Abstract
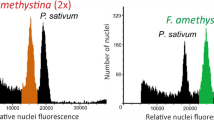
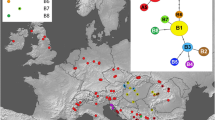
Polyploidy is a common feature of agricultural weeds and natural area invaders. There are few studies comparing related diploid and polyploid exotics, however, and it is unclear what ecological and genetic factors favor the establishment of weedy polyploids. This research characterizes the geographic distribution and phenotypic characteristics of diploid Hedera helix and tetraploid Hedera hibernica, European species that are invading North American forests. To confirm the taxonomic affinity of invasive plants, we sequenced five non-coding cpDNA regions for 108 individuals (105 populations) as well as reference samples representing all species in the genus Hedera. Because diploid H. helix and tetraploid H. hibernica are poorly distinguished by morphology and DNA sequence, we used flow cytometry to determine their distribution (585 individuals). More than 90 % of sampled plants had cpDNA sequences identical or similar to H. helix sensu lato and H. hibernica. Diploid H. helix was dominant on the U.S. east coast (78.5 % of sampled plants) while tetraploid H. hibernica was dominant on the U.S. west coast (72.2 % of sampled plants), mirroring the species’ occurrence in maritime versus continental climates of Europe. Moreover, for sympatric occurrences in the Pacific Northwest, H. hibernica was larger and more frequently reproductive than H. helix. In a 2-year garden experiment, tetraploid H. hibernica had substantial architectural differences compared to diploid H. helix, including larger (but less numerous) leaves and thicker (but less branched) stems. Field experiments are needed to evaluate “pre-adaptation” (directional ecological filtering) and other factors mediating the invasion of H. helix and H. hibernica.






Similar content being viewed by others
References
Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Inter 11:36–42
Ackerfield J, Wen J (2002) A morphometric analysis of Hedera L. (the ivy genus, Araliaceae) and its taxonomic implications. Adansonia 24:197–212
Ackerfield J, Wen J (2003) Evolution of Hedera (the ivy genus, Araliaceae): insights from chloroplast DNA data. Int J Plant Sci 164:593–602
Alexander JM, Kueffer C, Daehler CC, Edwards PJ, Pauchard A, Seipel T, MIREN Consortium (2011) Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc Natl Acad Sci USA 108:656–661
Amsellem L, Chevallier M-H, Hossaert-McKey M (2001) Ploidy level of the invasive weed Rubus alceifolius (Rosaceae) in its native range and in areas of introduction. Plant Syst Evol 228:171–179
Barrett SCH, Richardson BJ (1986) Genetics attributes of invading species. In: Grives RH, Burdon JJ (eds) Ecology of biological invasions: an Australian perspective. Australian Academy of Science, Canberra, pp 21–33
Bennett MD, Leitch IJ, Hanson L (1998) DNA amounts in two samples of angiosperm weeds. Ann Bot-Lond 82:121–134
Bingham ET (1980) Maximizing heterozygosity in autopolyploids. In: Lewis WH (ed) Polyploidy: biological relevance. Plenum, New York, pp 471–489
Broz AK, Manter DK, Bowman G, Müller-Schärer H, Vivanco JM (2009) Plant origin and ploidy influence gene expression and life cycle characteristics in an invasive weed. BMC Plant Biol 9:33–37
Bruneau A, Anderson GJ (1988) Reproductive biology of diploid and triploid Apios americana (Leguminosae). Am J Bot 75:1876–1883
Buggs RJA, Pannell JR (2007) Ecological differentiation and diploid superiority across a moving ploidy contact zone. Evolution 61:125–140
Chapman H, Robson B, Pearson ML (2004) Population genetic structure of a colonising, triploid weed, Hieracium lepidulum. Heredity 92:182–188
Clarke M, Reichard S, Hamilton C (2006) Prevalence of different horticultural taxa of ivy (Hedera spp., Araliaceae) in invading populations. Biol Invasions 8:149–157
