Abstract
Purpose
We examined the inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by a nitrogen-doped titanium dioxide (N-TiO2) visible-light photocatalyst that was activated via light irradiation in the natural environment and was safe for human use as a coating material.
Methods
The photocatalytic activity of glass slides coated with three types of N-TiO2 without metal or loaded with copper or silver and copper was investigated by measuring acetaldehyde degradation. The titer levels of infectious SARS-CoV-2 were measured using cell culture after exposing photocatalytically active coated glass slides to visible light for up to 60 min.
Results
N-TiO2 photoirradiation inactivated the SARS-CoV-2 Wuhan strain and this effect was enhanced by copper loading and further by the addition of silver. Hence, visible-light irradiation using silver and copper-loaded N-TiO2 inactivated the Delta, Omicron, and Wuhan strains.
Conclusion
N-TiO2 could be used to inactivate SARS-CoV-2 variants, including emerging variants, in the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the recent years, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has caused significant health and economic losses worldwide (Zhou et al. 2020). Constant inactivation of the virus in the environment is crucial to prevent the spread of SARS-CoV-2, which is transmitted mainly through aerosols emitted by infected persons (Prather et al. 2020). Infectious viruses have also been detected on the surfaces of objects in public places, such as hospitals (Feng et al. 2021), which are widely disinfected using alcohol or other agents (Kampf et al. 2005). However, the coating of various surfaces using solid antiviral compounds was shown to provide sustained antiviral effectiveness (van Doremalen et al. 2020). For antimicrobial coatings, the use of various compounds that can kill or inhibit the growth of pathogens, fungi, and viruses has been considered (Rai et al. 2020). Among these compounds, visible-light photocatalysts are promising as they can degrade organic molecules via oxidative chemicals generated by light irradiation from indoor lighting fixtures (Matsuura et al. 2021; Uema et al. 2021; Nakano et al. 2022; Prakash et al. 2022). The effectiveness of visible-light photocatalysts, titanium dioxide (TiO2) loaded without metal (Matsuura et al. 2021), tungsten trioxide (WO3) with unknown additives (Uema et al. 2021), and TiO2 loaded with nanoclusters of copper oxide (Nakano et al. 2022) for the inactivation of SARS-CoV-2 has been previously reported. However, the effect of additive metals on viral inactivation by main component oxides remains to be elucidated. The inactivation of SARS-CoV-2 using silver (Ag) nanoparticles has been proposed (Pilaquinga et al. 2021), although the effects of Ag as an additive for visible-light photocatalysts remain unclear. The present study revealed that the efficacy of SARS-CoV-2 inactivation was enhanced by loading Ag or copper (Cu) on nitrogen-doped titanium dioxide (N-TiO2), which is a visible-light photocatalyst (Asahi et al. 2001, 2014; Morikawa et al. 2006; Ohwaki et al. 2016). We also showed that N-TiO2 loaded with both Ag and Cu could inactivate the SARS-CoV-2 Delta and Omicron variants that temporarily dominated the pandemic.
Materials and Methods
N-TiO2 coating of glass surfaces
We evaluated three types of visible photocatalysts: N-TiO2 (Toyotsu Vehitecs Co., Ltd., VCT-01SQC), Cu-loaded Cu/N-TiO2 (Toyotsu Vehitecs Co., Ltd., VCTIIC-01SQC), and Cu + Ag/N-TiO2 (N-TiO2 loaded with both Cu and Ag; Toyotsu Vehitecs Co., Ltd., VCTIICA-01). These photocatalysts were synthesized based on the flowchart depicted in Fig. 1. N-TiO2 powder with 0.25% nitrogen doping for oxygen was obtained by annealing the anatase-type TiO2 powder with the nitrogen source (Asahi et al. 2001; Morikawa et al. 2006). The crystal structure of the powder was evaluated using X-ray diffraction (XRD) (SU3500, Rigaku, Japan) analysis, and the optical absorption edge was measured via ultraviolet–visible absorption spectroscopy (V-780, JASCO, Japan). Cu or Cu and Ag loading were prepared by impregnating N-TiO2 with an aqueous solution of Cu(NO3)2 or AgNO3 at room temperature, followed by stirring, washing, and filtration. Each powder was finely ground, water-slurried, and mixed with an organic binder to prepare the photocatalytic coating solutions with 6% solid content. The size distribution of the dispersed particles in the coating solution was evaluated using dynamic light scattering analysis (DLS) (Zetasizer Nano ZSP, Malvern Panaltical). Glass slides or 5-cm square glass plates (Matsunami Glass Ind., Ltd., S1111) were sonicated in acetone and ethanol for 10 min each and then dried at 80 °C for 1 h. Coating solution (0.8 mL) was dropped onto the washed glass and spin-coated at 750 rpm for 5 s. The samples were then heat-treated at 200 °C for 1 h and ultraviolet-irradiated using a black light for 1 h to remove surface deposits before use in the subsequent experiments.
