Abstract
Objective
To develop a safe and effective oral vaccine against Helicobacter pylori using its HpaA protein expressed in Lactococcus lactis.
Results
The gene encoding HpaA was obtained by PCR and ligated to pNZ8110-lysM following digestion with NaeI + SphI. The recombinant plasmid was transferred into E. coli for multiplication, and then into L. lactis. The recombinant L. lactis was induced to express HpaA, resulting in two products of 29 and 25 kDa, both of which yielded positive immunoreaction with mouse antisera against H. pylori, as confirmed by immunoblot assays. The 29 kDa product constituted 12% of the cell lysates. Oral inoculation with the engineered L. lactis evoked significantly elevated serum IgG level in mice (P < 0.05).
Conclusions
A novel engineered L. lactis strain was developed that efficiently produces whole HpaA protein with desired antigenicity and potent immunogenicity. It provides a basis for approaches to L. lactis-delivered anti-H. pylori vaccination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Helicobacter pylori infects more than half of the global population, causing a variety of diseases from gastritis to gastric cancer. The emergence of antibiotic-resistant strains, relatively high cost of therapies and patients’ compliance are significant problems, suggesting that an effective vaccine would be of great benefit (Mascellino et al. 2017).
Helicobacter pylori adhesin A (HpaA) is a lipoprotein located in the cellular outer membrane and flagellum sheath. Mucosal vaccination with HpaA plus cholera toxin was capable of inducing specific mucosal immune responses, thereby providing immune protective efficacy in mice (Flach et al. 2011). Formulation of multivalent vaccines by using certain antigenic epitopes of HpaA produced protective effects (Guo et al. 2017a, b). These data suggest the potential of HpaA for anti-H. pylori vaccination.
Probiotics like Lactococcus lactis have been used as oral vaccine delivery vehicles (Zhang et al. 2016; Gu et al. 2009). Lactococcus lactis is a Gram-positive bacterium, widely used in food processing with generally recognized safety. A recombinant L. lactis strain was engineered for expression of the N-terminus of HpaA in fusion with Neisseria meningitidis PorA, and oral vaccination with this strain induced increased specific serum IgG level in mice (Vasquez et al. 2015). We have tried producing a fusion of HpaA and Omp22 in L. lactis; however, only two undefined segments of the desired fusion protein were obtained (Zhang et al. 2016). Factually, the immune effects of L. lactis delivery of the single whole HpaA remain unclear. Therefore, we cloned the complete coding region of H. pylori hpaA gene into L. lactis. The resultant engineered strain was induced to express HpaA and used in oral vaccination of mice. This study established a novel L. lactis strain, which can express HpaA efficiently with desired immunoreactivity and potent immunogenicity, providing a critical basis for developing L. lactis-vectored anti-H. pylori vaccines.
Materials and methods
Bacteria and plasmids
The bacteria and plasmids used here were presented in Supplementary Table 1. In brief, L. lactis NZ3900 strain and pNZ8110 were provided by NIZO Food Research (Netherlands). The plasmid pNZ8110-lysM was constructed based on pNZ8110 with its sequence deposited in GenBank (Accession No. KY385375.1). Lactococcus lactis was cultured with GM17 (M17 broth supplied with 5 g glucose l−1) at 30 °C. Chloramphenicol was added to GM17 at 10 mg l−1 when cultivating bacteria containing pNZ8110 or its derivatives (Zhang et al. 2016).
Experimental animals
This project was given approval of animal experiments by the Institutional Review Board at Zhengzhou University. We complied with the ARRIVE guidelines when conducting this study. Specific-pathogen-free BALB/c mice, 6 w old, were purchased from the Henan Provincial Experimental Animal Center.
PCR of H. pylori hpaA
Genome DNA of H. pylori MEL-Hp27 was extracted using alkaline lysis methods, and used as PCR template for amplifying the whole coding region of hpaA gene. The PCR primers were 5′-CGTGCCGGCATGAAAGCAAATAATC-3′ (NaeI) and 5′–ACATGC ATGCTTATCGGTTTCT–3′ (SphI) in sequences. The rectangle wire frame shows the stop codon. The PCR parameters were 30 cycles of 94 °C for 60 s, 50 °C for 50 s and 72 °C for 50 s.
