Abstract
Invasions have often been linked to reduced biodiversity, but the role of non-native species in the decline of native species is ambiguous. In a 2003 survey of four California vineyard regions, exotic spiders (Cheiracanthium spp.) were more dominant in vineyards with lower spider species diversity and reduced spider abundance. There was no evidence for the role of species interactions in the invasion of Cheiracanthium spiders, however, as native spiders from the same feeding guild were most abundant in regions with high Cheiracanthium levels. Comparison with a survey conducted 10 years earlier indicated that the recent invader C. mildei simply represented an addition to the spider community, with no apparent change in proportions of the congener C. inclusum. Invasion success is discussed with respect to agricultural habitat, as results suggest that disturbed conditions in many vineyards may favor Cheiracanthium spp. and native wandering spiders while decreasing overall spider diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species threaten biodiversity in managed and natural ecosystems worldwide (Mack et al. 2000; Kolar and Lodge 2001). Invasions may result in species displacement (Parker et al. 1999; Levine et al. 2003; Yurkonis et al. 2005), although evidence is often indirect and difficult to obtain (Didham et al. 2005). It is still unclear whether the majority of invasions cause declines in biodiversity through the suppression of native species. Just as invasive species may be “drivers” of ecological change, they may also be “passengers” of anthropogenic changes to ecosystems that negatively impact native species (MacDougall and Turkington 2005). Highly disturbed, species-poor habitats may be more vulnerable to invasion than undisturbed habitats with high species diversity (Stachowicz et al. 1999; Shea and Chesson 2002; Kennedy et al. 2002). Weaker competition in species-poor habitats, due to an absence of ecologically similar native species, may allow invaders to establish more easily (Levine and D’Antonio 1999; Tilman 1999; Von Holle and Simberloff 2004; Yurkonis et al. 2005), leading to a negative association between invaders and ecologically similar native species. Alternatively, if non-native and native species respond positively to the same ecological conditions, invasions may be more successful in habitats with higher species diversity (Levine 2000). Under this mechanism, a positive correlation between native and non-native species would be expected.
Generalist arthropod predators include invasive species that are capable of affecting native species through a variety of direct and indirect pathways (Snyder and Evans 2006). The negative impacts of invasive ant species, for example, have been enormous (Holway et al. 2002). As generalist arthropod predators, spiders have the potential to affect native arthropod species; nevertheless, spiders have been largely overlooked as invasive species (but see Nyffeler et al. 1986; Hann 1990; Burger et al. 2001; Gruner 2005). Once established, invasive spiders may be viewed as either beneficial arthropods in agroecosystems, or as disruptive predators in native ecosystems. Documented displacements of native by invasive spider species are rare, although Hann (1990) reported an invasive web-building spider competed with and ultimately displaced an endemic web-building species in New Zealand.
Here, we present results from surveys that documented exotic Cheiracanthium spiders (Miturgidae) in California vineyards. Spiders comprise most arthropod predators in California vineyards (Costello and Daane 1999) and surveys conducted in the 1990s found that among the wandering spider species was a yellow sac spider of the genus Cheiracanthium (Costello and Daane 1995, 1999). There are two known Cheiracanthium species in the Americas—C. mildei L. Koch, a Mediterranean native that was first reported in New England in the late 1940s (Bryant 1952), and C. inclusum Hentz, which is present in Africa and the New World (Platnick 2008). Both are wandering nocturnal hunters that feed on a wide variety of prey (Peck and Whitcomb 1970; Mansour et al. 1980; Wise 1993). We predicted that the invasion success of Cheiracanthium spiders would be negatively correlated with the species richness and abundance of native spiders. In particular, we expected a negative association between Cheiracanthium spiders and ecologically similar native species (i.e., native wandering spiders).