Qualitative analysis on reactive oxygen radicals
Reactive oxygen species generated by photocatalysts was qualitatively analyzed using the spin-trap ESR (Electron Spin Resonance) method. 5,5-dimethyl-1-pyrroline N-oxide (DMPO) (Tokyo Chemical Industry Co., Ltd.) was used as the spin-trapping reagent. DMPO was diluted with water to 5 mol/dm3, purified using spherical activated carbon (AS ONE CORPORATION), and finally used as a spin-trap solution. After spreading 100 μL of the solution on each N-TiO2-coated glass plate at a depth of ~ 4 cm2 and irradiated with visible light at 10,000 lx for 15 min, ~ 75 μL of the reaction solution was collected and measured using X-band ESR (Bruker E500). The measurement conditions were microwave power, 3.17 mW; microwave frequency, 9.82 GHz; modulation amplitude, 0.2 mT; modulation frequency, 100 kHz; time constant, 30 ms; receiver gain, 60; magnetic field scan, 20 mT; and scan, 10.
Observation using scanning electron microscopy and quantification by inductively coupled plasma
The surface condition of the glass coated with N-TiO2 was examined via scanning electron microscopy (SEM) (Hitachi High-Tech Co., Regulus 8230). The coated glass slides were cut into 10-mm squares, further coated with osmium, and secondary electron images were captured at an acceleration voltage of 2 keV. Acid dissolution of N-TiO2 coated on the glass surface was performed and the Ti content of each sample was quantified via inductively coupled plasma optical emission spectroscopy (ICP–OES) (Hitachi High-Tech Science Co., PS3520UVDDII). The Cu and Ag contents were quantified using ICP mass spectrometry (ICP–MS) (Agilent Technologies, Agilent 8900). The TiO2 content was estimated by calculating the Ti content, and the total TiO2 + Cu + Ag content was used as the sample total weight.
Photocatalytic activity evaluation
The photocatalytic activity of N-TiO2 was evaluated using an acetaldehyde degradation test. The 5-cm square glass plates coated with each type of N-TiO2 were placed in 500-mL glass containers, filled with air composition levels of O2/N2 gas and 60 ppm acetaldehyde gas, sealed, and left in the dark for 1 h, which was considered 0 h. The concentration of acetaldehyde and its degradation product, CO2, were measured via gas chromatography (Shimadzu Co., GC-14B) under conditions of visible-light irradiation. The fluorescent light of illuminance at 10,000 lx (T-10A, KONICA MINOLTA, INC.) and light power of 550 μW/cm2 (UVR-2 UD40 detector, TOPCON CO.) with cut film to omit ultraviolet light at < 400 nm was used as a visible-light source. No changes were observed in light intensity during the pre- and post-experiments. The rate of CO2 production was calculated via linear regression after subtracting background due to uncoated glass.
Enzyme-linked immunosorbent assay analysis of binding affinity between S-protein and ACE2
Binding affinity assay between S-protein and ACE2 was performed using a partially modified protocol of the COVID-19 Spike-ACE2 binding assay kit II (RayBiotech, Inc.). Briefly, 100 μL of a solution of Fc-tagged SARS-CoV-2 spike receptor binding domain protein (S-protein) diluted with the attached buffer (1× Assay Diluent) was placed on a photocatalyst-coated glass slide under a fluorescent lamp covered with a 400 nm cut filter and irradiated with 10,000 lx light for 0, 15, or 60 min at ~25 °C. The glass slide was placed in a plastic petri dish and covered to prevent drying. The light-irradiated glass slide was transferred to a 50 mL centrifuge tube and centrifuged at 1000 rpm for 30 s to collect the solution. Then, the slide was washed with 100 μL assay solution, which was collected by centrifugation at 1000 rpm for 30 s. The collected reaction solution was placed into 96-well plates whose bottoms were coated with recombinantly expressed ACE2, and enzyme-linked immunosorbent assay was performed. The absorbance was measured at 450 nm at 0, 15, and 60 min (designated A0, A15, and A60, respectively). The percentage of denatured S-protein undetectable by the antibody at 15 and 60 min was calculated as (A0 − A15)/A0 and (A0 − A60)/A0, respectively. Four samples were measured per experiment and all experiments were repeated twice. The results were reported as mean and standard deviation.