Consaul LL, Gillespie LJ, Waterway MJ (2008) Systematics of North American arctic diploid Puccinellia (Poaceae): morphology, DNA content, and AFLP markers. Syst Bot 33:251–261
Czarnecki D (2010) Polyploids in Lantana camara: possible origins, uses for breeding, and association with invasiveness. HortScience 45:488–495
Green A, Ramsey T, Ramsey J (2011) Phylogeny and biogeography of ivies (Hedera spp., Araliaceae), a polyploid complex of woody vines. Syst Bot 36:1114–1127
Greilhuber J, Temsch EM, Loureiro JCM (2007) Nuclear DNA content measurement. In: Doležel J, Greilhuber J, Suda J (eds) Flow cytometry with plant cells. Analysis of genes, chromosomes, and genomes. Wiley-VCH Verlag GmbH & Co., Weinheim, pp 67–101
Husband B (2004) The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biol J Linn Soc 82:537–546
Jacobsen P (1954) Chromosome numbers in the genus Hedera L. Hereditas 40:252–255
Kessler B, Reches S (1977) Structural and functional changes of chromosomal DNA during aging and phase change in plants. Chromosom Today 6:237–246
König C, Ebert I, Greilhuber J (1987) A DNA cytophotometric and chromosome banding study in Hedera helix (Araliaceae), with reference to differential DNA replication associated with juvenile-adult phase change. Genome 3:498–503
Kron P, Suda J, Husband BC (2007) Applications of flow cytometry to evolutionary and population biology. Annu Rev Ecol Evol Syst 38:847–876
Kubátová B, Trávnícek P, Bastlová D, Curn V, Jarolímová V, Suda J (2008) DNA ploidy-level variation in native and invasive populations of Lythrum salicaria at a large geographical scale. J Biogeogr 35:167–176
Leitch IJ, Bennett MD (2004) Genome downsizing in polyploid plants. Biol J Linn Soc 82:651–663
Levin DA (1975) Minority cytotype exclusion in local plant populations. Taxon 24:35–43
Levin DA (1983) Polyploidy and novelty in flowering plants. Am Nat 122:1–25
Levin DA (2002) The role of chromosome change in plant evolution. Oxford University Press, New York
Lui K, Thompson FL, Eckert CG (2005) Causes and consequences of extreme variation in reproductive strategy and vegetative growth among invasive populations of a clonal aquatic plant, Butomus umbellatus L. (Butomaceae). Biol Invasions 7:427–444
Mahelka V, Suda J, Jarolímová V, Trávníček P, Krahulec F (2005) Genome size discriminates between closely related taxa Elytrigia repens and E. intermedia (Poaceae: Triticeae) and their hybrid. Folia Geobot 40:367–384
Marchant CJ, MacFarlane RM (1980) Chromosome polymorphism in triploid populations of Fritillaria lanceolata Pursh (Liliaceae) in California. Bot J Linn Soc 81:135–154
McAllister HA, Rutherford A (1983) The species of ivy. Ivy J 9:45–54
McAllister HA, Rutherford A (1990) Hedera helix L. and H. hibernica (Kirchner) Bean (Araliaceae) in the British Isles. Watsonia 18:7–15
Obermayer R, Greilhuber J (2000) Genome size in Hedera helix L.—a clarification. Caryologia 53:1–4
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Ann Rev Genet 34:401–437
Pandit MK, Tan HTW, Bisht MS (2006) Polyploidy in invasive plant species of Singapore. Bot J Linn Soc 151:395–403
Pandit MK, Pocock JO, Kunin WE (2011) Ploidy influences rarity and invasiveness in plants. J Ecol 99:1108–1115
Parisod C, Holderegger R, Brochmann C (2010) Evolutionary consequences of autopolyploidy. New Phytol 186:5–17
Queller DC, Strassmann JE, Hughes CR (1993) Microsatellites and kinship. Trends Ecol Evol 8:285–288
Ramsden C, Bériault K, Bogart P (2006) A nonlethal method of identification of Ambystoma laterale, A. jeffersonianum and sympatric unisexuals. Mol Ecol Notes 6:261–264
Ramsey J (2011) Polyploidy and ecological adaptation in wild yarrow. Proc Natl Acad Sci USA 108:7096–7101
