Abstract
Objectives
To enhance the efficiency of influenza virosome-mediated gene delivery by engineering this virosome.
Results
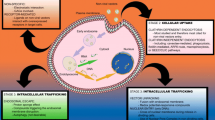
A novel chimeric influenza virosome was constructed containing the glycoprotein of Vesicular stomatitis virus (VSV-G), along with its own hemagglutinin protein. To optimize the transfection efficiency of both chimeric and influenza cationic virosomes, HEK cells were transfected with plasmid DNA and virosomes and the transfection efficiency was assessed by FACS analysis. The chimeric virosome was significantly more efficient in mediating transfection for all amounts of DNA and virosomes compared to the influenza virosome.
Conclusions
Chimeric influenza virosome, including VSV-G, is superior to the conventional influenza virosome for gene delivery.







Similar content being viewed by others
References
Barsoum J, Brown R, McKee M, Boyce FM (1997) Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther 8:2011–2018
Carneiro FA, Bianconi ML, Weissmüller G et al (2002) Membrane recognition by vesicular stomatitis virus involves enthalpy-driven protein-lipid interactions. J Virol 76:3756–3764
De Jonge J, Holtrop M, Wilschut J, Huckriede A (2006a) Reconstituted influenza virus envelopes as an efficient carrier system for cellular delivery of small-interfering RNAs. Gene Ther 13:400–411
De Jonge J, Schoen P, terVeer W et al (2006b) Use of a dialyzable short-chain phospholipid for efficient solubilization and reconstitution of influenza virus envelopes. Biochim Biophys Acta 1758:527–536
De Jonge J, Leenhouts JM, Holtrop M et al (2007) Cellular gene transfer mediated by influenza virosomes with encapsulated plasmid DNA. Biochem J 405:41–49
Gaudin Y, Tuffereau C, Segretain D et al (1991) Reversible conformational changes and fusion activity of rabies virus glycoprotein. J Virol 65:4853–4859
Gurusinghe S, Young P, Michelsen J, Strappe P (2015) Suppression of dedifferentiation and hypertrophy in canine chondrocytes through lentiviral vector expression of Sox9 and induced pluripotency stem cell factors. Biotechnol Lett 37:1495–1504
Irvine DJ, Swartz MA, Szeto GL (2013) Engineering synthetic vaccines using cues from natural immunity. Nat Mater 12:978–990
Machida K, Imataka H (2015) Production methods for viral particles. Biotechnol Lett 37:753–760
Mizuarai S, Ono K, Yamaguchi K et al (2001) Production of transgenic quails with high frequency of germ-line transmission using VSV-G pseudotyped retroviral vector. Biochem Biophys Res Commun 286:456–463
Ohkuma S, Poole B (1978) Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 75:3327–3331
Park S, Pyo C-W, Choi S-Y (2014) High-efficiency lentiviral transduction of primary human CD34+ hematopoietic cells with low-dose viral inocula. Biotechnol Lett 37:1–8
Paternostre MT, Lowy RJ, Blumenthal R (1989) pH-dependent fusion of reconstituted vesicular stomatitis virus envelopes with vero cells: measurement by dequenching of fluorescence. FEBS Lett 243:251–258
Puri A, Booy FP, Doms RW et al (1990) Conformational changes and fusion activity of influenza virus hemagglutinin of the H2 and H3 subtypes: effects of acid pretreatment. J Virol 64:3824–3832
Schoen P, Chonn A, Cullis PR et al (1999) Gene transfer mediated by fusion protein hemagglutinin reconstituted in cationic lipid vesicles. Gene Ther 6:823–832
Acknowledgment
This work was supported by a grant from Pasteur Institute, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest
Additional information
Yahya Mohammadzadeh and Narges Rasouli are equal first authors.
Rights and permissions
About this article
Cite this article
Mohammadzadeh, Y., Rasouli, N., Aref, M.H.S. et al. A novel chimeric influenza virosome containing Vesicular stomatitis G protein as a more efficient gene delivery system. Biotechnol Lett 38, 1321–1329 (2016). https://doi.org/10.1007/s10529-016-2108-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2108-1