Constructing recombinant pNZ8110
The recombinant plasmid pNZ8110-lysM-hpaA was constructed through NaeI + SphI enzyme digestion and ligation reaction of hpaA and pNZ8110-lysM, and transferred into E. coli MC1061 using the heat-shock method. Then the plasmid was separated from E. coli, and introduced into L. lactis NZ3900 via electroporation (Zhang et al. 2009).
Production of mouse antisera against H. pylori
Helicobacter pylori NCTC11637 was cultured, collected and disrupted ultrasonically. Mice were immunized using mixture of the H. pylori cell lysate supernatant and Freund’s adjuvant by subcutaneous route as described before (Zhang et al. 2009). The mouse sera were collected by retro-orbital bleeding 1 w after immunization.
HpaA expression and identification
The engineered lactococcal strain, designated as NZ3900/pNZ8110-lysM-hpaA, was cultured using GM17, and expression of HpaA was induced using 40 µg nisin l−1 for 5 h (Chen et al. 2011). The culture supernatant was sampled as described elsewhere (Sun et al. 2017). The bacterial cell wall proteins were prepared from 10 ml of the bacterial culture. The cultivated bacteria were pelleted by centrifugation at 4 °C and 4.3 × 103 g for 5 min, washed using TES solution (200 g sucrose l−1, 1 mmol EDTA l−1, 50 mmol Tris/HCl l−1) for twice, and then suspended in 200 μl TES-LMR (TES containing 100 mg RNase l−1,30 g lysozyme l−1), kept at 37 °C for 2 h mixing at intervals for several times. The supernatant was separated by centrifugation at 25,000×g 4 °C for 10 min, added ice pre-cooling trichloroacetic acid at 160 g l−1, kept in ice for 20 min. The precipitate was obtained by centrifugation at 11,500×g and 4 °C for 10 min, suspended in 100 μl 50 mM NaOH and kept at 4 °C overnight. The supernatant was used as the cell wall protein samples. Preparation of cell lysate samples and western blot assays were carried out as described before (Zhang et al. 2009).
Immunization and sampling
The mice were divided at random into three groups (six mice each). The pNZ8110 group, HpaA group and PBS group were treated by oral gavage with 200 µl NZ3900/pNZ8110 (5 × 1014 CFU l−1), NZ3900/pNZ8110-lysM-hpaA (5 × 1014 CFU l−1) and PBS, respectively, for four times at weekly intervals. The sera and intestinal juice were sampled 2 weeks post-immunization (Sun et al. 2017).
Assessment of antibodies
As an ELISA detector antigen for antibody assay, recombinant HpaA (rHpaA) was obtained by purification from isopropyl thiogalactoside (IPTG)-induced E. coli TB1/pMAL-c2x-hpaA via amylose resin affinity chromatography (Huang et al. 2013). The serum IgG and intestinal SIgA were assayed using ELISA (Zhang et al. 2016).
Data analysis
Statistical analysis was performed using the software SPSS17.0. One-way variance analysis and Bonferroni test were used for detection of difference among the groups. Statistically significant difference was determined at P < 0.05.
Results
Amplification of hpaA
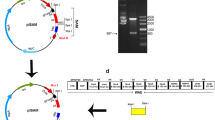
The coding region of hpaA was obtained by PCR from H. pylori MEL-Hp27, having a length of 783 base pairs (bp) (Fig. 1a) and a sequence as shown in GenBank (DQ353891), as determined by agarose gel electrophoresis and sequencing.
Agarose gel electrophoresis of hpaA PCR product (a) and identification of the constructed plasmid by nuclease digestion (b). a Lane 1 DNA markers. Lane 2,3,4 hpaA PCR product. Lane 5 negative control. b Lane 1 DNA ladder. Lane 2 hpaA PCR product. Lane 3 NaeI + SphI digestion product of pNZ8110-lysM-hpaA. Lane 4 NaeI + SphI digestion product of pNZ8110-lysM. Lane 5 pNZ8110-lysM-hpaA digested with SphI
Engineering L. lactis
The hpaA was ligated to pNZ8110-lysM and cloned into E. coli MC1061, from which the recombinant plasmid pNZ8110-lysM-hpaA was extracted and transferred into L. lactis NZ3900. The recombinant plasmid was extracted from the L. lactis transformants, and underwent NaeI + SphI enzyme digestion, resulting in two products of approximately 780 and 3.7 × 103 bp (lane 3 in Fig. 1b). The gene sequencing showed that the cloned gene in the recombinant plasmid possessed the desired sequence. These findings demonstrate a successfully constructed recombinant L. lactis strain, which was designated as L. lactis NZ3900/pNZ8110-lysM-hpaA. The structure of pNZ8110-lysM- hpaA was shown in Fig. 2.