Materials and Methods
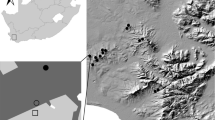
Spider composition was surveyed in four major California vineyard regions in June and July 2003 (Fig. 1). The sampling period was selected to match the seasonal period when most vineyard spiders are adults or large immatures (Costello and Daane 1999), in order to facilitate species identification. Thirty-eight vineyard blocks were each sampled once: seven Central Coast wine grapes (San Luis Obispo County); eleven North Coast wine grapes (Napa and Sonoma Counties); twelve San Joaquin Valley raisin or table grapes (Madera, Fresno, and Kern Counties); and eight Northern Interior wine grapes (San Joaquin and Sacramento Counties). To examine the occurrence of Cheiracanthium spiders in vineyards outside California, five additional wine grape vineyards were sampled in northwestern Oregon (Benton County) in October 2006.
Proportions of exotic Cheiracanthium spp. (black section of pie chart) and native spiders collected in 2003. Samples sites were within four California vineyard regions: a North Coast (Napa and Sonoma Counties); b Central Coast (San Luis Obispo County); c Northern Interior (San Joaquin and Sacramento Counties); d San Joaquin Valley (Madera, Fresno and Kern Counties)
As was typical of the regional patterns of land use, Central Coast vineyards were surrounded primarily by oak woodland, while Northern Interior and San Joaquin Valley vineyards were surrounded by other agricultural blocks (vineyards and/or stone fruit orchards). Surrounding habitat in the North Coast region included other vineyards, oak woodland and grasslands. Oregon vineyards were surrounded by other vineyards or pine forests.
Spiders were sampled using a “beat” sampling method, as described by Costello and Daane (1997). Foliage between grape vines was shaken and beaten over a 1 m2 cloth funnel for 30 s; spiders fell into a plastic bag attached with binder clips to the bottom of the funnel. In the field, collected samples were immediately deposited into a cooler to discourage spider-spider predation. Eighteen samples were taken from each site. Samples were collected from six vineyard rows; sampled rows were five rows apart, and three samples were taken per row at intervals of ten vines. All samples were sorted in the laboratory; spiders were deposited in 70% alcohol and identified using keys developed by Roth (1993) or Ubick et al. (2005). Most spiders were identified to species or genus. Immatures from some families (e.g., Araneidae, Linyphiidae) could not be sorted beyond the family level; these spiders were instead sorted to morphospecies.
To assess changes over time in Cheiracanthium abundance, samples from four vineyards in the 2003 survey were compared with samples collected from the same four vineyards in a 1993 survey (Costello and Daane 1995). The beat collection method was used in both surveys, and samples were taken during the same month in both years (June). The presence of C. mildei was not reported in these earlier surveys. To exclude the possibility that C. mildei had been overlooked, voucher specimens from 1993 surveys were examined to confirm species identity.
Data analyses
Differences between California regions in proportions of Cheiracanthium and native wandering spiders, species richness, and total spider abundance were compared using Kruskal–Wallis tests. Nonparametric multiple comparison tests were performed using the kruskalmc function in R version 2.7.0 (R Development Core Team 2008). To examine spider community structure in vineyards, spiders were assigned to guilds following classifications in Uetz et al. (1999), with the exception of the “diurnal stalker” guild, which we refer to here as “jumping spiders” (Salticidae), since these were the only spiders representing this guild in our samples. Guild structure was compared between regions using the Bray-Curtis similarity index.
We used regression analysis to assess relationships between dominance of Cheiracanthium spiders (expressed as proportion of Cheiracanthium to total spiders) and species richness and total spider abundance. Data for California and Oregon were analyzed separately. It should be noted that species richness is necessarily correlated with abundance. We were unable to rarefy species richness, however, since the minimum number of spiders per vineyard was low (n = 6) and rarefaction curves tend to converge at low abundances (Gotelli and Colwell 2001). Nonetheless, we feel that the number of species present may be a useful ecological measure in itself. All vineyards where Cheiracanthium was not present (n = 10) were excluded from these analyses. Proportional data were arcsine square-root transformed (Systat 2007). Total spider abundance was log-transformed to meet assumptions of normality. For analyses of California data, region and insecticide use (high-input/low-input) were initially included as dichotomous dummy variables, but were insignificant at P < 0.05 and the data are not included here. Spearman rank correlation was used to assess the association between abundances of Cheiracanthium spp. and native wandering spiders across California sites, with the cor.test function in R.