Inactivation of SARS-CoV-2 and variants using N-TiO2 plates
SARS-CoV-2 JP/TY/WK-521/2020 (WK-521; Wuhan strain, GenBank Sequence Accession: LC522975), SARS-CoV-2 hCoV/Japan/TY11-927-P1/2021 (TY11-927; Delta variant, GISAID variant name: EPI_ISL_2158617), and SARS-CoV-2 hCoV-19/Japan/TY38-873/2021 (TY38-873; Omicron variant, GISAID variant name: EPI_ISL_7418017) were kindly provided by Drs. Masayuki Saijo, Mutsuyo Takayama-Ito, Masaaki Sato, and Ken Maeda of the National Institute of Infectious Disease (Tokyo Japan). The viruses were propagated once at the Shiga University of Medical Science in VeroE6/TMPRSS2 cells (JCRB Cell Bank, Osaka, Japan). VeroE6/TMPRSS2 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS) and 50 μg/mL each of penicillin, streptomycin, and G418 in 96-well plates for 3 days. The viral solution (500 μL) was placed on 25-cm2 glass plates coated with N-TiO2 and then exposed to visible-light for the indicated times. The experiment was conducted in a draft chamber with constant negative pressure from exhaust air to ensure safety, and the temperature of the samples was maintained at ~25 °C. A dilution series of the recovered viral solution was prepared and inoculated onto confluent VeroE6/TMPRSS2 cells that had been washed twice with 100 µL of Hanks’ Balanced Salt Solution (HBSS) (Nacalai Tesque). The final concentrations of the viral solutions were ~104 to 102 TCID50/mL, and which were higher than the concentration (101 TCID50/mL) that confirmed human infection (Killingley et al. 2022). The cells were incubated for 1 h at 37 °C, the solution was then removed via aspiration, and the wells were washed once with 100 µL HBSS media. The wash solution was removed via aspiration, 100 µL DMEM supplemented with 0.1% bovine serum albumin (BSA) was added, and the cells were cultured in an incubator at 37 °C for 3 days. Cytopathic effects were observed under a microscope, and the mean tissue culture infectious dose (TCID50) was determined. All viral experiments were performed in the Biosafety Level 3 facility of the Research Center for Animal Life Science, Shiga University of Medical Science. Four samples were measured per experiment and all experiments were repeated twice. The results were calculated as mean and standard deviation.
Results
Synthesis of N-TiO2 particles
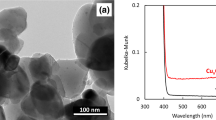
Three types of N-TiO2 were synthesized using titanium dioxide powder as a raw material to prepare the coatings (Fig. 1). The synthesized N-TiO2 was in the anatase phase with an average crystallite size of 20 nm (Supplementary Fig. 1) and exhibited optical absorption in the visible-light region of 400–520 nm (Supplementary Fig. 2). Dynamic light scattering analysis of the particle size revealed that all the particles exhibited a similar distribution, with a peak at ~ 28 nm (Fig. 2). These particles were used in further experiments.
Reactive oxygen species produced by the light irradiation of N-TiO2
The free radical species produced by water molecules via the photocatalytic reaction of N-TiO2 were analyzed using spin-trap ESR (Figure 3). The three ESR spectra of N-TiO2 showed the same pattern comprising four signals with amplitude ratios of 1:2:2:1, which were determined to be derived from the adduct of the OH radicals that reacted with the spin-trapping agent DMPO, whereas the other spectral patterns, such as signals derived from superoxide anion and alkyl radicals (Finkelstein et al. 1982; Makino et al. 1982), were not detected (Figure 3). Under this light irradiation condition, the chief reactive oxygen species in the reaction solution was found to be OH radicals. The signal intensity of the reaction solution of N-TiO2 without metal support was < 1/10 of those of the metal-supported N-TiO2 particles, although not exactly quantitative (Figure 3).
Structure and composition of N-TiO2 coated on the glass surface
Glass surfaces coated with three types of N-TiO2 showed agglomerated particles of ~ 20 nm in diameter and pores that were several tens of nm in size (Figure 4). The surface loading of N-TiO2 and film thickness were ~ 0.5 g/m2 and ~ 200 nm, respectively, based on the weight increment after coating. The size of the SARS-CoV-2 virus particles ranged from 60 to 140 nm (Zhu et al. 2020); therefore, the viral particles were considered to contact N-TiO2 at the surface layer without entering the layer when the virus solution was dropped onto the coated glass surface. The compositional analysis of the N-TiO2 coated on the glass surface using ICP–OES and ICP–MS showed that it contained trace amounts of Cu. Cu/N-TiO2 contained 3.3 mass% Cu while Ag+Cu/N-TiO2 contained 1.6 mass% Cu and 0.1 mass% Ag (Table 1).