L. lactis expression of HpaA
HpaA expression in the engineered strain was induced when the OD600 of culture = 0.3 using 40 μg nisin l−1 and lasted for 5 h. As shown in SDS-PAGE patterns, there were two protein strips with enhanced density, located at 29 and 25 kDa, in both the cell lysate (lane 3 in Fig. 3) and cell wall protein samples (lane 7 in Fig. 3) of NZ3900/pNZ8110-lysM-hpaA, compared with the samples of NZ3900/pNZ8110-lysM (lane 2, 6 in Fig. 3). The percentage of the 29 kDa protein was estimated to be 12% in the cell lysate proteins of the recombinant strain. In the samples of the culture supernatant, no desired expression product of HpaA was detected (lane 4, 5 in Fig. 3).
SDS-PAGE of expression products of the nisin-induced lactococcal strains. Lane 1 protein markers. Lane 2 cell lysate ptoreins of NZ3900/pNZ8110-lysM. Lane 3 cell lysate proteins of NZ3900/pNZ8110-lysM-hpaA. Lane 4 culture supernatant of NZ3900/pNZ8110-lysM. Lane 5 culture supernatant of NZ3900/pNZ8110-lysM-hpaA. Lane 6 cell wall proteins of NZ3900/pNZ8110-lysM. Lane 7 cell wall proteins of NZ3900/pNZ8110-lysM-hpaA. The 29 and 25 kDa protein strips were denser in the cell lysate and cell wall protein samples of NZ3900/pNZ8110-lysM-hpaA than in the controls
Immunoreactivity of L. lactis expressed HpaA
One week after immunization with solvent H. pylori cell lysate antigens, the mouse sera were collected from mice, and used in the following westernblot analysis for identification of the immunoreactivity of the L. lactis-expressed HpaA. As results, both the 29 and 25 kDa proteins in the cell lysate and cell wall samples of NZ3900/pNZ8110-lysM-hpaA yielded positive immunoreaction with the mouse anti-H. pylori sera, while no positive strips were found at the corresponding locations in the controls (Fig. 4). No remarkable differential strips were recognized in culture supernatant samples between the two strains. These results demonstrated successful expression of the whole HpaA (29 kDa) in the engineered L. lactis.
Westernblot analysis of L. lactis expression products of HpaA using mouse antisera against H. pylori. Lane 1 protein markers. Lane 2 cell lysate sample of NZ3900/pNZ8110-lysM-hpaA. Lane 3 cell lysate sample of NZ3900/pNZ8110-lysM. Lane 4 culture supernatant of NZ3900/pNZ8110-lysM-hpaA. Lane 5 culture supernatant of NZ3900/pNZ8110-lysM. Lane 6 cell wall proteins of NZ3900/pNZ8110-lysM-hpaA. Lane 7 cell wall proteins of NZ3900/pNZ8110-lysM. Both the 29 and 25 kDa proteins in the cell lysate and cell wall samples of NZ3900/pNZ8110-lysM-hpaA yielded positive immunoreaction with the mouse anti-H. pylori sera
Assessment of specific antibodies
The rHpaA was purified from the IPTG-induced E. coli TB1/pMAL-c2x-hpaA with approx. 90% purity. Two weeks after the oral immunization with the engineered L. lactis, mouse sera and intestinal juice were sampled. ELISA assays were performed for detection of the anti-HpaA antibodies in the samples. As shown in Fig. 5, the NZ3900/pNZ8110-lysM-hpaA immunized group has significantly enhanced serum anti-HpaA IgG levels, as compared with the groups treated with NZ3900/pNZ8110 and PBS, respectively (P < 0.05). However, no statistical difference was found in mucosal SIgA antibody levels among the three groups.
