Abstract
Objectives
Catalytic promiscuity, or the ability to catalyze a secondary reaction, provides new opportunities for industrial biocatalysis by expanding the range of biocatalytic reactions. Some nitrilases converting nitriles to amides, referred to as the secondary activity, show great potential for amides production. And our goal was exploiting the amide-forming potential of nitrilases.
Results
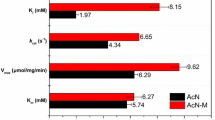
In this study, we characterized and altered the secondary activity of nitrilase from Acidovorax facilis 72 W (Nit72W) towards different substrates. We increased the secondary activity of Nit72W towards 2-cyanopyridine by 196-fold and created activity toward benzonitrile and p-nitrophenylacetonitrile by modifying the active pocket. Surprisingly, the best mutant, W188M, completely converted 250 mM 2-cyanopyridine to more than 98% 2-picolinamide in 12 h with a specific activity of 90 U/mg and showed potential for industrial applications.
Conclusions
Nit72W was modified to increase its secondary activity for the amides production, especially 2-picolinamide.




Similar content being viewed by others
References
Babtie A, Tokuriki N, Hollfelder F (2010) What makes an enzyme promiscuous? Curr Opin Chem Biol 14:200–207
Case DA et al (2005) The Amber biomolecular simulation programs. J Comput Chem 26:1668–1688
Casnati A et al (2005) Calixarene-based picolinamide extractants for selective An/Ln separation from radioactive waste. Eur J Org Chem 2005:2338–2348
Devamani T et al (2016) Catalytic promiscuity of ancestral esterases and hydroxynitrile lyases. J Am Chem Soc 138:1046–1056
Devine PN, Howard RM, Kumar R, Thompson MP, Truppo MD, Turner NJ (2018) Extending the application of biocatalysis to meet the challenges of drug development. Nat Rev Chem 2:409–421
Fernandes BCM et al (2006) Nitrile hydratase activity of a recombinant nitrilase. Adv Synth Catal 348:2597–2603
Godin AM et al (2011) Antinociceptive and anti-inflammatory activities of nicotinamide and its isomers in different experimental models. Pharmacol Biochem Be 99:782–788
Gong JS et al (2016) Engineering of a fungal nitrilase for improving catalytic activity and reducing by-product formation in the absence of structural information. Catal Sci Technol 6:4134–4141
Gutmann A, Nidetzky B (2016) Unlocking the potential of leloir glycosyltransferases for applied biocatalysis: Efficient synthesis of uridine 5′-diphosphate-glucose by sucrose synthase. Adv Synth Catal 358:3600–3609
Humble MS, Berglund P (2011) Biocatalytic Promiscuity. Eur. J Org Chem 2011:3391–3401
Hur S, Bruice TC (2003) The near attack conformation approach to the study of the chorismate to prephenate reaction. Proc Natl Acad Sci USA 100:12015–12020
Jiang S, Zhang L, Yao Z, Gao B, Wang H, Mao X, Wei D (2017) Switching a nitrilase from Syechocystis sp. PCC6803 to a nitrile hydratase by rationally regulating reaction pathways. Catal Sci Technol 7:1122–1128
Kawabata T, Ogino T, Mori M, Awai M (1992) Effects of nicotinamide and its isomers on iron-induced renal damage. Acta Pathol Jpn 42:469–475
Kiziak C, Klein J, Stolz A (2007) Influence of different carboxy-terminal mutations on the substrate-, reaction- and enantiospecificity of the arylacetonitrilase from Pseudomonas fluorescens EBC191. Protein Eng Des Sel 20:385–396
Kiziak C, Stolz A (2009) Identification of amino acid residues responsible for the enantioselectivity and amide formation capacity of the Arylacetonitrilase from Pseudomonas fluorescens EBC191. Appl Environ Microbiol 75:5592–5599
Liang C et al (2016) Engineering a carbohydrate-processing transglycosidase into glycosyltransferase for natural product glycodiversification. Sci Rep 6:21051
Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C (2015) ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J Chem Theory Comput 11:3696–3713
Martinkova L, Kren V (2010) Biotransformations with nitrilases. Curr Opin Chem Biol 14:130–137
Nedrud DM, Lin H, Lopez G, Padhi SK, Legatt GA, Kazlauskas RJ (2014) Uncovering divergent evolution of alpha/beta-hydrolases: a surprising residue substitution needed to convert Hevea brasiliensis hydroxynitrile lyase into an esterase. Chem Sci 5:4265–4277
Ogata S, Takeuchi M, Teradaira S, Yamamoto N, Iwata K, Okumura K, Taguchi H (2002) Radical Scavenging Activities of Niacin-Related Compounds. Biosci Biotech Bioch 66:641–645
Philpott HK, Thomas PJ, Tew D, Fuerst DE, Lovelock SL (2018) A versatile biosynthetic approach to amide bond formation. Green Chem 20:3426–3431
Schmidt S et al (2015) An enzyme cascade synthesis of epsilon-caprolactone and its oligomers. Angew Chem Int Ed 54:2784–2787
Singla P, Bhardwaj RD (2019) Enzyme promiscuity – A light on the “darker” side of enzyme specificity. Biocatal Biotransfor 38:81–92
Sosedov O, Baum S, Burger S, Matzer K, Kiziak C, Stolz A (2010) Construction and application of variants of the Pseudomonas fluorescens EBC191 arylacetonitrilase for increased production of acids or amides. Appl Environ Microbiol 76:3668–3674
Sosedov O, Stolz A (2014) Random mutagenesis of the arylacetonitrilase from Pseudomonas fluorescens EBC191 and identification of variants, which form increased amounts of mandeloamide from mandelonitrile. Appl Microbiol Biotechnol 98:1595–1607
Sosedov O, Stolz A (2015) Improvement of the amides forming capacity of the arylacetonitrilase from Pseudomonas fluorescens EBC191 by site-directed mutagenesis. Appl Microbiol Biotechnol 99:2623–2635
Thuku RN, Brady D, Benedik MJ, Sewell BT (2009) Microbial nitrilases: versatile, spiral forming, industrial enzymes. J Appl Microbiol 106:703–727
Wang JM, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174
Yamamoto H, Okamoto H (1980) Protection by picolinamide, a novel inhibitor of poly (ADP-ribose) synthetase, against both streptozotocin-induced depression of proinsulin synthesis and reduction of NAD content in pancreatic islets. Biochem Bioph Res Co 95:474–481
Zhang LJ, Yin B, Wang C, Jiang SQ, Wang HL, Yuan YA, Wei DZ (2014) Structural insights into enzymatic activity and substrate specificity determination by a single amino acid in nitrilase from Syechocystis sp PCC6803. J Struct Biol 188:93–101
Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (No. 2017M621390), the Fundamental Research Funds for the Central Universities (No. 222201814037), and National Natural Science Foundation of China (No. 21676090).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Jiang, S., Sun, Y. et al. Switching the secondary and natural activity of Nitrilase from Acidovorax facilis 72 W for the efficient production of 2-picolinamide. Biotechnol Lett 43, 1617–1624 (2021). https://doi.org/10.1007/s10529-021-03137-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03137-7