Abstract
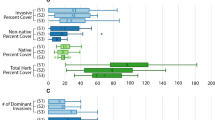
The effects of invasive plants on plants native to areas that are being invaded can be quite variable, depending on the species of the invasive plant involved as well as the physical characteristics of the location being invaded. My study focuses on the effects of Phragmites australis Linnaeus (common reed) and Lythrum salicaria L. (purple loosestrife) on the same native plant community. Uninvaded plots dominated by native plants Typha angustifolia L. (narrowleaf cattail) and Typha latifolia L. (broadleaf cattail) served as the control. I surveyed percent cover of species during early summer and midsummer for 3 years in six Hudson River freshwater tidal wetlands (sites). Differences in species richness, composition and abundance were small, but significant among invaded and uninvaded plots and among sites. However, these differences remained significant when data for dominant species (invasive and native) were removed. Differences in native plant species abundance were attributed to invasive plant species-specific characteristics and differences in species richness and composition were attributed to physical location (zonation) in these freshwater tidal marshes. “Invasive” status of a dominant plant species was less important in invasive plant–native plant interactions than species-specific characteristics and zonation. Further research into the effects of site and land-use on invasive plant impacts is recommended.



Similar content being viewed by others
References
Bais HP, Vepachedu R, Gilroy S et al (2003) Allelopathy and exotic plant invasions: from molecules and genes to species interactions. Science 301(5638):1377–1380. doi:10.1126/science.1083245
Baker HG (1986) Patterns of plant invasions in North America. In: Mooney HA, Drake JA (eds) Ecology of biological invasions of North America and Hawaii. Springer-Verlag, New York
Bashkin M, Stohlgren TJ, Otsuki Y, Lee M, Evangelista P, Belnap J (2003b) Soil characteristics and plant exotic species invasions in the Grand Staircase-Escalante National Monument, Utah, USA. Appl Soil Ecol 22(1):67–77. doi:10.1016/S0929-1393(02)00108-7
Bertness MD, Hacker SD (1994) Physical stress and positive associations among marsh plants. Am Nat 144(3):363–372. doi:10.1086/285681
Biddlestone AJ, Gray KR, Thorairagan K (1991) A botanical approach to the treatment of wastewaters. J Biotechnol 17(3):209–220. doi:10.1016/0168-1656(91)90012-K
Bradford MA, Schumacher HB, Catovsky S et al (2007) Impacts of invasive plant species on riparian plant assemblages: interactions with elevated atmospheric carbon dioxide and nitrogen deposition. Oecologia 152(4):791–803. doi:10.1007/s00442-007-0697-z
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27(4):326–349. doi:10.2307/1942268
Call LJ, Nilsen ET (2005) Analysis of interactions between the invasive tree-of-heaven (Ailanthus altissima) and the native black locust (Robinia pseudoacacia). Plant Ecol 176:275–285. doi:10.1007/s11258-004-0338-0
Callaway RM, Ridenour WM, Laboski T et al (2005) Natural selection for resistance to the allelopathic effects of invasive plants. J Ecol 93(3):576–583. doi:10.1111/j.1365-2745.2005.00994.x
Chambers RM (1997) Porewater chemistry associated with Phragmites and Spartina in a Connecticut marsh. Wetlands 17:360–367
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot 64(3–4):261–273. doi:10.1016/S0304-3770(99)00055-8
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Coops H, Vandervelde G (1995) Seed dispersal, germination and seedling growth of 6 helophyte species in relation to water-level zonation. Freshw Biol 34(1):13–20. doi:10.1111/j.1365-2427.1995.tb00418.x
Corbin JD, D’Antonio CM (2004) Competition between native perennial and exotic annual grasses: implications for an historical invasion. Ecology 85(5):1273–1283. doi:10.1890/02-0744
Cully AC, Cully JF, Hiebert RD (2003) Invasions of exotic plant species in tallgrass prairie fragments. Conserv Biol 17(4):990–998. doi:10.1046/j.1523-1739.2003.02107.x
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu Rev Ecol Syst 23:63–87