Assessment of the serum IgG and intestinal SIgA in mice. *P < 0.05, compared with the controls treated with NZ3900/pNZ8110 and PBS, respectively. This figure shows that the group received oral gavage with the engineered L. lactis producing HpaA has significantly enhanced serum IgG level, in comparison with the controls. The SIgA antibody level is higher in the mice immunized using NZ3900/pNZ8110-lysM-hpaA than in the controls, but the difference was statistically insignificant (P > 0.05)
Discussion
As an etiological agent of a variety of gastric and even extra-gastric diseases, H. pylori has drawn increased attention since it was first observed in 1984. Vaccination is a promising preventive measure and therapy for H. pylori infection. However, H. pylori vaccine development has not progressed as expected (Mirzaei et al. 2017). The important but controversial issues in present studies include determination of protective immunogens, safe adjuvant and vaccine delivery vehicles (Mirzaei et al. 2017). HpaA has been identified as a protective antigen while L. lactis has been proved as a safe vehicle for orally administered vaccines (Liu et al. 2017; Sun et al. 2017), suggesting that engineering of L. lactis to deliver HpaA might be a considerable step toward the efficient and safe H. pylori vaccine.
Lactococcus lactis has been used as a cell factory for a variety of exogenous proteins in part due to its outstanding safety. However, the productivity might be rather low when using L. lactis for expression of certain H. pylori proteins like HspA (Zhang et al. 2015). The difference in expression regulation mechanism among various bacterial species, especially between gram positive and negative species, might contribute to this phenomenon. In this study, HpaA was expressed as 29 and 25 kDa proteins, both clearly visuable in SDS-PAGE and recognized by H. pylori specific antisera in western blots, indicating high production efficiency of HpaA in the engineered L. lactis and application potential for delivery of this crucial antigen. The 29 kDa product should be the whole HpaA protein, and the 25 kDa protein might be an incomplete or partially degraded expression product, as inferred by sequence analysis using software Omiga 2.0.
Since subcellular location of antigens in bacteria might greatly affect the immune efficacy, we assayed the cell lysate, culture supernatant and cellular wall proteins using SDS-PAGE and western blots. The vector plasmid pNZ8110-lysM conjugated with NZ3900 was supposed to be able to express exogenous proteins targeting at various subcellular locations, such as cytoplasmic, secretory and surface-exposed, by using different gene inserting sites in the plasmid and whether or not deleting stop codons in the cloned genes. As HpaA is a membrane lipoprotein with lipid attachment site at the 28th amino acid (cysteine) of the N terminus, and thereby possesses capacity of adherence to cellular membrane, we cloned the hpaA gene comprising the stop codon, and inserted it downstream of the Usp45 signal peptide and upstream of the cellular wall anchor motif lysM. SDS-PAGE showed that HpaA was detectable in the cell lysate and cell wall proteins, but not in the culture supernatant samples, suggesting mostly cytoplasmic accumulation of the expression product. Western blot results demonstrated immunoreactivity of the expression product. The expression regulation mechanism of L. lactis is worth further investigation.
Oral administration of purified H. pylori HpaA conferred a remarkable immune effect but, to an extent, this depended on conjugated use of mucosal adjuvant like cholera toxin. To take the advantage of the immune adjuvant activity and protection of L. lactis from digestion by digestive juice, we delivered HpaA to the gastrointestinal mucosal immune sites via oral vaccination with the engineered L. lactis. As observed, the group administrated with the recombinant strain had significantly enhanced serum IgG level than the controls, indicating that HpaA was efficiently delivered to the immune sites, stimulating the mucosal immune system, and provoking humoral immune responses. The intestinal SIgA level is higher in the HpaA treated group than the controls, but the difference cannot be considered as statistically significant, most probably owing to the relatively less sample sizes of the groups (n = 6, each group).
Conclusion
This study successfully constructed an engineered L. lactis strain, which can efficiently produce HpaA and deliver HpaA to the mucosal immune site by oral administration, evoking significant serum specific IgG responses. These observations form a critical foundation for developing Lactococcus-delivered oral anti-H. pylori vaccines.