Visible photocatalytic activity of N-TiO2
A 5-cm square glass slide coated with each N-TiO2 was placed in a 500-mL sealed container to measure acetaldehyde degradation activity under visible-light irradiation (Fig. 5). For all three types of N-TiO2, irradiating the coated glass pieces with visible-light decreased the concentration of acetaldehyde in a time-dependent manner, whereas the concentration of the reaction product, CO2, increased (Fig. 5). The rate of CO2 formation by metal-loaded N-TiO2 was ~ 2.5 times faster than that of N-TiO2 without metal loading. The rates of CO2 formation were almost equal for Cu/N-TiO2 (0.903 ppm/h) and Ag + Cu/TiO2 (0.939 ppm/h) (Table 2).
Inhibition of the binding of S-protein with ACE2 by N-TiO2
We examined whether the binding of SARS-CoV-2 S-proteins involved in infection with Angiotensin Converting enzyme 2 (ACE2) was inhibited by the photocatalytic activity of N-TiO2 coated on the glass surface (Fig. 6). When the three types of N-TiO2 were irradiated with visible-light at 10,000 lx, ~ 70% and > 90% of the S-protein in the solution were not detected by the antibody after 15 and 60 min, respectively, whereas the protein concentration in the reaction solution remained unchanged. After 15- and 60-min light irradiation, there were no significant differences in the proportion of S-protein denaturation between N-TiO2 without metal loading and Cu/N-TiO2 (p = 0.095 and 0.785) or N-TiO2 without metal loading and Ag + Cu/N-TiO2 (p = 0.068 and 0.238) that became undetectable with the antibody.
Denaturation of S-protein by three types of N-TiO2 irradiated with visible-light. White bars indicate N-TiO2, light gray bars indicate Cu/N-TiO2, and dark gray bars indicate Ag + Cu/TiO2. No significant differences at the 5% level were observed between N-TiO2 and Cu/N-TiO2 or Ag + Cu/N-TiO2 under both the 15- and 60-min light irradiation conditions
Effects of N-TiO2 supported by Ag and Cu on SARS-CoV-2 inactivation
The light- and time-dependent inactivation of SARS-CoV-2 (WK-521) by N-TiO2 with or without metals was investigated using cell culture experiments (Figure 7). To test whether the initial viral titers (7356 TCID50/mL) decreased from zero time in N-TiO2 without metal, it was compared with one of the other samples between the two groups using one-sided t-test at a 5% significance level. As a result, no significant difference was detected between the 15- and 60-min dark conditions (p = 0.074 and 0.068, respectively); however, a significant difference was detected between the 15 and 60-min light conditions (p = 0.039 and 0.010, respectively) (Figure 7 w/o). Based on these results, it was observed that N-TiO2 without metal could not inactivate SARS-CoV-2 in the dark. Furthermore, under light conditions, some viruses were inactivated after > 15 min of light exposure in this study. Although no significant differences were detected, the mean viral titer at 60 min (2750 TCID50/mL) was lower than at 15 min in the light condition (3750 TCID50/mL). Significant reductions in the viral titers were observed in the Cu/N-TiO2 plates under all conditions (Figure 7 Cu). Viral titers decreased by ~ 80% for 15 and 60 min in the dark condition and 15 min in the light condition (p = 0.013, 0.013, and 0.016, respectively), and by ~ 3% at 60 min in the light condition (p = 0.006). In the light condition, the viral titer at 60 min was significantly reduced to ~ 16% compared with that at 15 min (p = 0.006); however, no significant difference was observed in the dark condition (p = 0.430). The inactivation of SARS-CoV-2 was also observed on Ag+Cu/N-TiO2 plates under all conditions (Figure 7 Ag+Cu). In Ag+Cu/N-TiO2, time-dependent virus inactivation was also detected under dark conditions, with viral titers reaching ~ 24% at 15 min and 4.5% at 60 min compared with that at 0 min (p = 0.005 and 0.003, respectively). The light dependence of viral inactivation was also found to be 9.6% at 15 min compared with that at 0 min and < 1% at 60 min under the light conditions (p = 0.004 and 0.002, respectively).