Ramsey J, Schemske DW (1998) Pathways, mechanisms and rates of polyploidy formation in flowering plants. Annu Rev Ecol Evol Syst 29:467–501
Ramsey J, Schemske DW (2002) Neopolyploidy in flowering plants. Annu Rev Ecol Evol Syst 33:589–639
Reichard S (2000) Hedera helix. In: Randall JM, Bossard C (eds) Noxious wildland weeds of California. University of California Press, Berkeley, pp 212–216
Rice W (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rose PQ (1996) The gardener’s guide to growing ivies. David and Charles, London
Rumney GR (1968) Climatology and the world’s climates. Macmillan, New York
Rutherford A, McAllister HA, Mill RR (1993) New ivies from the Mediterranean area and Macronesia. Plantsman 15:115–128
Schäffner KH, Nagl W (1979) Differential DNA replication involved in transition from juvenile to adult phase in Hedera helix (Araliaceae). Plant Syst Evol Suppl 2:105–110
Schlaepfer DR (2010) Why only tetraploid Solidago gigantea (Asteraceae) became invasive: a common garden comparison of ploidy levels. Oecologia 163:661–670
Schlaepfer DR, Edwards PJ, Widmer A, Billeter R (2008) Phylogeography of native ploidy levels and invasive tetraploids of Solidago gigantea. Mol Ecol 17:5245–5256
Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J, Siripun KC, Winder CT, Schilling EE, Small RL (2005) The tortoise and the hare II: relative utility of 21 non-coding chloroplast DNA sequences for phylogenetic analysis. Am J Bot 92:142–166
Shishkin BK (1973) Flora of the USSR Volume XVI Umbelliflorae. Keter Press, Jerusalem
Soltis PS, Soltis DE (2009) The role of hybridization in plant speciation. Annu Rev Plant Biol 60:561–588
Sulgrove SM (1984) The great ivy debate: the status of hibernica. Ivy J 10:33–45
Te Beest M, Le Roux JJ, Richardson DM, Brysting AK, Suda J, Kubešová M, Pyšek P (2012) The more the better? The role of polyploidy in facilitating plant invasions. Ann Bot 109:19–45
Thomas LK (1998) Topographic alterations, forest structure, and invasion by English ivy (Hedera helix L.) in the Rock Creek Floodplain, Washington, D.C. Nat Area J 18:164–168
Treier UA, Broennimann O, Normand S, Guisan A, Schaffner U, Steinger T, Muller-Scharer H (2009) Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa. Ecology 90:1366–1377
Valcárcel V, Fix O, Vargas P (2003) Chloroplast and nuclear evidence for multiple origins of polyploids and diploids of Hedera (Araliaceae) in the Mediterranean basin. Mol Phylogenet Evol 27:1–20
Verlaque R, Aboucaya A, Fridlender A (2002) Invasive alien flora of France: ecology, life-forms and polyploidy. Bot Helv 112:121–136
Acknowledgments
We thank Paul Kron and Brian Husband for assistance with flow cytometry; Erin Fox, Owen Hardy, Rob Laport, and Emily Reiss for help in the field and molecular lab; and Washington State Parks for logistical support and permission to conduct this research. Photo imagery is courtesy of the Washington State Department of Natural Resources, ©1995. Brian Husband, J.J. Le Roux, John Jaenike, Paul Kron, Hugh McAllister, Robert Minckley, Roger del Moral, Dick Olmstead, Suzanne Pierot, Sarah Reichard, Allison Rutherford, Sabina Sulgrove, Russell Windle, and two anonymous reviewers provided helpful comments on a draft of this manuscript. This research was supported by research grants from The American Ivy Society (to TR), Sigma Xi (to TR and to AG), and a National Science Foundation CAREER award (DEB-0953551) and International Research Fellowship (to JR).
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Green, A.F., Ramsey, T.S. & Ramsey, J. Polyploidy and invasion of English ivy (Hedera spp., Araliaceae) in North American forests. Biol Invasions 15, 2219–2241 (2013). https://doi.org/10.1007/s10530-013-0446-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0446-7