To assess whether invasion levels of Cheiracanthium spiders changed between 1993 and 2003, proportions of C. inclusum and both Cheiracanthium spiders together were compared between years using Mann–Whitney tests.
Results
A total of 1367 spiders were collected from California vineyards, belonging to 17 families and 30 species (Table 1). Cheiracanthium spiders occurred at 28 out of the 38 vineyards sampled and comprised more than 25% of the spider composition at eight vineyards (Fig. 1). Cheiracanthium mildei and C. inclusum accounted for 4.2 and 2.6% of spiders overall. Native wandering spiders included three species: Anyphaena pacifica Banks, Hibana incursa Chamberlin (both Anyphaenidae) and Trachelas pacificus Chamberlin (Corinnidae). The majority of identified species were restricted to North America (Platnick 2008) and are, therefore, likely to be native. Only five of the species collected show a wider distribution: Salticus scenicus Clerck (Salticidae) and Misumena vatia Clerck (Thomisidae), which are Holarctic; Badumna longinqua L. Koch (Desidae), which is present in Australia, New Zealand and the New World, but was represented by only one individual in our surveys; and the two Cheiracanthium species, C. mildei and C. inclusum. Of these, only B. longinqua and C. mildei are known to be exotic, while the presence of C. inclusum in Africa and the New World (Platnick 2008) strongly suggests that it is also an invader to North America (Cheiracanthium is primarily an Old World genus; C. mildei and C. inclusum are the only Cheiracanthium species known to be present in the Americas).
There was wide variation among the 38 vineyards in total number of spiders collected (range: 6–146) and number of spider species per vineyard (range: 4–20). There were also stark differences in the spiders that dominated each vineyard region (Fig. 2). The only spider guild that was consistently represented in all regions was the jumping spiders (Salticidae). Funnel weavers, orb weavers, and diurnal wandering spiders were not represented in most vineyards. According to the Bray-Curtis index, North Coast and Northern Interior vineyards showed the greatest similarity in guild structure of all regions within California (Table 2). Spider guild composition in the San Joaquin Valley was not similar to any other region, and was most dissimilar to the Central Coast (Table 2). Guild structure and species composition of Oregon vineyards was very similar to North Coast vineyards (Table 2). Cheiracanthium spiders were present in all vineyards sampled in Oregon.
Regional differences in species richness within California were only marginally significant (Kruskal–Wallis test, H = 6.82, P = 0.078), although the Central Coast had the highest number of species per vineyard, while the San Joaquin Valley had the lowest (Fig. 3). There were significant differences between regions in total numbers of spiders per vineyard (H = 9.71, P = 0.02), percentages of Cheiracanthium spiders (H = 15.78, P < 0.01) and percentages of native wandering spiders (H = 14.42, P < 0.01). Vineyards in the San Joaquin region contained the lowest numbers of spiders and spider species of all regions within California, the highest proportions of Cheiracanthium spiders and native wandering spiders (Fig. 3). The Central Coast, in contrast, showed the highest numbers of spiders and spider species, but had the lowest numbers of both Cheiracanthium spiders and native wandering spiders.
Influence of four different grape-growing regions on a percentage of Cheiracanthium spiders, b percentage of native wandering spiders, c species richness, and d total number of spiders. Data are means ± SE. Different letters above means indicate that they are significantly different (nonparametric multiple comparison tests, P < 0.05)
Across California, there were significant negative relationships between proportion of Cheiracanthium spiders and both total number of spiders per vineyard and species richness (Fig. 4). Vineyards with low spider abundance and low species richness tended to have higher proportions of Cheiracanthium spiders. There was a marginally positive correlation, however, between Cheiracanthium spiders and native wandering spiders (Spearman rank correlation, r = 0.30, P = 0.07).