Inactivation of SARS-CoV-2 (WK-521, Wuhan strain) following visible-light irradiation of three types of N-TiO2. SARS-CoV-2 virus solution on each N-TiO2-coated glass was irradiated to visible-light for 0, 15, or 60 min in the dark (D) or light (L). The y-axis shows the mean plus standard deviations of the infectious virus titers. Asterisks indicate significant differences of p < 0.01 when comparing paired titers, indicated by solid or dotted lines, using one-sided Welch t-test. Pairs showing significant differences at p < 0.05 when comparing viral titers with one-sided Welch t-test are indicated by white circles (at zero time and the sample) and asterisks (pairs indicated by solid or dotted lines)
Inactivation of SARS-CoV-2 delta and omicron variants
The volume of the infectious viral titers of the SARS-CoV-2 Wuhan, Delta, and Omicron variants were determined using cell culture assay following the visible-light irradiation of Ag+Cu/N-TiO2-coated glass plates for 60 min. Significant decreases in infectious titers due to light irradiation for 60 min were detected for all three variants compared with the reference titers at 0 min (Figure 8). Although a direct comparison among variants could not be conducted owing to the different initial viral concentrations, > 99% of the Wuhan strain, 88.8% of the Delta variant, and 98.6% of the Omicron variant were inactivated following 60 min of light irradiation compared with the viral titers observed at 0 min.
Inactivation of SARS-CoV-2 variants following visible-light irradiation of Ag + Cu/N-TiO2. Each bar represents the mean titers of infectious viruses after 0 and 60 min of light irradiation in each variant. The upper side of the standard deviation is indicated. Asterisks indicate significant differences of p < 0.01 using a one-sided Welch t-test when comparing the tiers after 0 and 60 min of light irradiation in each variant. W Wuhan, D Delta and O Omicron
Discussion
The photocatalysis of N-TiO2 resulted in the degradation of acetaldehyde (Figure 5), conformational changes in S-proteins (Figure 6), and inactivation of SARS-CoV-2 (Figure 7 and Figure 8) in this study. In previous studies, SARS-CoV-2 inactivation by visible photocatalysts TiO2 (Matsuura et al. 2021) and WO3 (Uema et al. 2021) was considered to be primarily due to reactive oxygen species generated via light irradiation. Therefore, we hypothesized the following three main reaction fields for N-TiO2 involved in viral inactivation.
-
1)
Holes on the titanium dioxide surface that oxidizes and decomposes organic matter common to the three types of N-TiO2.
-
2)
OH radicals produced via water oxidation by the holes.
-
3)
OH radicals generated by the Fenton reaction in the presence of copper through the production of superoxide anions by the combination of excited electrons and oxygen.
We discuss which reaction field was more effective in virus inactivation by N-TiO2 in the followings.
The photocatalytic degradation of organic compounds, such as acetaldehyde in an aqueous solution, is considered to occur primarily in the hole reaction field created on the photocatalyst surface where these molecules are adsorbed (Zhang and Nosaka 2015), whereas some of them are thought to occur due to OH radicals diffusing into the solution (Asahi et al. 2014). From the results of the acetaldehyde degradation presented in Fig. 5, the holes involved in the oxidative decomposition of organic compounds in N-TiO2 without metal support were found to be ~ 40% of those in N-TiO2 with metal support under the same light irradiation conditions. Since electrons excited by visible-light move quickly from N-TiO2 to Cu in Cu-loaded N-TiO2 (Kato et al. 2010), we inferred that this difference was attributable to the combination between excited electrons and holes in probability. As no difference was observed in the inhibition of S-protein and ACE protein binding in Fig. 6, the hole reaction field in this experiment was found to be saturated. In the viral inactivation experiment in Fig. 7, the inactivation activity of N-TiO2 without metal support was observed to be only 1/10 of that of N-TiO2 with metal support. Based on these results, the mechanisms of action of metal-loaded N-TiO2 were considered to be direct inactivation via protein adsorption of Cu and Ag and indirect promotion of inactivation. As another mechanism, the inactivation of certain viral biomolecules by oxidation via the OH radicals derived from the Fenton reaction was also considered.
Recently, TiO2 loaded with copper oxide nanoclusters has been reported to inactivate SARS-CoV-2 even in dark conditions, and light irradiation further enhances this effect (Nakano et al. 2022). This visible photocatalytic virus inactivation was partially caused by Cu(I) species, and the mechanism was considered effective for protein adsorption owing to the solid-phase properties of Cu (Sunada et al. 2012). SARS-CoV-2 inactivation using Ag nanoparticles has also been reported, and the mechanism is considered to be via protein adsorption as well (Pilaquinga et al. 2021). Adsorption to Cu or Ag could also be considered to attract viral particles near the hole reaction field on the N-TiO2 surface, resulting in the efficient oxidative degradation of S-proteins or other biochemicals of the virus by the holes.
