Abstract
Purpose
We screened nitrilases with significant nitrile hydratase activity to exploit their potential in benzylic amide biosynthesis. We also investigated the factors affecting their hydration activity to support further research on benzylic amide production by nitrilase.
Methods
A sequence-based screening method using previously reported crucial positions identified to be essential for amide-forming capacity of nitrilase (referred to as “amide-formation hotspots”) as molecular probes to identify putative amide-forming nitrilases.
Results
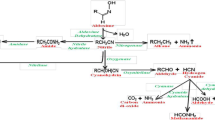
Based on the previously reported “amide-formation hotspots,” we identified a nitrilase NitPG from Paraburkholderia graminis DSM 17151 that could produce a significant amount of mandelamide toward mandelonitrile and exhibited general hydration activity toward various benzylic nitriles. The time-course experiment with NitPG demonstrated that amide was also a true reaction product of nitrilase, suggesting that the nitrile catalysis by amide-forming nitrilase could be a post-transition state bifurcation-mediated enzymatic reaction. Further research demonstrated that low temperature, metal ion addition, and specific substrate structure could profoundly improve the amide formation capability of nitrilase.
Conclusions
NitPG with broad hydration activity is a potential candidate for the enzymatic synthesis of benzylic amides for biotechnological applications. Studying the effect of nitrilase hydration activity could promote our understanding of the factors that influence amide and acid distribution.





Similar content being viewed by others
References
Arfi T, Nigam VK (2020) Studies on a thermostable nitrilase from Staphylococcus Sp and its In-silico characterisation. Biologia 75(12):2421–2432. https://doi.org/10.2478/s11756-020-00554-3
Badoei-Dalfard A, Ramezani-Pour N, Karami Z (2016) "Production and characterization of a nitrilase from pseudomonas aeruginosa RZ44 and its potential for nitrile biotransformation. Iran J Biotechnol 14(3):142–153. https://doi.org/10.15171/ijb.1179
Bhalla TC, Kumar V, Kumar V, Thakur N (2018) Nitrile metabolizing enzymes in biocatalysis and biotransformation. Appl Biochem Biotechnol 185(4):925–946. https://doi.org/10.1007/s12010-018-2705-7
Chen J, Zheng RC, Zheng YG, Shen YC (2009) Microbial transformation of nitriles to high-value acids or amides. Adv Biochem Eng Biotechnol 113:33–77. https://doi.org/10.1007/10_2008_25
Cheng Z, Xia Y, Zhou Z (2020) Recent advances and promises in nitrile hydratase: from mechanism to industrial applications. Front Bioeng Biotechnol 8:352. https://doi.org/10.3389/fbioe.2020.00352
Constable DJC et al (2007) Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem 9(5):411–420. https://doi.org/10.1039/b703488c
Duca D, Rose DR, Glick BR (2014) Characterization of a nitrilase and a nitrile hydratase from Pseudomonas sp. strain UW4 that converts indole-3-acetonitrile to indole-3-acetic acid. Appl Environ Microbiol 80(15):4640–4649. https://doi.org/10.1128/AEM.00649-14
Fan HY et al (2017) A novel nitrilase from Ralstonia eutropha H16 and its application to nicotinic acid production. Bioprocess Biosyst Eng 40(8):1271–1281. https://doi.org/10.1007/s00449-017-1787-x
Fernandes BCM et al (2006) Nitrile hydratase activity of a recombinant nitrilase. Adv Synth Catal 348(18):2597–2603. https://doi.org/10.1002/adsc.200600269
García-Álvarez R, Crochet P, Cadierno V (2013) Metal-catalyzed amide bond forming reactions in an environmentally friendly aqueous medium: nitrile hydrations and beyond. Green Chem 15(1):46–66. https://doi.org/10.1039/c2gc36534k
Gong JS et al (2017) Nitrile-converting enzymes as a tool to improve biocatalysis in organic synthesis: recent insights and promises. Crit Rev Biotechnol 37(1):69–81. https://doi.org/10.3109/07388551.2015.1120704
Günther J et al (2018) The nitrilase PtNIT1 catabolizes herbivore-induced nitriles in Populus trichocarpa. BMC Plant Biol 18(1):251. https://doi.org/10.1186/s12870-018-1478-z
Hare SR, Tantillo DJ (2017) Post-transition state bifurcations gain momentum – current state of the field. Pure Appl Chem 89(6):679–698. https://doi.org/10.1515/pac-2017-0104
Jiang S et al (2017) Switching a nitrilase from Syechocystis sp. PCC6803 to a nitrile hydratase by rationally regulating reaction pathways. Catal Sci Technol 7(5):1122–1128. https://doi.org/10.1039/c7cy00060j
Kaplan O et al (2006) Purification and characterization of a nitrilase from Aspergillus niger K10. Appl Microbiol Biotechnol 73(3):567–575. https://doi.org/10.1007/s00253-006-0503-6
