive (Jaffé et al. 2009). With the estimated density of 2.4–3.2 colonies per km2 in Germany (Moritz et al. 2007) this would provide queens with 11–15 drone-delivering colonies from within reach. Jaffé et al. (2009) reported median numbers of colonies in reach of virgin queens to be 10–37 in Europe (10–17 in Germany). Thus, starting with 25 colonies and having (due to partial losses) ~ 15 colonies left that do develop well next spring, we have a mating population/colony density very similar to the situation in nature [although numbers are higher in Africa and the Mediterranean (Jaffé et al. 2009)]. The size of the populations should be large enough to avoid inbreeding, and possible bottle-necks will be compensated by balancing selection on the sex locus. This will increase the frequency of rare sex alleles, as was shown in A. cerana, after its invasion in Australia (Gloag et al. 2016; Ding et al. 2017). An additional benefit of working in a local area is the possibility of adaptation to the local environment and forage seasonality (Strange et al. 2007), as well as to local disease variants (Blacquière and Panziera 2018). A recent transplantation experiment in which colonies of different origins in Europe were compared on reciprocal locations showed how important this may be: local colonies were always performing better than non-local colonies due to strong genotype–environment interactions (Büchler et al. 2014; Meixner et al. 2014). Is it important to consider that most invasions of bee-parasites and diseases have occurred in conjunction with trade of bees and queen bees (Mutinelli 2011; Owen 2017). Brosi et al. (2017) explained how these immunity-offending practices increase the vulnerability of the bee stock. Because our approach works at a local scale, the chances of importing or exporting diseases and parasites will eventually decrease.
DBBB may increase and conserve the functional genetic diversity of the honey bee population
The western honey bee in large scale apicultural settings was found to have a very high genetic diversity, higher than in ‘original’ endemic populations in Europe (Harpur et al. 2012; Oldroyd 2012), caused by mixing colonies from different original lineages, e.g. M lineage (A. m. iberica and A. m. mellifera) and C lineage (A. m. carnica and A. m. ligustica) from Europe (Honey bee Genome Sequencing Consortium 2006). Although this high diversity may look favourable, it might actually be a threat for locally adapted subspecies or populations. Extremely high allelic diversity might slow down balancing selection for polygenic traits. Alternatively, but not mutually exclusive, mating of adapted queens with drones from non-adapted colonies may also increase risks of colony death. Therefore, De La Rúa et al. (2013) strongly call for strict conservation measures to preserve functional genetic diversity through conservation of local subspecies. This conservation will even work at a smaller (more local) scale if our proposed approach will conserve locally adapted populations of bee colonies. This will overall increase the functional genetic diversity of the honey bee, at country- and continent level (which is the sum of local diversities, in contrast to an artificially maintained diversity by a limited number of breeders).
DBBB may help swarms to establish, thereby contributing to re-introduction of honey bees into the wild
A significant proportion of western honey bee, A. mellifera populations has always been non-managed (Africa; Jaffé et al. 2009). On feral colonies strong selection pressures act, not only by V. destructor. Being feral may, however, also reduce certain selection pressures in comparison to being managed (Loftus et al. 2016). In cases where the feral population is sufficiently isolated from managed colonies, this can lead to resistance (e.g. Seeley 2007; Le Conte et al. 2007). Often swarms from managed colonies, which become feral, soon die (Thompson et al. 2014), probably because those colonies do not possess genes enabling survival in the absence of mite treatments. However, swarms from the naturally selected population will be more likely to survive because they have acquired resilience to V. destructor. Darwin’s Black Bee Box might therefore shift the balance between managed and feral colonies by increasing the chances for survival and re-establishment of wild honey bee populations.
DBBB may help the invasion of Varroa destructor to become a successful naturalization
After an invasive species has established, the preferred and chosen path of targeted management, including control/mitigation, may become more costly and troublesome than accepting the inevitable fate that the species has become ‘ours’ (Epstein 2017). However, being established also implies that the invasive species has become part of ‘our’ nature, which also opens the challenge for the invasive species to adapt and fit in a new niche as a naturalized species (Blackburn et al. 2011). For the established invasive species V. destructor, we propose to gradually abandon targeted management measures and hand the initiative over to natural selection. We therefore call upon beekeepers and scholars to follow our Darwin’s Black Bee Box trials, to be started now and in the years to come.