ESR measurements using the spin-trapping agent DMPO can qualitatively identify multiple reactive oxygen species generated in water (Finkelstein et al. 1980). In the presence of water, the photocatalyst holes react with water molecules to produce free radical species (Kakuma et al. 2015). Using the ESR spectral pattern, only OH radicals were detected in all the samples and no OOH and alkyl radicals, among others, derived from superoxide anions were identified (Figure 3). The amplitude intensity of the ESR spectra derived from OH radicals was stronger in Cu-loaded N-TiO2 than in N-TiO2 without metal loading. This difference was not considered to be caused by the hole binding to water, but rather from the Fenton reaction that occurred in the presence of copper. OH radicals are strong oxidants with an oxidation potential of 2.81 eV, which is sufficiently high to inactivate the virus (Koppenol and Liebman 1984; Nakamura et al. 2020). In the viral inactivation experiments shown in Figure 7, both metal-loaded N-TiO2 inactivated SARS-CoV-2 in a light-irradiated and time-dependent manner. Collectively, these results suggest that the oxidative effect of OH radicals on some viral biomolecules may be the chief cause of their inactivation. In previous studies, the fragmentation of viral RNA was observed in addition to the photocatalytic degradation of S-proteins (Matsuura et al. 2021; Nakano et al. 2022), and OH radicals may be involved in both the oxidative degradations.
We revealed an additive effect of metals (Cu and Ag) with N-TiO2 on the inactivation of SARS-CoV-2. A comparison of the inactivation activity of N-TiO2 and Cu/N-TiO2 (Fig. 7) suggested that inactivation was enhanced by the presence of Cu owing to photoirradiation-induced increased generation of reactive oxygen species. The time-dependent inactivation by Ag + Cu/N-TiO2 under dark conditions was attributed to Ag (Fig. 7). Furthermore, the most effective inactivation of SARS-CoV-2 was observed when Ag + Cu/N-TiO2 was exposed to light for 60 min. Taken together, these results suggest that N-TiO2 triggers light-induced SARS-CoV-2 inactivation and is enhanced by Cu loading and further enhanced by the addition of Ag. The promotion of viral inactivation may result from multiple action mechanisms. Surface-coated copper and silver have been known to exert direct antiviral activity against numerous types of viruses, with or without viral envelopes (Thurman et al. 1989; Rai et al. 2020). Solid-state silver has also been reported to inactivate enveloped influenza A viruses as well as SARS-CoV-2 more strongly than the same amount of copper (Minoshima et al. 2016). This finding supports the results shown in Fig. 4, which revealed a stronger inactivation activity of Ag + Cu/N-TiO2 compared with Cu/N-TiO2 in dark conditions. Another possible mechanism of action is that viral particles are adsorbed by Cu or Ag and inactivated at active sites near the photocatalytic surface, such as holes and OH radicals generated by light irradiation in the vicinity, and the difference in the inactivation activities of Cu/N-TiO2 and Ag + Cu/N-TiO2 may reflect the difference in affinity of Cu and Ag toward the viral surface.
The SARS-CoV-2 Delta variant was reported to spread more rapidly than the Wuhan strain (Kupferschmidt and Wadman 2021). Furthermore, the Omicron variant was reported to show more effective transmission (Torjesen 2021; Dehury et al. 2021). These variants temporarily became predominant, suggesting that emerging variants may be prevalent in future. In the present study, visible-light irradiation of Ag + Cu/N-TiO2 inactivated three SARS-CoV-2 strains, including the Wuhan, Delta, and Omicron variants (Fig. 5). Amino acid substitutions in the S-protein, which binds to ACE2 on the human cell membrane of both the Delta and Omicron variants on the surface of virus may alter the structure or charge state of the S-protein and affect its inactivation (Han et al. 2022). Despite some differences in the amino acid sequences of the S-proteins, the inactivation mechanism was partially considered to occur due to some changes of these proteins by hole and OH radicals generated via photocatalytic activity, as was the case with the Wuhan strain. If the adsorption properties against viral particles differ for Ag and Cu, Ag + Cu/N-TiO2 may be applicable to a wide range of mutations, and thus may inactivate new SARS-CoV-2 variants.
Data availability
The datasets were included in electric supplementary materials.