Relationship between the proportion Cheiracanthium spiders of total spiders (arcsine square-root transformed) in each vineyard with a the total number of spiders (log10-transformed) and b the number of spider species in each respective vineyard. Data are from 2003 collections; vineyards where Cheiracanthium spiders were not present were excluded from analyses
There was also a marginally significant negative relationship between proportion of Cheiracanthium spiders and total spider numbers per vineyard in Oregon (R 2 = 0.75, P = 0.058; arcsine √proportion Cheiracanthium = 1.42 − 0.66*log(spider number), while the relationship between proportion Cheiracanthium and species richness was negative but nonsignificant (R 2 = 0.49, P = 0.19; arcsine √proportion Cheiracanthium = 0.73 − 0.03*number species).
In comparisons of San Joaquin Valley samples from 1993 and 2003, a striking change in species incidence and proportional representation occurred within the Cheiracanthium spiders. C. mildei was not represented in samples from 1992 to 1993, but was the dominant Cheiracanthium species in 2003. In our reexamination of voucher specimens from 1992 to 1993, all Cheiracanthium spiders were confirmed to be C. inclusum, as reported by Costello and Daane (1995). Percentages of Cheiracanthium spiders in 1993 and 2003 were 15.8 ± 5.1 and 57.1 ± 12.0 (means ± SE), respectively, and were significantly higher in 2003 (Mann–Whitney test, P < 0.05). Percentages of C. inclusum were 15.8 ± 5.1 in 1993 and 19.3 ± 13.5 in 2003, and were not significantly different between years (P > 0.05).
Discussion
In California, Cheiracanthium spiders appeared to have been most successful in vineyards where abundance and diversity of native species was depleted. To examine the consistency of this pattern, we also sampled vineyards outside California, and showed that a similar, marginally significant relationship between proportions of Cheiracanthium spiders and total spider abundance was present in Oregon. The role of species interactions in the invasion of Cheiracanthium spiders was ambiguous, however. Cheiracanthium spiders were most successful in California regions where native wandering spiders were also abundant, and there was even a marginally positive correlation between abundances of Cheiracanthium and native wandering spiders. The comparison of spider species composition in San Joaquin Valley vineyards between 1993 and 2003 also yielded little evidence for species interactions in the invasion of Cheiracanthium spiders. Although invaders are likely to interact most strongly with ecologically similar native species (Prieur-Richard et al. 2000; Symstad 2000), the presence of both Cheiracanthium species in many of the same vineyards in 2003 suggests that these species may coexist in vineyards. The large increase in the proportion of Cheiracanthium spiders between years appears to be due to the addition of C. mildei to the spider fauna.
This pattern runs contrary to the view that invasive species should be more successful in ecosystems where functionally similar native species are absent (e.g., Von Holle and Simberloff 2004). Other studies examining invasions of generalist arthropod predators have uncovered similar results. An invasive carabid beetle, for example, appeared to coexist with native carabids (Niemelä and Spence 1991; Niemalä et al. 1997). Similarly, Burger et al. (2001) found a positive correlation between numbers of exotic spiders and native spiders, and concluded that non-native spiders represented a supplement to native spider diversity. High prey availability may prevent predator-predator competition from occurring, as seemed to be the case with a non-native coccinellid beetle, which suppressed a native coccinellid at low prey densities but not at high prey densities (Obrycki et al. 1998). The setting of this study may also have precluded species interactions; long-term dynamics may not be possible in agricultural systems, due to disturbance from insecticide applications and other farming practices (Janssen et al. 2006). The roles of short- and long-term dynamics in the relatively recent arrival of C. mildei are also difficult to determine, as C. mildei has either been present in these sites for less than 10 years, or has not become established over the long-term, and has repeatedly recolonized vineyards after dispersal events or population declines.