DBBB might work for other invasive parasitic species too
Since evolution by natural selection is universal for all species, it seems evident that this type of approach could in principle be used for other invasive species as well (e.g. for Tropilaelaps spp., de Guzman et al. 2017, and for Nosema ceranae, Fries 2010). Indeed, in many cases invasive parasite species are controlled by using pesticides, thereby preventing adaptation of the novel hosts (reviewed by Dunn and Hatcher 2015). Given that our scheme can be adapted to these other cases of invasive parasite species in a meaningful way, taking advantage of natural selection might constitute one sustainable approach for dealing with established parasites and biological invasions in the future.
References
Aizen MA, Garibaldi LA, Cunningham SA, Klein AM (2008) Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr Biol 18:1572–1575
Anderson DL, Trueman JWH (2000) Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp Appl Acarol 24:165–189
Avalos A, Pan H, Li C, Acevedo-Gonzalez JP, Rendon G, Fields CJ, Brown PJ, Giray T, Robinson GE, Hudson ME, Zhang G (2017) A soft selective sweep during rapid evolution of gentle behaviour in an Africanized honey bee. Nat Commun 8(1550):1–9
Blackburn TM, Pysek P, Bacher S, Carlton JT, Duncan RP, Jarosik V, Wilson JRU, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339
Blacquière T, Panziera D (2018) A plea for use of honey bees’ natural resilience in beekeeping. Bee World 95:34–38
Brosi BJ, Delaplane KS, Boots M, De Roode JC (2017) Ecological and evolutionary approaches to managing honey bee disease. Nat Ecol Evol 1:1250–1262
Büchler R, Costa C, Hatjina F, Andonov S, Meixner MD, Le Conte Y, Uzunov A, Berg S, Bienkowska M, Bouga M, Drazic M, Dyrba W, Kryger P, Panasiuk B, Pechhacker H, Petrov P, Kezic N, Korpela S, Wilde J (2014) The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. J Apic Res 53(2):205–214
De Guzman LI, Williams GR, Khongphinitbunjong K, Chantawannakul P (2017) Ecology, life history, and management of Tropilaelaps mites. J Econ Entomol 110(2):319–332
De La Rúa P, Jaffé R, Muñoz I, Serrano J, Moritz RFA, Kraus FB (2013) Conserving genetic diversity in the honey bee: comments on Harpur et al. (2012). Mol Ecol 22:3208–3210
Delaplane KS, Pietravalle S, Brown MA, Budge GE (2015) Honey bee colonies headed by hyperpolyandrous queens have improved brood rearing efficiency and lower infestation rates of parasitic Varroa mites. PLoS ONE 10(12):e0142985. https://doi.org/10.1371/journal.pone.0142985
Ding G, Xu H, Oldroyd BP, Gloag RS (2017) Extreme polyandry aids the establishment of invasive populations of a social insect. Heredity 119:381–387
Dunn A, Hatcher MJ (2015) Parasites and biological invasions: parallels, interactions, and control. Trends Parasitol 31(5):189–199
Eickwort GC (1994) Evolution and life-history patterns of mites associated with bees. In: Houck MA (ed) Mites. Ecological and evolutionary analysis of life history patterns. Chapman & Hall, London, pp 218–251
Ellis JD, Munn PA (2005) The worldwide health status of honey bees. Bee World 86:88–101
Epstein G (2017) Invasive alien species management: a personal impasse. Front Environ Sci. https://doi.org/10.3389/fenvs.2017.00068
Fries I (2010) Nosema ceranae in European honey bees (Apis mellifera). J Inv Path 103:S73–S79
Fries I, Imdorf A, Rosenkranz P (2006) Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie 37:564–570
Genersch E, von der Ohe W, Kaatz H, Schroeder A, Otten C, Büchler R, Berg S, Ritter W, Mühlen W, Gisder S, Meixner M, Liebig G, Rosenkranz P (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41:332–352
Gloag R, Ding G, Christie JR, Buchmann G, Beekman M, Oldroyd BP (2016) An invasive social insect overcomes genetic load at the sex locus. Nat Ecol Evol 1:0011
