Abstract
Purpose of work
Plants synthesize and accumulate secondary metabolites as defensive volatiles against diverse stresses. We aim to unravel the jasmonate-inducible volatile de novo synthetic metabolites in plants using a deuterium-labeling technique.
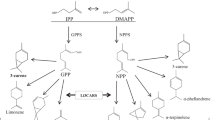
Jasmonic acid and its methyl ester (MeJA) are well-documented for inducing defensive volatiles. Here, we have developed an efficient deuterium oxide (D2O)-based labeling approach to determine the extent of de novo synthetic metabolites in a model plant A. bidentata bidentata. The labeling approach was demonstrated on quantitative profiling of terpene volatile organic compounds (VOCs) elicited by airborne MeJA in Achyranthes plants. We show, for the first time that airborne MeJA-elicited terpene VOCs are predominantly and differentially de novo synthesized except for a homoterpene, (3E)-4,8-dimethyl-1,3,7-nonatriene, which is weakly and least labelled with deuterium. D2O is therefore an efficient labeling source for investigating de novo synthetic metabolites of terpene VOCs in planta.


Similar content being viewed by others
References
Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defenses. Biochim Biophys Acta 1734:91–111
Boland W, Gälber A (1989) Biosynthesis of homo-terpenes in higher plants. Helv Chim Acta 72:247–253
Degenhardt J, Gershenzon J (2000) Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210:815–822
Dicke M, van Loon JJA (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl 97:237–249
Dicke M, van Loon JJA, Soler R (2009) Chemical complexity of volatiles from plants induced by multiple attack. Nature Chem Biol 5:317–324
Fonseca S, Chini A, Hamberg M et al (2009) (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nature Chem Biol 5:344–350
Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105:7100–7105
Köllner TG, Schnee C, Gershenzon J, Degenhardt J (2004) The variability of sesquiterpenes emitted from two zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereo selective multiple product enzymes. Plant Cell 16:1115–1131
Paré P, Tumlinson J (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114:1161–1167
Schnee C, Köllner TJ, Held M, Turlings TCJ, Gershenzon J, Degenhard J (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 24:1129–1134
Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16:2117–2127
Suza WP, Rowe ML, Hamberg M, Staswick PE (2010) A tomato enzyme synthesize (+)-7-iso-jasmonuyl-L-isoleicine in wound leaves. Planta 231:717–728
Takabayashi J, Sabelis MW, Janssen A, Shiojiri K, van Wijk M (2006) Can plants betray the presence of multiple herbivore species to predators and parasitoids? The role of learning in phytochemical information networks. Eco Res 21:3–8
Tamogami S, Rakwal R, Agrawal GK (2008) Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem Biophys Res Commun 376:723–727
Tamogami S, Agrawal GK, Rakwal R (2010) An in planta technique for cis-/trans-stereochemical analysis of jasmonoyl isoleucine. J Plant Physiol 167:933–937
Tamogami S, Rakwal R, Agrawal GK (2011a) Jasmonates to jasmolites in plants: past, present and future. Adv Bot Res 60:309–348
Tamogami S, Takahashi Y, Abe M, Noge K, Rakwal R, Agrawal GK (2011b) Conversion of airborne nerolidol to DMNT emission requires additional signals in A. bidentata bidentata. FEBS Lett 585:1807–1813
Tamogami S, Noge K, Abe M, Agrawal GK, Rakwal R (2012) Methyl jasmonate is transported to distal leaves via vascular process metabolizing itself into JA-Ile and triggering VOCs emission as defensive metabolites. Plant Signal Behav 7:1–4
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Acknowledgments
ST appreciates Japan Society for the Promotion of Science for a research fund (JSPS; ID Number C-24580165). GKA appreciates Japan Society for the Promotion of Science (JSPS; ID Number S-10182) for research at NIAS and collaborations therein with ST and RR. RR acknowledges the great support of Professor Yoshihiro Shiraiwa (Provost, Faculty of Life and Environmental Sciences, University of Tsukuba) and Prof. Seiji Shioda and Dr. Tetsuo Ogawa (Department of Anatomy I, Showa University School of Medicine) in promoting interdisciplinary research and unselfish encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamogami, S., Noge, K., Abe, M. et al. Deuterium labeling for investigating de novo synthesis of terpene volatiles in Achyranthes bidentata . Biotechnol Lett 35, 1247–1252 (2013). https://doi.org/10.1007/s10529-013-1201-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1201-y