References
Blanchard TG, Czinn SJ (2015) Current status and prospects for a Helicobacter pylori vaccine. Gastroenterol Clin North Am 44:677–689
Chen SY, Zhang RG, Duan GC, Shi JX (2011) Food-grade expression of Helicobacter pylori ureB subunit in Lactococcus lactis and its immunoreactivity. Curr Microbiol 62:1726–1731
Flach CF, Svensson N, Blomquist M, Ekman A, Raghavan S, Holmgren J (2011) A truncated form of HpaA is a promising antigen for use in a vaccine against Helicobacter pylori. Vaccine 29:1235–1241
Gu Q, Song D, Zhu M (2009) Oral vaccination of mice against Helicobacter pylori with recombinant Lactococcus lactis expressing urease subunit B. FEMS Immunol Med Microbiol 56:197–203
Guo L, Yang H, Tang F et al (2017a) Oral immunization with a multivalent epitope-based vaccine, based on NAP, Urease, HSP60, and HpaA, provides therapeutic effect on H. pylori infection in mongolian gerbils. Front Cell Infect Microbiol 7:349
Guo L, Zhang J, Cui L et al (2017b) Crystallization and X-ray analysis of the extracellular adhesion domain of Helicobacter pylori adhesin A: the significance of the cation composition in the crystallization precipitant. Acta Crystallogr F Struct Biol Commun 73:202–208
Huang X, Xu B, Duan G, Song C (2013) The rOmp22-HpaA fusion protein confers protective immunity against Helicobacter pylori in mice. Curr Microbiol 67:487–492
Liu W, Tan Z, Liu H et al (2017) Nongenetically modified Lactococcus lactis-adjuvanted vaccination enhanced innate immunity against Helicobacter pylori. Helicobacter 22:e12426
Mascellino MT, Porowska B, De Angelis M, Oliva A (2017) Antibiotic susceptibility, heteroresistance, and updated treatment strategies in Helicobacter pylori infection. Drug Des Dev Ther 11:2209–2220
Mirzaei N, Poursina F, Moghim S, Rashidi N, Ghasemian Safaei H (2017) The study of H. pylori putative candidate factors for single- and multi-component vaccine development. Crit Rev Microbiol 43:631–650
Sun N, Zhang R, Duan G, Peng X, Wang C, Fan Q, Chen S, Xi Y (2017) An engineered food-grade Lactococcus lactis strain for production and delivery of heat-labile enterotoxin B subunit to mucosal sites. BMC Biotechnol 17:25
Vasquez AE, Manzo RA, Soto DA et al (2015) Oral administration of recombinant Neisseria meningitidis PorA genetically fused to H. pylori HpaA antigen increases antibody levels in mouse serum, suggesting that PorA behaves as a putative adjuvant. Hum Vaccin Immunother 11:776–788
Zhang XJ, Duan GC, Zhang RG, Fan QT (2009) Optimized expression of Helicobacter pylori ureB gene in Lactococcus lactis NICE system and experimental study on its immunoreactivity. Curr Microbiol 58:308–314
Zhang XJ, Feng SY, Li ZT, Feng YM (2015) Expression of Helicobacter pylori hspA gene in Lactococcus lactis NICE system and experimental study on its immunoreactivity. Gastroenterol Res Pract 2015:750932
Zhang R, Duan G, Shi Q, Chen S, Fan Q, Sun N, Xi Y (2016) Construction of a recombinant Lactococcus lactis strain expressing a fusion protein of Omp22 and HpaA from Helicobacter pylori for oral vaccine development. Biotechnol Lett 38:1911–1916
Acknowledgements
This study was financially supported by the China Postdoctoral Science Foundation (200801273) and National Natural Science Foundation of China (81773495).
Author contributions
R. Zhang and G. Duan designed the project, interpreted the data and wrote the manuscript. W. Chen constructed the engineered L. lactis strain. C. Wang and W. Chen performed expression of HpaA, SDS-PAGE and Western blot analysis. Q. Shi, S. Chen and Q. Fan carried out the animal experimentation.
Supporting information
Supplementary Table 1—Bacteria and plasmids used.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, R., Wang, C., Cheng, W. et al. Delivery of Helicobacter pylori HpaA to gastrointestinal mucosal immune sites using Lactococcus lactis and its immune efficacy in mice. Biotechnol Lett 40, 585–590 (2018). https://doi.org/10.1007/s10529-017-2502-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2502-3