Guarna MM, Hoover SE, Huxter E, Higo H, Moon K-M, Domanski D, Bixby MEF, Melathopoulos AP, Ibrahim A, Peirson M, Desai S, Micholson D, White R, Borchers CH, Currie RW, Pernal SF, Foster LJ (2017) Peptide biomarkers used for the selective breeding of a complex polygenic trait in honey bees. Sci Rep 7(8381):1–10
Harpur BA, Minaei S, Kent CF, Zayed A (2012) Management increases genetic diversity of honey bees via admixture. Mol Ecol 21:4414–4421
Honey Bee Genome Sequencing Consortium (2006) Insights into social insects from the genome of the honey bee Apis mellifera. Nature 443:931–949
Jaffé R, Dietemann V, Allsopp MH, Costa C, Crewe RM, Dall’Olio R, De La Rúa P, El-Niweiri MAA, Fries I, Kezic N, Meusel MS, Paxton RJ, Shaibi T, Stolle E, Moritz RFA (2009) Estimating the density of honeybee colonies across their natural range to fill the gap in pollinator decline censuses. Conserv Biol 24(2):583–593
Jandricic SE, Otis GW (2003) The potential for using male selection in breeding honey bees resistant to Varroa destructor. Bee World 84:155–164
Jensen AB, Palmer KA, Chaline N, Raine NE, Tofilski A, Martin SJ, Pederson BV, Boomsma JJ, Ratnieks FLW (2005) Quantifying honey bee mating range and isolation in semi-isolated valleys by DNA microsatellite paternity analysis. Conserv Genet 6:527–537
Kefuss J, Vanpoucke J, Bolt M, Kefuss C (2016) Selection for resistance to Varroa destructor under commercial beekeeping conditions. J Apic Res 54:563–576
Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B 274:303–313
Kraus B, Page RE (1995) Effect of Varroa jacobsoni (Mesostigmata: Varroidae) on feral Apis mellifera (Hymenoptera: Apidae) in California. Environm Entomol 24(6):1473–1480
Kruitwagen A, van Langevelde F, van Dooremalen C, Blacquiere T (2017) Naturally selected honey bee (Apis mellifera) colonies resistant to Varroa destructor do not groom more intensively. J Apic Res 56:354–365
Le Conte Y, de Vaublanc G, Crauser D, Jeanne F, Rousselle J-C, Bécard J-M (2007) Honey bee colonies that have survived Varroa destructor. Apidologie 38:566–572
Locke B (2016) Natural Varroa mite-surviving Apis mellifera honey bee populations. Apidologie 47:467–482
Loftus JC, Smith ML, Seeley TD (2016) How honey bee colonies survive in the wild: testing the importance of small nests and frequent swarming. PLoS ONE 11(3):e0150362. https://doi.org/10.1371/journal.pone.0150362
McMullan J (2018) Adaptation in honey bee (Apis mellifera) colonies exhibiting tolerance to Varroa destructor in Ireland. Bee World 95(2):39–43
Meixner MD, Francis RM, Gajda A, Kryger P, Andonov S, Uzunov A, Topolska G, Costa C, Amiri E, Berg S, Bienkowska M, Bouga M, Büchler R, Dyrba W, Gurgulova K, Hatjina F, Ivanova E, Janes M, Kezic N, Korpela S, Le Conte Y, Panasiuk B, Pechhacker H, Tsoktouridis G, Vaccari G, Wilde J (2014) Occurrence of parasites and pathogens in honey bee colonies used in a European genotype–environment—interactions experiment. J Apic Res 53:215–229
Moritz RFA, Härtel S, Neumann P (2005) Global invasions of the western honey bee (Apis mellifera L.) and consequences for biodiversity. Ecoscience 12:289–301
Moritz RFA, Kraus FB, Kryger P, Crewe RM (2007) The size of wild honey bee populations (Apis mellifera) and its implications for the conservation of honey bees. J Insect Conserv 11:391–397
Mutinelli F (2011) The spread of pathogens through trade in honey bees and their products (including queen bees and semen): overview and recent developments. Sci Tech Rev Off Int Epizoot (Paris) 30:257–271
Neumann P, Blacquière T (2017) The Darwin cure for apiculture? Natural selection and managed honey bee health. Evol Appl 10:226–230
Neumann P, Carreck C (2010) Honey bee colony losses: A global perspective. J Apic Res 49:1–6
Neumann P, Moritz RFA, van Praagh J (1999a) Queen mating-frequency in different types of honey bee mating apiaries. J Apic Res 38:11–18
