Abstract
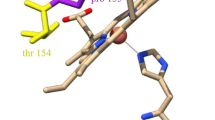
The major O2-insensitive nitroreductase (NfsA) of Escherichia coli shares low sequence homology but similar biochemical and structural features with NfsB, the E. coli minor O2-insensitive nitroreductase. A structural comparison revealed Phe42 was present in the active site of NfsA but not NfsB. F42Y, F42N and F42A were generated and had decreased activity toward nitrofurazone by 52, 96, and 99 %, respectively. The kinetic parameters for other nitroaromatic substrates were also determined. Compared to wild type, the mutants did not have significantly altered K ms, but had dramatically decreased k cat and k cat/K m values. Far-UV CD spectral analysis of the mutants suggested that there were no significant conformational changes however F42A and F42N had changes from 208 to 222 nm, which was attributed to loss of helix content. These findings revealed that Phe42 is important for maintaining NfsA activity and structure.







Similar content being viewed by others
References
Anlezark GM, Melton RG, Sherwood RF, Coles B, Friedlos F, Knox RJ (1992) The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954)I. Purification and properties of a nitroreductase enzyme from Escherichia coli-a potential enzyme for antibody-directed enzyme prodrug therapy (ADEPT). Biochem Pharmacol l44:2289–2295
Friedlos F, Court S, Ford M, Denny WA, Springer C (1998) Gene-directed enzyme prodrug therapy: quantitative bystander cytotoxicity and DNA damage induced by CB1954 in cells expressing bacterial nitroreductase. Gene Ther 5(1):105–112
Grove JI, Lovering AL, Guise C, Race PR, Wrighton CJ, White SA, Hyde EI, Searle PF (2003) Generation of Escherichia coli nitroreductase mutants conferring improved cell sensitization to the prodrug CB1954. Cancer Res 63:5532–5537
Haynes CA, Koder RL, Miller AF, Rodgers DW (2002) Structures of nitroreductase in three states. J Biol Chem 277:11513–11520
Jaberipour M, Vass SO, Guise CP, Grove JI, Knox RJ, Hu L, Hyde EI, Searle PF (2010) Testing double mutants of the enzyme nitroreductase for enhanced cell sensitisation to prodrugs: effects of combining beneficial single mutations. Biochem Pharmacol 79:102–111
Knox RJ, Friedlos F, Sherwood RF, Melton RG, Anlezark GM (1992) The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954)II. A comparison of an Eschericia coli nitroreductase and Walker DT diaphorase. Biochem Pharmacol 44:2297–2301
Kobori T, Sasaki H, Lee WC, Zenno S, Saigo K, Murphy ME, Tanokura M (2001) Structure and site-directed mutagenesis of a flavoprotein from Escherichia coli that reduces nitrocompounds: alteration of pyridine nucleotide binding by a single amino acid substitution. J Biol Chem 276:2816–2823
Lovering AL, Hyde EI, Searle PF, White SA (2001) The structure of Escherichia coli nitroreductase complexed with nicotinic acid: three crystal forms at 1.7 Å, 1.8 Å and 2.4 Å resolution. J Mol Biol 309:203–213
María DR, Pérez-Reinado E, Castillo F, Moreno-Vivián C (2008) Reduction of polynitroaromatic compounds: the bacterial nitroreductases. FEMS Microbiol Rev 32:474–500
Prosser GA, Copp JN, Syddall SP, Williams EM, Smaill JB, Wilson WR, Patterson AV, Ackerley DF (2010) Discovery and evaluation of Escherichia coli nitroreductases that activate the anti-cancer prodrug CB1954. Biochem Pharmacol 79:678–687
Race PR, Lovering AL, Green RM, Ossor A, White SA, Searle PF, Wrighton CJ, Hyde EI (2005) Structural and mechanistic studies of Escherichia coli nitroreductase with the antibiotic nitrofurazone. J Biol Chem 280:13256–13264
Race PR, Lovering AL, White SA, Grove JI, Searle PF, Wrighton CW, Hyde EI (2007) Kinetic and structural characterisation of Escherichia coli nitroreductase mutants showing improved efficacy for the prodrug substrate CB1954. J Mol Biol 368:481–492
San KY, Bennett GN, Berrios-Rivera SJ (2002) Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng 4:182–192
Vass SO, Jarrom D, Wilson WR, Hyde EI, Searle PF (2009) E. coli NfsA: an alternative nitroreductase for prodrug activation gene therapy in combination with CB1954. Brit J Cancer 100:1903–1911
Zenno S, Koike H, Kumar AN, Jayaraman R, Tanokura M, Saigo K (1996) Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J Bacteriol 178:4508–4514
Zigansbin AM, Gerlacb R, Borch T, Naumov AV, Naumova RP (2007) Production of eight different bydride complexes and nitrite release from 2,4,6-trinitrotoluene by Yarrowia lipolytica. Appl Environ Microb 73(24):7898–7905
Acknowledgments
The authors acknowledge the financial support provided by the National Key Project for Basic Research (2010CB126102), the National High Technology Research and Development Program of China (2011AA10A204), Science and Technology Project of Liaoning Province (2012225113), The Fundamental Research Funds for the Central Universities (DUT13JB08) and Dalian Municipal Science and Technology Program (2013E13SF114).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Zhan, J., Bai, J. et al. Residue Phe42 is critical for the catalytic activity of Escherichia coli major nitroreductase NfsA. Biotechnol Lett 35, 1693–1700 (2013). https://doi.org/10.1007/s10529-013-1262-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1262-y