References
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-dopedtitanium oxides. Science 293:269–271. https://doi.org/10.1126/science.1061051
Asahi R, Morikawa T, Irie H, Ohwaki T (2014) Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: designs, developments, and prospects. Chem Rev 114:9824–9852. https://doi.org/10.1021/cr5000738
Dehury B, Raina V, Misra N, Suar M (2021) Effect of mutation on structure, function and dynamics of receptor binding domain of human SARS-CoV-2 with host cell receptor ACE2: a molecular dynamics simulations study. J Biomol Struct Dyn 39:7231–7245. https://doi.org/10.1080/07391102.2020.1802348
Feng B, Xu K, Gu S, Zheng S, Zou Q, Xu Y, Yu L, Lou F, Yu F, Jin T, Li Y, Sheng J, Yen HL, Zhong Z, Wei J, Chen Y (2021) Multi-route transmission potential of SARS-CoV-2 in healthcare facilities. J Hazard Mater 402:123771. https://doi.org/10.1016/j.jhazmat.2020.123771
Finkelstein E, Rosen GM, Rauckman EJ (1980) Spin trapping. Kinetics of the reaction of superoxide and hydroxyl radicals with nitrones. J Am Chem Soc 102:4994–4999. https://doi.org/10.1021/ja00535a029
Finkelstein E, Rosen GM, Rauckman EJ (1982) Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol Pharmacol 21:262–265
Han P, Li L, Liu S, Wang Q, Zhang D, Xu Z, Han P, Li X, Peng Q, Su C, Huang B, Li D, Zhang R, Tian M, Fu L, Gao Y, Zhao X, Liu K, Qi J, Gao GF, Wang P (2022) Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell 185:630–640. https://doi.org/10.1007/BF00167182
Kakuma Y, Nosaka A, Nosaka Y (2015) Difference in TiO2 photocatalytic mechanism between rutile and anatase studied by the detection of active oxygen and surface species in water. Phys Chem Chem Phys 17:18691–18698
Kampf G, Grotheer D, Steinmann J (2005) Efficacy of three ethanol-based hand rubs against feline calicivirus, a surrogate virus for Norovirus. J Hosp Infect 60:144–149. https://doi.org/10.1016/j.jhin.2004.12.005
Katoh R, Furube A, Yamanaka K, Morikawa T (2010) Charge separation and trapping in N-doped TiO2 photocatalysts: a time-resolved microwave conductivity study. J Phys Chem Lett 1:3261–3265. https://doi.org/10.1021/jz1011548
Killingley B, Mann AJ, Kalinova M, Boyers A, Goonawardane N, Zhou J, Lindsell K, Hare SS, Brown J, Frise R, Smith E, Hopkins C, Noulin N, Löndt B, Wilkinson T, Harden S, McShane H, Baillet M, Gilbert A, Jacobs M, Charman C, Mande P, Nguyen-Van-Tam JS, Semple MG, Read RC, Ferguson NM, Openshaw PJ, Rapeport G, Barclay WS, Catchpole AP, Chiu C (2022) Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med 28:1031–1041. https://doi.org/10.1038/s41591-022-01780-9
Koppenol WH, Liebman JF (1984) The oxidizing nature of the hydroxyl radical. A comparison with the Ferry1 Ion (FeO2+). J Phys Chem 88:99–101
Kupferschmidt K, Wadman M (2021) Delta variant triggers new phase in the pandemic. Science 372:1375–1376. https://doi.org/10.1126/science.372.6549.1375
Makino K, Mossoba MM, Riesz P (1982) Chemical effects of ultrasound on aqueous solutions. Evidence for hydroxyl and hydrogen free radicals (OH and H ) by spin trapping. J Am Chem Soc 104:3537–3539. https://doi.org/10.1021/ja00376a064
Matsuura R, Lo CW, Wada S, Somei J, Ochiai H, Murakami T, Saito N, Ogawa T, Shinjo A, Benno Y, Nakagawa M, Takei M, Aida Y (2021) SARS-CoV-2 disinfection of air and surface contamination by TiO2 photocatalyst-mediated damage to viral morphology, RNA, and protein. Viruses 13:942. https://doi.org/10.3390/v13050942
Minoshima M, Lu Y, Kimura T, Nakano R, Ishiguro H, Kubota Y, Hashimoto K, Sunada K (2016) Comparison of the antiviral effect of solid-state copper and silver compounds. J Hazard Mater 312:1–7. https://doi.org/10.1016/j.jhazmat.2016.03.023
Morikawa T, Irokawa Y, Ohwaki T (2006) Enhanced photocatalytic activity of TiO2−xNx loaded with copper ions under visible-light irradiation. Appl Cat A: General 314:123–127. https://doi.org/10.1016/j.apcata.2006.08.011
Nakamura S, Ando N, Sato M, Ishihara M (2020) Ultraviolet irradiation enhances the microbicidal activity of silver nanoparticles by hydroxyl radicals. Int J Mol Sci 21:3204–3215
Nakano R, Yamaguchi A, Sunada K, Nagai T, Nakano A, Suzuki Y, Yano H, Ishiguro H, Miyauchi M (2022) Inactivation of various variant types of SARS-CoV-2 by indoor-light-sensitive TiO2-based photocatalyst. Sci Rep 12:5804. https://doi.org/10.1038/s41598-022-09402-7