Neumann P, van Praagh J, Moritz RFA, Dustmann J (1999b) Testing reliability of a potential island mating apiary using DNA-microsatellites. Apidologie 30:257–276
Neumann P, Yañez O, Fries I, de Miranda JR (2012) Varroa invasion and virus adaptation. Trends Parasitol 28:353–354
Oddie MAY, Dahle B, Neumann P (2017) Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ 5:e3956. https://doi.org/10.7717/perj.3956
Oddie M, Büchler R, Dahle B, Kovacic M, Le Conte Y, Locke B, de Miranda JR, Mondet F, Neumann P (2018) Rapid parallel evolution overcomes global honey bee parasite. Sci Rep 8:7704. https://doi.org/10.1038/s41598-018-26001-7
Oldroyd BP (1999) Coevolution while you wait: Varroa jacobsoni, a new parasite of western honey bees. Trends Ecol Evol 14(8):312–315
Oldroyd BP (2012) Domestication of honey bees was associated with expansion of genetic diversity. Mol Ecol 21:4409–4411
Oliver R (2018) http://scientificbeekeeping.com/selective-breeding-for-mite-resistance-1000-hives-100-hours/. Last approach Apr 10, 2019
Owen R (2017) Role of human action in the spread of honey bee (Hymenoptera: Apidea) pathogens. J Econom Entomol 110:797–801
Panziera D, Van Langevelde F, Blacquière T (2017) Varroa sensitive hygiene contributes to naturally selected Varroa resistance in honey bees. J Apic Res 56:635–642
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Inv Path 103:S96–S119
Seeley TD (1995) The wisdom of the hive. Harvard University Press, London
Seeley TD (2007) Honey bees of the Arnot Forest: a population of feral colonies persisting with Varroa destructor in the north-eastern United States. Apidologie 38:19–29
Seeley TD (2017) Life-history traits of wild honey bee colonies living in forests around Ithaca, NY, USA. Apidologie 48:743–754
Strange JP, Garnery L, Sheppard WS (2007) Persistence of the Landes ecotype of Apis mellifera mellifera in southwest France: confirmation of a locally adaptive annual brood cycle trait. Apidologie 38:259–267
Thompson CE, Biesmeijer JC, Allnutt TR, Pietravalle S, Budge GE (2014) Parasite pressures on feral honey bees (Apis mellifera sp.). PLoS ONE 9(8):e105164. https://doi.org/10.1371/journal.pone.0105164
Uzunov A, Brascamp EW, Büchler R (2017) The basic concept of honey bee breeding programs. Bee World 94(3):84–87
Van der Weijden W, Leeuwis R, Bol P (2007) Biological globalisation: bio-invasions and their impacts on nature, the economy and public health. KNNV Publishing, Utrecht. ISBN 978-90-5011-243-7
Van Stratum P (2016) Vitale bijen hebben de toekomst. Bijenhouden 10(5):14–15
Villa JD, Bustamante DM, Dunkley JP, Escobar LA (2008) Changes in honey bee (Hymenoptera: Apidae) colony swarming and survival pre- and post-arrival of Varroa destructor (Mesostigmata: Varroidae) in Louisiana. Ann Entomol Soc Am 101(5):867–871
Woolhouse ME, Haydon DT, Antia R (2005) Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol Evol 20(5):238–244. https://doi.org/10.1016/j.tree.2005.02.009
Acknowledgements
We thank Amanda Norton for critical comments on a late draft of this paper.
Funding
The ideas which led to the writing of this perspective paper are partly based on the experience and results obtained in a 10 years natural selection trial, for which TB acknowledges the Dutch Ministry of Economic Affairs (now Ministry of Agriculture, Nature and Food Quality) and the European Commission for Financial support (Projects NL 05/2.2; NL 08/2.1; NP 11/2.1; NP 14-6.1; NP 17.1). In addition, the ideas have been discussed and fine-tuned within the COLOSS (https://coloss.org/) Task Force ‘Survivors’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Blacquière, T., Boot, W., Calis, J. et al. Darwinian black box selection for resistance to settled invasive Varroa destructor parasites in honey bees. Biol Invasions 21, 2519–2528 (2019). https://doi.org/10.1007/s10530-019-02001-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02001-0