Notwithstanding these considerations, regional dominance of Cheiracanthium spiders appears to have scaled negatively with increased prominence of natural habitat in the landscape (San Joaquin Valley > Northern Interior > North Coast > Central Coast), while total spider abundance and spider diversity showed an opposing pattern. High percentages of non-crop habitats surrounding crop systems can influence spider composition (Schmidt and Tscharntke 2005) and diversity (Clough et al. 2005). Here, we did not directly measure the amount of non-crop habitat in each region, but we suspect that the complexity of the surrounding landscape may be important in explaining regional patterns in both spider diversity and the invasion success of Cheiracanthium spiders. It should be noted that we are unable to rule out the influence of other factors that could have to contributed to observed patterns, such as propagule pressure, which may influence the success of invasions more than characteristics of invaded communities (Levine 2000; Von Holle and Simberloff 2005), or possible differences between regions in numbers of spider predators such as birds, which Gruner (2005) showed could profoundly influence the success of spider invasions.
However, the negative relationships between proportions of Cheiracanthium spiders and both numbers of spiders and species richness are likely to reflect the adaptability of Cheiracanthium spiders, as was noted by Costello and Daane (1995). Invasive species may be passengers of large-scale changes in environmental conditions that negatively affect native species (Shea and Chesson 2002; MacDougall and Turkington 2005), and the conversion of landscapes to vineyard monoculture may allow Cheiracanthium spiders to thrive at the expense of native spider diversity. Vineyard monoculture may not be a hostile environment for native wandering spiders, however. The co-occurrence of Cheiracanthium spiders with native wandering spiders may reflect similar responses to disturbance. The adaptability of Cheiracanthium spiders may not result solely from their status as exotic species, but may be characteristic of wandering spiders in general. Non-native and native species can respond positively to the same environmental conditions (Levine 2000; Burger et al. 2001), and the wandering spider guild as a whole may flourish under conditions that are hostile to other spider species.
References
Bryant EB (1952) Redescription of Cheiracanthium mildei L Koch, a recent spider immigrant from Europe. Psyche 58:120–123
Burger JC, Patten MA, Prentice TR, Redak RA (2001) Evidence for spider community resilience to invasion by non-native spiders. Biol Conserv 98:241–249
Clough Y, Kruess A, Kleijn D, Tscharntke T (2005) Spider diversity in cereal fields: comparing factors at local, landscape and regional scales. J Biogeogr 32:2007–2014
Costello MJ, Daane KM (1995) Spider (Aranaeae) species composition and seasonal abundance in San Joaquin Valley grape vineyards. Environ Entomol 24:823–831
Costello MJ, Daane KM (1997) Comparison of sampling methods used to estimate spider (Araneae) species abundance and composition in grape vineyards. Environ Entomol 26:142–149
Costello MJ, Daane KM (1999) Abundance of spiders and insect predators on grapes in central California. J Arachnol 27:531–538
Didham RK, Tylianakis JM, Hutshinson MA, Ewers RM, Gemmell NJ (2005) Are invasive species the drivers of ecological change? Trends Ecol Evol 20:470–474
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Gruner DS (2005) Biotic resistance to an invasive spider conferred by generalist insectivorous birds on Hawai`i Island. Biol Invasions 7:541–546
Hann SW (1990) Evidence for the displacement of an endemic New Zealand spider, Latrodectus katipo Powell by the South African species Steatoda capensis Hann (Araneae: Theridiidae). N Z J Zool 17:295–308
Holway DA, Lach L, Suarez AV, Tsutsui TD, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233
Janssen A, Montserrat M, HilleRisLambers R, de Roos AM, Pallini A, Sabelis MW (2006) Intraguild predation usually does not disrupt biological control. In: Brodeur J, Boivin G (eds) Trophic guild interactions in biological control. Springer, Dordrecht, pp 21–44
Kennedy TA, Naeem S, Howe KM, Knops JMH, Tilman D, Reich P (2002) Biodiversity as a barrier to ecological invasion. Nature 417:636–638