Edwards KR, Adams MS, Kvĕt J (1995) Invasion history and ecology of Lythrum salicaria in North America. In: Pyšek P, Prach K, Rejmánek M et al (eds) Invasions: general aspects and special problems. SPB Academic Publishing, Netherlands
Falco LA, Fernandez GCJ, Nowak RS (2007) Variation in establishment of a non-native annual grass influences competitive interactions with Mojave Desert perennials. Biol Invasions 9:293–307. doi:10.1007/s10530-006-9033-5
Findlay S, Groffman P (1997) Effects of marsh vegetation on sediment biogeochemistry: a baseline for restoration. A final report to NOAA
Gaudet CL, Keddy PA (1988) A comparative approach to predicting competitive ability from plant traits. Nature 334(6169):242–243. doi:10.1038/334242a0
Gorchov DL, Trisel DE (2003) Competitive effects of the invasive shrub, Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of native tree seedlings. Plant Ecol 166:13–24. doi:10.1023/A:1023208215796
Gordon DR (1998) Effects of invasive non-indigenous plant species on ecosystem processes: lessons from Florida. Ecol Appl 8(4):975–989. doi:10.1890/1051-0761(1998)008[0975:EOINIP]2.0.CO;2
Hager HA, McCoy KD (1998) The implications of accepting untested hypotheses: a review of the effects of purple loosestrife (Lythrum salicaria) in North America. Biodivers Conserv 7(8):1069–1079. doi:10.1023/A:1008861115557
Hager HA, Vinebrook RD (2004) Positive relationships between invasive purple loosestrife (Lythrum salicaria) and plant species diversity and abundance in Minnesota wetlands. Can J Bot 82(6):763–773. doi:10.1139/b04-052
Hawarthbrockman MJ, Murkin HR, Clay RT (1993) Effects of shallow flooding on newly established purple loosestrife seedlings. Wetlands 13(3):224–227
Hierro JL, Callaway RM (2003) Allelopathy and exotic plant invasion. Plant Soil 256(1):29–39. doi:10.1023/A:1026208327014
Keller BEM (2000) Plant diversity in Lythrum, Phragmites, and Typha marshes, Massachusetts, U.S.A. Wetlands Ecol Manage 8:391–401. doi:10.1023/A:1026505817409
Kourtev PS, Ehrenfeld JG, Haggblom M (2003) Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biol Biochem 35(7):895–905. doi:10.1016/S0038-0717(03)00120-2
Lempe J, Stevens KJ, Peterson RL (2001) Shoot responses of six Lythricaceae species to flooding. Plant Biol 3(2):186–193. doi:10.1055/s-2001-12901
Lenssen J, Menting F, van der Putten W et al (1999) Control of plant species richness and zonation of functional groups along a freshwater flooding gradient. Oikos 86(3):523–534. doi:10.2307/3546656
Lesica P, DeLuca TH (2004) Is tamarisk allelopathic? Plant Soil 267(1–2):357–365. doi:10.1007/s11104-005-0153-y
Maskell LC, Firbank LG, Thompson K et al (2006) Interactions between non-native plant species and the floristic composition of common habitats. J Ecol 94:1052–1060. doi:10.1111/j.1365-2745.2006.01172.x
Meyerson LA, Saltonstall K, Windham L et al (2000) A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecol Manage 8:89–103. doi:10.1023/A:1008432200133
Minchinton TE, Bertness MD (2003) Disturbance-mediated competition and the spread of Phragmites australis in a coastal marsh. Ecol Appl 13(5):1400–1416. doi:10.1890/02-5136
Morgan EC, Overholt WA (2005) Potential allelopathic effects of Brazilian pepper (Schinus terebinthifolius Raddi, Anacardiaceae) aqueous extract on germination and growth of selected Florida native plants. J Torrey Bot Soc 132(1):11–15. doi:10.3159/1095-5674(2005)132[11:PAEOBP]2.0.CO;2
Nieder WC, Barnaba E, Findlay SEG, Hoskins SB, Holochuck N, Blair EA (2004) Distribution and abundance of submerged aquatic vegetation and Trapa natans in the Hudson River. J Coast Res 45:150–161
Odum WE, Smith TJ III, Hoover JK et al (1984) The ecology of tidal freshwater marshes of the United States East Coast: a community profile. U.S. Fish and Wildlife Service, FWS OBS-83(17)
Pennings SC, Grant MB, Bertness MD (2005) Plant zonation in low-latitude salt marshes: disentangling the roles of flooding, salinity and competition. J Ecol 93(1):159–167. doi:10.1111/j.1365-2745.2004.00959.x