Kiziak C, Conradt D, Stolz A, Mattes R, Klein J (2005) Nitrilase from Pseudomonas fluorescens EBC191: cloning and heterologous expression of the gene and biochemical characterization of the recombinant enzyme. Microbiology (reading) 151(Pt 11):3639–3648. https://doi.org/10.1099/mic.0.28246-0
Kiziak C, Stolz A (2009) Identification of Amino Acid Residues Responsible for the Enantioselectivity and Amide Formation Capacity of the Arylacetonitrilase from Pseudomonas fluorescens EBC191. Appl Environ Microbiol 75(17):5592–5599. https://doi.org/10.1128/aem.00301-09
Martinkova L, Kren V (2010) Biotransformations with nitrilases. Curr Opin Chem Biol 14(2):130–137. https://doi.org/10.1016/j.cbpa.2009.11.018
Petrickova A, Sosedov O, Baum S, Stolz A, Martinkova L (2012) Influence of point mutations near the active site on the catalytic properties of fungal arylacetonitrilases from Aspergillus niger and Neurospora crassa. J Mol Catal B 77:74–80. https://doi.org/10.1016/j.molcatb.2012.01.005
Pitzer J, Steiner K (2016) Amides in nature and biocatalysis. J Biotechnol 235:32–46. https://doi.org/10.1016/j.jbiotec.2016.03.023
Prasad S, Bhalla TC (2010) Nitrile hydratases (NHases): at the interface of academia and industry. Biotechnol Adv 28(6):725–741. https://doi.org/10.1016/j.biotechadv.2010.05.020
Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. https://doi.org/10.1093/nar/gku316
Sheppard AN, Acevedo O (2009) Multidimensional exploration of valley-ridge inflection points on potential-energy surfaces. J Am Chem Soc 131(7):2530–2540. https://doi.org/10.1021/ja803879k
Sosedov O, Stolz A (2015) Improvement of the amides forming capacity of the arylacetonitrilase from Pseudomonas fluorescens EBC191 by site-directed mutagenesis. Appl Microbiol Biotechnol 99(6):2623–2635. https://doi.org/10.1007/s00253-014-6061-4
Stolz A, Eppinger E, Sosedov O, Kiziak C (2019) “Comparative Analysis of the Conversion of Mandelonitrile and 2-Phenylpropionitrile by a Large Set of Variants Generated from a Nitrilase Originating from Pseudomonas fluorescens EBC191.” Molecules. https://doi.org/10.3390/molecules24234232
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Vesela AB, Petrickova A, Weyrauch P, Martinkova L (2013) Heterologous expression, purification and characterization of arylacetonitrilases from Nectria haematococca and Arthroderma benhamiae. Biocatal Biotransform 31(1):49–56. https://doi.org/10.3109/10242422.2012.758117
Wajant H, Effenberger F (2002) Characterization and synthetic applications of recombinant AtNIT1 from Arabidopsis thaliana. Eur J Biochem 269(2):680–687. https://doi.org/10.1046/j.0014-2956.2001.02702.x
Wang HL, Sun HH, Wei DZ (2013) Discovery and characterization of a highly efficient enantioselective mandelonitrile hydrolase from Burkholderia cenocepacia J2315 by phylogeny-based enzymatic substrate specificity prediction. BMC Biotechnol. https://doi.org/10.1186/1472-6750-13-14
Winkler M, Meischler D, Klempier N (2007) Nitrilase-catalyzed enantioselective synthesis of pyrrolidine- and piperidinecarboxylic acids. Adv Synth Catal 349(8–9):1475–1480. https://doi.org/10.1002/adsc.200700040
Yamada H, Kobayashi M (1996) Nitrile hydratase and its application to industrial production of acrylamide. Biosci Biotechnol Biochem 60(9):1391–1400. https://doi.org/10.1271/bbb.60.1391
Zhang ZJ, Xu JH, He YC, Ouyang LM, Liu YY (2011) Cloning and biochemical properties of a highly thermostable and enantioselective nitrilase from Alcaligenes sp. ECU0401 and its potential for (R)-(-)-mandelic acid production. Bioprocess Biosyst Eng 34(3):315–322. https://doi.org/10.1007/s00449-010-0473-z
Funding
This work is supported by the grant from the National Key Research and Development Program of China (Grant No. 2021YFC2102100).
Author information
Authors and Affiliations
Contributions
KZ and TZP: conceived and designed the study. KZ: conducted the literature search and performed the experiments. KZ and LZW: were involved in the analysis and interpretation of data. KZ: drafted the manuscript. HLW, YHR and DZW: The study was supervised and tutored. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interet
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, K., Pan, T., Wang, L. et al. Screening and characterization of a nitrilase with significant nitrile hydratase activity. Biotechnol Lett 44, 1163–1173 (2022). https://doi.org/10.1007/s10529-022-03291-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-022-03291-6