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Levine JM (2000) Species diversity and biological invasions: relating local process to community pattern. Science 288:852–854
Levine JM, D’Antonio CM (1999) Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87:15–26
Levine JM, Vila M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond Series B 270:775–781
MacDougall AS, Turkington R (2005) Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86:42–55
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzazz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Mansour F, Rosen D, Shulov A, Plaut HN (1980) Evaluation of spiders as biocontrol agents of Spodoptera littoralis larvae on apple in Israel. Oecol Appl 1:225–232
Niemalä J, Spence JR, Carcamo H (1997) Establishment and interactions of carabid populations: an experiment with native and introduced species. Ecography 20:643–652
Niemelä J, Spence JR (1991) Distribution and abundance of an exotic ground-beetle (Carabidae): a test of community impact. Oikos 62:351–359
Nyffeler M, Dondale CD, Redner JH (1986) Evidence for displacement of a North American spider, Steatoda borealis (Hentz), by the European species S. bipunctata (Linnaeus) (Araneae: Theridiidae). Can J Zool 64:867–874
Obrycki JJ, Giles KL, Ormord AM (1998) Interactions between an introduced and indigenous coccinellid species at different prey densities. Oecologia 117:279–285
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Peck WB, Whitcomb WH (1970) Studies on the biology of a spider, Cheiracanthium inclusum (Hentz). Univ Ark Agric Exp Stn Bull 753:1–76
Platnick NI (2008) The world spider catalog, version 8.5. New York, American Museum of Natural History (http://research.amnh.org/entomology/spiders/catalog/index.html)
Prieur-Richard AH, Lavorel S, Grigulis K, Dos Santos A (2000) Plant community diversity and invasibility by exotics: invasion of Mediterranean old fields by Conyza bonariensis and Conyza canadensis. Ecol Lett 3:412–422
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Roth VD (1993) Spider genera of North America. American Arachnological Society, Gainesville
Schmidt MH, Tscharntke T (2005) Landscape context of sheetweb spider (Araneae: Linyphiidae) abundance in cereal fields. J Biogeogr 32:467–473
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176
Snyder WE, Evans EW (2006) Ecological effects of invasive arthropod generalist predators. Annu Rev Ecol Evol Syst 37:95–122
Stachowicz JJ, Whitlatch RB, Osman RW (1999) Species diversity and invasion resistance in a marine ecosystem. Science 286:1577–1579
Symstad AJ (2000) A test of the effects of functional group richness and composition on grassland invasibility. Ecology 81:99–109
Systat (2007) SYSTAT Version 12.0. Systat Software Inc, Evanston
Tilman D (1999) The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80:1455–1474
Ubick D, Paquin P, Cushing PE, Roth V (2005) Spiders of North America: an identification manual. American Arachnological Society, Gainesville
Uetz GW, Halaj J, Cady AB (1999) Guild structure of spiders in major crops. J Arachnol 27:270–280
Von Holle B, Simberloff D (2004) Testing Fox’s assembly rule: does plant invasion depend on recipient community structure? Oikos 105:551–563
Von Holle B, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212–3218
Wise DH (1993) Spiders in ecological webs. Cambridge University Press, Cambridge
Yurkonis KA, Meiners SJ, Wachholder BE (2005) Invasion impacts diversity through altered community dynamics. J Ecol 93:1053–1061
Acknowledgments
This work was supported with grants from the National Science Foundation Dissertation Improvement Grant DEB 0710434, the Robert van den Bosch Memorial Scholarship, and the California Table Grape Commission and American Vineyard Foundation. We thank vineyard managers for use of their farms, and Derek Fehrer and Luke Powell for help in the field and laboratory. We also acknowledge Daniel Simberloff and two anonymous reviewers whose helpful suggestions greatly improved this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hogg, B.N., Gillespie, R.G. & Daane, K.M. Regional patterns in the invasion success of Cheiracanthium spiders (Miturgidae) in vineyard ecosystems. Biol Invasions 12, 2499–2508 (2010). https://doi.org/10.1007/s10530-009-9659-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9659-1