Ohwaki T, Saeki S, Aoki K, Morikawa T (2016) Evaluation of photocatalytic activities and characteristics of Cu- or Fe-modified nitrogen-doped titanium dioxides for applications in environmental purification. Jpn J Appl Phys 55:01AA05
Pilaquinga F, Morey J, Torres M, Seqqat R, Piña MLN (2021) Silver nanoparticles as a potential treatment against SARS-CoV-2: a review. Wiley Interdiscip Rev Nanomed Nanobiotechnol 13:e1707. https://doi.org/10.1002/wnan.1707
Prakash J, Cho J, Mishra YK (2022) Photocatalytic TiO2 nanomaterials as potential antimicrobial and antiviral agents: scope against blocking the SARS-COV-2 spread. Micro Nano Eng 14:100100
Prather KA, Wang CC, Schooley RT (2020) Reducing transmission of SARS-CoV-2. Science 368:1422–1424. https://doi.org/10.1126/science.abc6197
Rai PK, Usmani Z, Thakur VK, Gupta VK, Mishra YK (2020) Tackling COVID-19 pandemic through nanocoatings: confront and exactitude. Curr Res Green Sustain Chem 3:100011
Sunada K, Minoshima M, Hashimoto K (2012) Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J Hazard Mater 235–236:265–270. https://doi.org/10.1016/j.jhazmat.2012.07.052
Thurman R, Bitton GCP (1989) The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. CRC Crit Rev Environ Control 18:295–315. https://doi.org/10.1080/10643388909388351
Torjesen I (2021) Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ 375:n2943. https://doi.org/10.1136/bmj.n2943
Uema M, Yonemitsu K, Momose Y, Ishii Y, Tateda K, Inoue T, Asakura H (2021) Effect of the photocatalyst under visible-light irradiation in SARS-CoV-2 stability on an abiotic surface. Biocontrol Sci 26:119–125. https://doi.org/10.4265/bio.26.119
van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382:1564–1567. https://doi.org/10.1056/NEJMc2004973
Zhang J, Nosaka Y (2015) Photocatalytic oxidation mechanism of methanol and the other reactants in irradiated TiO2 aqueous suspension investigated by OH radical detection. Appl Catal B: Environ 166–167:32–36
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. https://doi.org/10.1038/s41586-020-2012-7
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. https://doi.org/10.1056/NEJMoa2001017
Acknowledgements
Toyota Central R&D Labs, Inc. financially supported this research, and Takashi Matsuyama, Shu Saeki, Satoru Kosaka, Yoriko Matsuoka, and Takao Imaeda were employees of Toyota Central R&D Labs, Inc. for the duration of this study. Yasushi Itoh has no financial relationships to disclose.
Supporting information
Figure S1—XRD patterns of three types of photocatalyst. The crystal structure of all synthesized powder is the anatase phase (JCPDS file 21-1272). The crystallite diameters of all synthesized powder are calculated to be about 20 nm from Scherrer’s equation.
Figure S2—The optical absorption spectra of three types of photocatalyst and the base TiO2 powder. The vertical axis was Kubelka-Munk corrected based on the diffuse reflection spectrum data.
Figure S3—Spectrum of a light source using a fluorescent lamp with a UV-cut film.
Funding
Takashi Matsuyama, Shu Saeki, Satoru Kosaka, Yoriko Matsuoka, Yoshifumi Aoki, and Takao Imaeda have received research support from Toyota Central R&D Labs, Inc.
Author information
Authors and Affiliations
Contributions
All authors except for YA contributed to the study conception and design. Material preparation and data collection and analysis were performed by TM, SS, SK, YM, YA, YI, and TI. The first draft of the manuscript was written by Takashi Matsuyama and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Yasushi Itoh has no financial relationships to disclose. Takashi Matsuyama, Shu Saeki, Satoru Kosaka, Yoriko Matsuoka, Yoshifumi Aoki, and Takao Imaeda were employees of Toyota Central R&D Labs, Inc. for the duration of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsuyama, T., Saeki, S., Kosaka, S. et al. Inactivation of SARS-CoV-2 variants by nitrogen-doped titanium dioxide loaded with metals as visible-light photocatalysts. Biotechnol Lett 45, 551–561 (2023). https://doi.org/10.1007/s10529-023-03361-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-023-03361-3