Abstract
Objectives
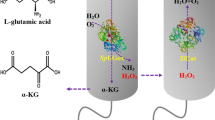
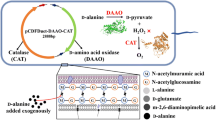
To improve the production of α-ketoglutaric acid (α-KG) from l-glutamate by whole-cell biocatalysis.
Results
A novel and highly active l-glutamate oxidase, SmlGOX, from Streptomyces mobaraensis was overexpressed and purified. The recombinant SmlGOX was approx. 64 kDa by SDS-PAGE. SmlGOX had a maximal activity of 125 ± 2.7 U mg−1 at pH 6.0, 35 oC. The apparent Km and Vmax values of SmlGOX were 9.3 ± 0.5 mM and 159 ± 3 U mg−1, respectively. Subsequently, a co-expression plasmid containing the SmlGOX and KatE genes was constructed to remove H2O2, and the protein levels of SmlGOX were improved by codon optimization. Finally, by optimizing the whole-cell transformation conditions, the production of α-KG reached 77.4 g l−1 with a conversion rate from l-glutamate of 98.5% after 12 h.
Conclusions
An efficient method for the production of α-KG was established in the recombinant Escherichia coli, and it has a potential prospect in industrial application.





Similar content being viewed by others
References
Angov E, Legler PM, Mease RM (2011) Adjustment of codon usage frequencies by codon harmonization improves protein expression and folding. Meth Mol Biol 705:1–13
Arima J, Tamura T, Kusakabe H, Ashiuchi M, Yagi T, Tanaka H, Inagaki K (2003) Recombinant expression, biochemical characterization and stabilization through proteolysis of an l-glutamate oxidase from Streptomyces sp. X-119-6. J Biochem 134:805–812
Arima J, Sasaki C, Sakaguchi C, Mizuno H, Tamura T, Kashima A, Kusakabe H, Sugio S, Inagaki K (2009) Structural characterization of l-glutamate oxidase from Streptomyces sp. X-119-6. FEBS J 276:3894–3903
Góth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Hossain GS, Li J, Shin HD, Chen RR, Du G, Liu L, Chen J (2014a) Bioconversion of l-glutamic acid to alpha-ketoglutaric acid by an immobilized whole-cell biocatalyst expressing l-amino acid deaminase from Proteus mirabilis. J Biotechnol 169:112–120
Hossain GS, Li J, Shin HD, Liu L, Wang M, Du G, Chen J (2014b) Improved production of alpha-ketoglutaric acid (alphα-KG) by a Bacillus subtilis whole-cell biocatalyst via engineering of l-amino acid deaminase and deletion of the alphα-KG utilization pathway. J Biotechnol 187:71–77
Huang HJ, Liu LM, Li Y, Du GC, Chen J (2006) Redirecting carbon flux in Torulopsis glabrata from pyruvate to alpha-ketoglutaric acid by changing metabolic co-factors. Biotechnol Lett 28:95–98
Liu L, Hossain GS, Shin HD, Li J, Du G, Chen J (2013) One-step production of alpha-ketoglutaric acid from glutamic acid with an engineered l-amino acid deaminase from Proteus mirabilis. J Biotechnol 164:97–104
Liu Q, Cheng H, Ma X, Xu N, Liu J, Ma Y (2016) Expression, characterization and mutagenesis of a novel glutamate decarboxylase from Bacillus megaterium. Biotechnol Lett 38:1107–1113
Niu P, Dong X, Wang Y, Liu L (2014) Enzymatic production of alpha-ketoglutaric acid from l-glutamic acid via l-glutamate oxidase. J Biotechnol 179:56–62
Stottmeister U, Aurich A, Wilde H, Andersch J, Schmidt S, Sicker D (2005) White biotechnology for green chemistry: fermentative 2-oxocarboxylic acids as novel building blocks for subsequent chemical syntheses. J Ind Microbiol Biotechnol 32:651–664
Sukhacheva MV, Netrusov AI (2000) Streptomyces sp. Z-11-6, a novel producer of extracellular l-glutamate oxidase. Microbiology 69:14–16
Wachiratianchai S (2004) Isolation, purification, and characterization of l-glutamate oxidase from Streptomyces sp. 18G. Electron J Biotechnol 7:275–281
Wang Y, Rao B, Yan H, Han R, Li L, Liao P, Ma L (2017) High-level expression of l-glutamate oxidase in Pichia pastoris using multi-copy expression strains and high cell density cultivation. Protein Expr Purif 129:108–114
Yovkova V, Otto C, Aurich A, Mauersberger S, Barth G (2014) Engineering the alpha-ketoglutarate overproduction from raw glycerol by overexpression of the genes encoding NADP + -dependent isocitrate dehydrogenase and pyruvate carboxylase in Yarrowia lipolytica. Appl Microbiol Biotechnol 98:2003–2013
Zhang D, Liang N, Shi Z, Liu L, Chen J, Du G (2009) Enhancement of α-ketoglutarate production in Torulopsis glabrata: redistribution of carbon flux from pyruvate to α-ketoglutarate. Biotechnol Bioprocess 14:134–139
Zhou J, Zhou H, Du G, Liu L, Chen J (2010) Screening of a thiamine-auxotrophic yeast for alpha-ketoglutaric acid overproduction. Lett Appl Microbiol 51:264–271
Zhou J, Yin X, Madzak C, Du G, Chen J (2012) Enhanced alpha-ketoglutarate production in Yarrowia lipolytica WSH-Z06 by alteration of the acetyl-CoA metabolism. J Biotechnol 161:257–264
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 31500044, 31571029, 31501475), the Natural Science Foundation of Tianjin (15JCYBJC30300, 15JCTPJC55400), the Key Projects in the Tianjin Science and Technology Pillar Program (No. 11ZCZDSY08600), and the “Hundred Talents Program” of the Chinese Academy of Sciences.
Supporting information
Supplementary Table 1—Strains and plasmids used.
Supplementary Table 2—Primers used.
Supplementary Table 3—The substrate specificity of SmLGOX.
Supplementary Fig. 1—Effects of metal ions on the activity of the recombinant SmLGOX. The relative activity was normalized as percentage activity of the control sample (125.2 ± 2.7 U mg−1). The data are presented as mean ± standard deviation (SD) from three independent experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qingdai Liu and Xiaoqian Ma have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2017_2314_MOESM2_ESM.tif
Supplementary Fig. 1 Effects of metal ions on the activity of the recombinant SmLGOX. The relative activity was normalized as percentage activity of the control sample (125.2 ± 2.7 U mg−1). The data are presented as mean ± standard deviation (SD) from three independent experiments. (TIFF 7626 kb)
Rights and permissions
About this article
Cite this article
Liu, Q., Ma, X., Cheng, H. et al. Co-expression of l-glutamate oxidase and catalase in Escherichia coli to produce α-ketoglutaric acid by whole-cell biocatalyst. Biotechnol Lett 39, 913–919 (2017). https://doi.org/10.1007/s10529-017-2314-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2314-5