Pyšek P, Prach K, Smilauer P (1995) Relating invasion success to plant traits: an analysis of the Czech Alien Flora. In: Pyšek P, Prach K, Rejmánek M et al (eds) Wade plant invasions: general aspects and special problems. SPB Academic Publishing, Netherlands
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, New York
Rand TA, Russell FL, Louda SM (2004) Local- vs. landscape-scale indirect effects of an invasive weed on native plants. Weed Technol 18:1250–1254 (supplement S)
Rawinski TJ, Malecki RA (1984) Ecological relationships among purple loosestrife, cattail and wildlife at the Montezuma National Wildlife Refuge. N Y Fish Game J 31:81–87
Rooth JE, Stevenson JC, Cornwell JC (2003) The influence of 5 and 20 yr-old Phragmites populations on rates of accretion in an oligohaline tidal marsh of Chesapeake Bay. Estuaries 26(2B):475–483
Roy J (1990) In search of the characteristics of plant invaders. In: di Castri F, Hansen AJ, Debussche M (eds) Biological invasions in Europe and the Mediterranean. Kluwer, Dordrecht
Russell FL, Louda SM, Rand TA et al (2007) Variation in herbivore-meditated indirect effects of an invasive plant on a native plant. Ecology 88(2):413–423. doi:10.1890/0012-9658(2007)88[413:VIHIEO]2.0.CO;2
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA 99(4):2445–2449. doi:10.1073/pnas.032477999
Saltonstall K (2003) Microsatellite variation within and among North American lineages of Phragmites australis. Mol Ecol 12:1689–1702. doi:10.1046/j.1365-294X.2003.01849.x
Silliman BR, Bertness MD (2004) Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conserv Biol 18(5):1424–1434. doi:10.1111/j.1523-1739.2004.00112.x
Simberloff D (2003) Confronting introduced species: a form of xenophobia? Biol Invasions 5:179–192. doi:10.1023/A:1026164419010
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. W.H. Freeman and Company, New York
Tecco PA, Gurvich DE, Díaz S et al (2006) Positive interaction between invasive plants: the influence of Pyracantha angustifolia on the recruitment of native and exotic woody species. Austral Ecol 31:293–300. doi:10.1111/j.1442-9993.2006.01557.x
Thelen GC, Vivanco JM, Newingham B et al (2005) Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives. Ecol Lett 8(2):209–217. doi:10.1111/j.1461-0248.2004.00713.x
Treberg MA, Husband BC (1999) Relationship between the abundance of Lythrum salicaria (purple loosestrife) and plant species richness along the Bar River, Canada. Wetlands 19(1):118–125
Vandenbrink FWB, Vandervelde GW, Bosman W et al (1995) Effects of substrate parameters on growth-responses of 8 helophyte species in relation to flooding. Aquat Bot 50(1):79–97. doi:10.1016/0304-3770(95)00452-6
Vandermaarel E (1995) Vicinism and mass effect in a historical perspective. J Veg Sci 6(3):445–446. doi:10.2307/3236245
Vitousek PM, Walker LR, Whittaker LD et al (1987) Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science 238:802–804. doi:10.1126/science.238.4828.802
Windham L, Meyerson LA (2003) Effects of common reed (Phragmites australis) expansions on nitrogen dynamics of tidal marshes of the Northeastern U.S. Estuaries 26(2B):452–464
Winogrond HG (1997) Invasion of Phragmites australis in the tidal marshes of the Hudson River. M.S. Thesis, Bard College, Annandale, New York
Winogrond H, Kiviat E (1997) The historical distribution of Phragmites australis within the Hudson River. In: Nieder WC, Waldman JR (eds) Final reports of the Tibor T. Polgar fellowship program, 1996, Sect. VI. Hudson River Foundation, New York, 29 pp
Zonneveld IS (1995) Vicinism and mass effect. J Veg Sci 5:441–444. doi:10.2307/3236244
Acknowledgments
My thanks to Rick Ostfeld, Charlie Janson, Catherine Graham and Jessica Gurevitch for editorial comments and support. This article is based on research funded by a NOAA OCRM Graduate Research Fellowship, Tibor T. Polgar Fellowship and the Slobodkin Award for Ecological Research (Stony Brook University).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGlynn, C.A. Native and invasive plant interactions in wetlands and the minimal role of invasiveness. Biol Invasions 11, 1929–1939 (2009). https://doi.org/10.1007/s10530-008-9370-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-008-9370-7